Multiblock Analysis of Risk Factors and Management Areas of Calf Mortality in Large-Scale Dairy Herds
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Herd Recruitment
2.2. On-Farm Data Collection
2.3. Sample Collection
2.4. Laboratory Analyses and Data Interpretation
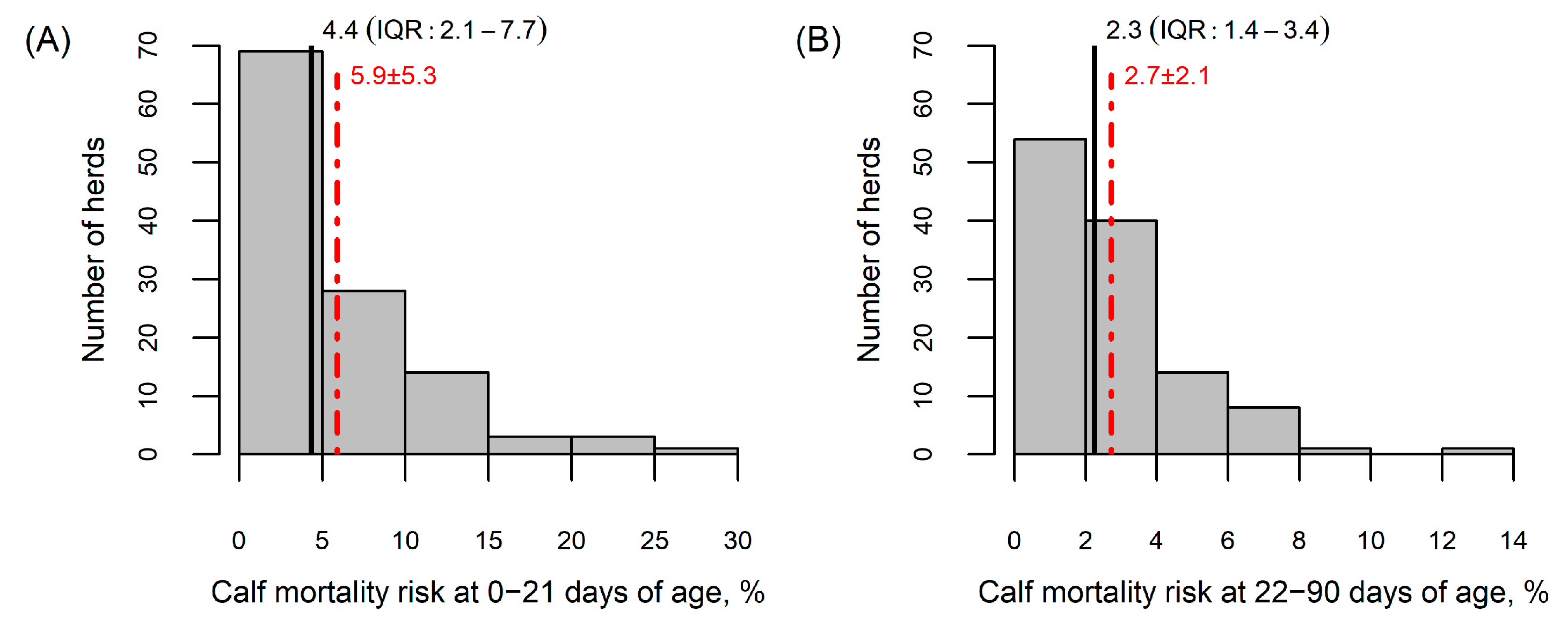
2.5. Mortality Calculations and Farm-Level Data
2.6. Data Editing
2.7. Statistical Analysis
3. Results
3.1. General Characteristics
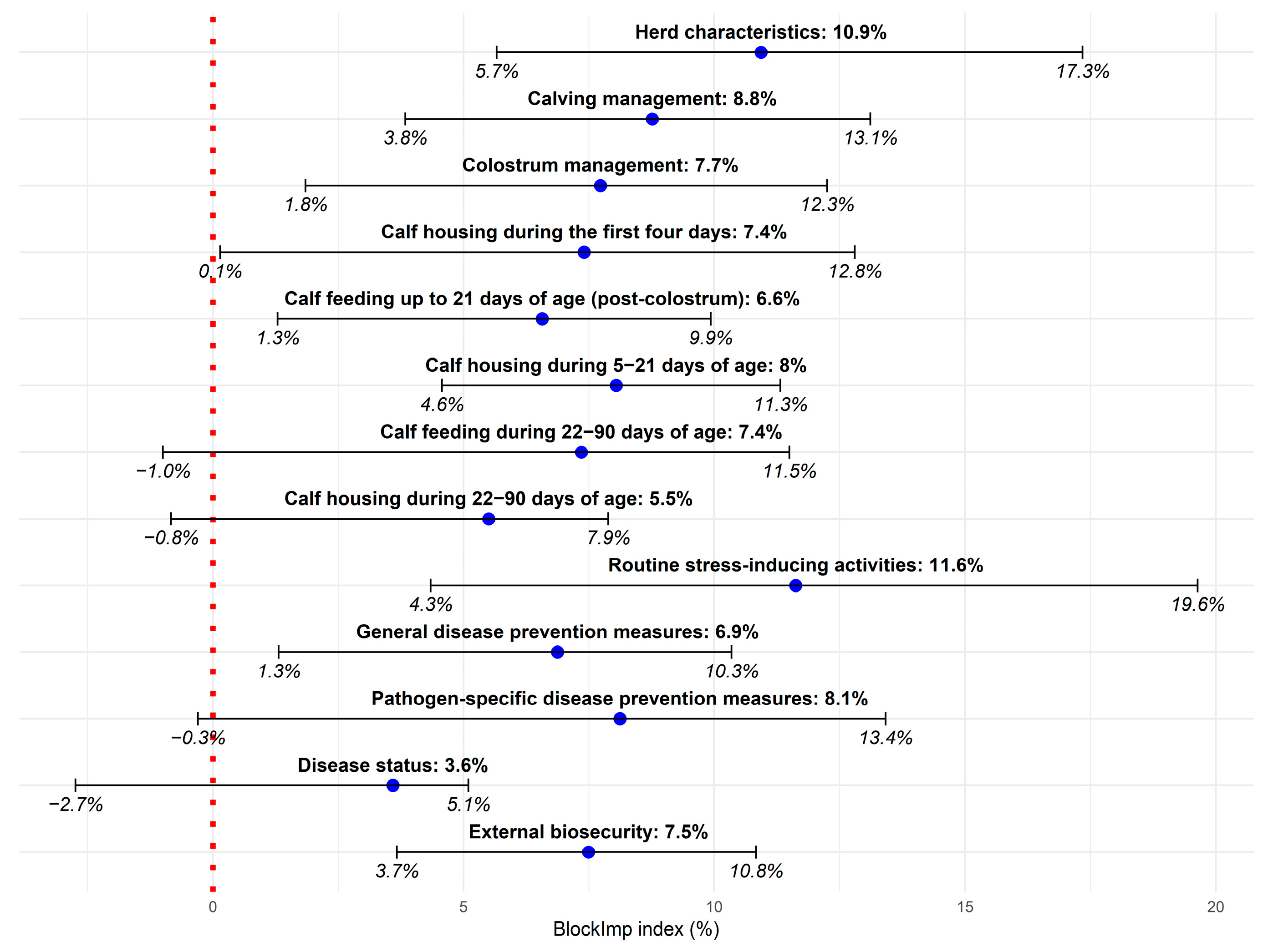
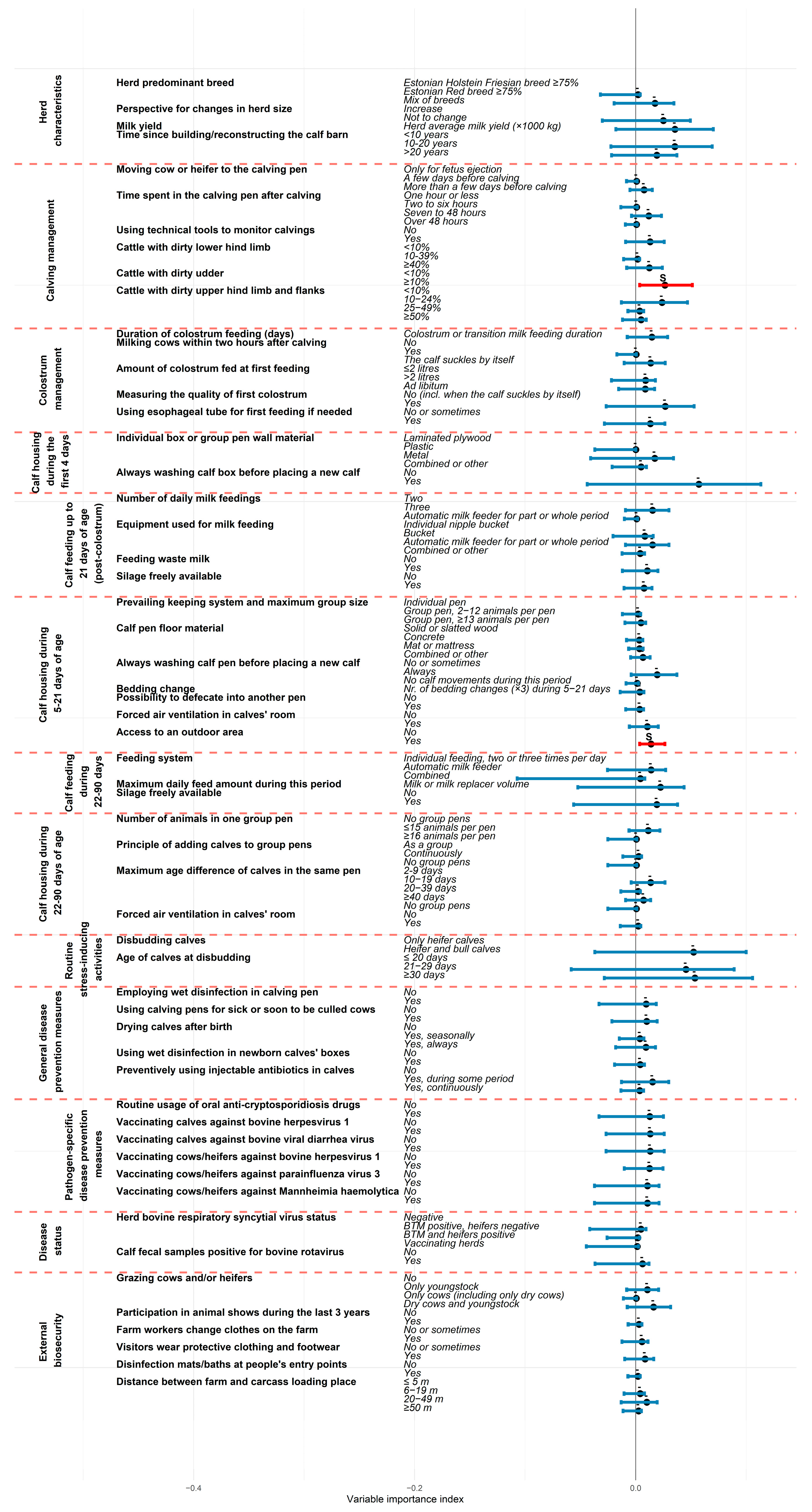
3.2. Importance of Farming Areas and Individual Risk Factors in Explaining Overall Preweaning Calf Losses
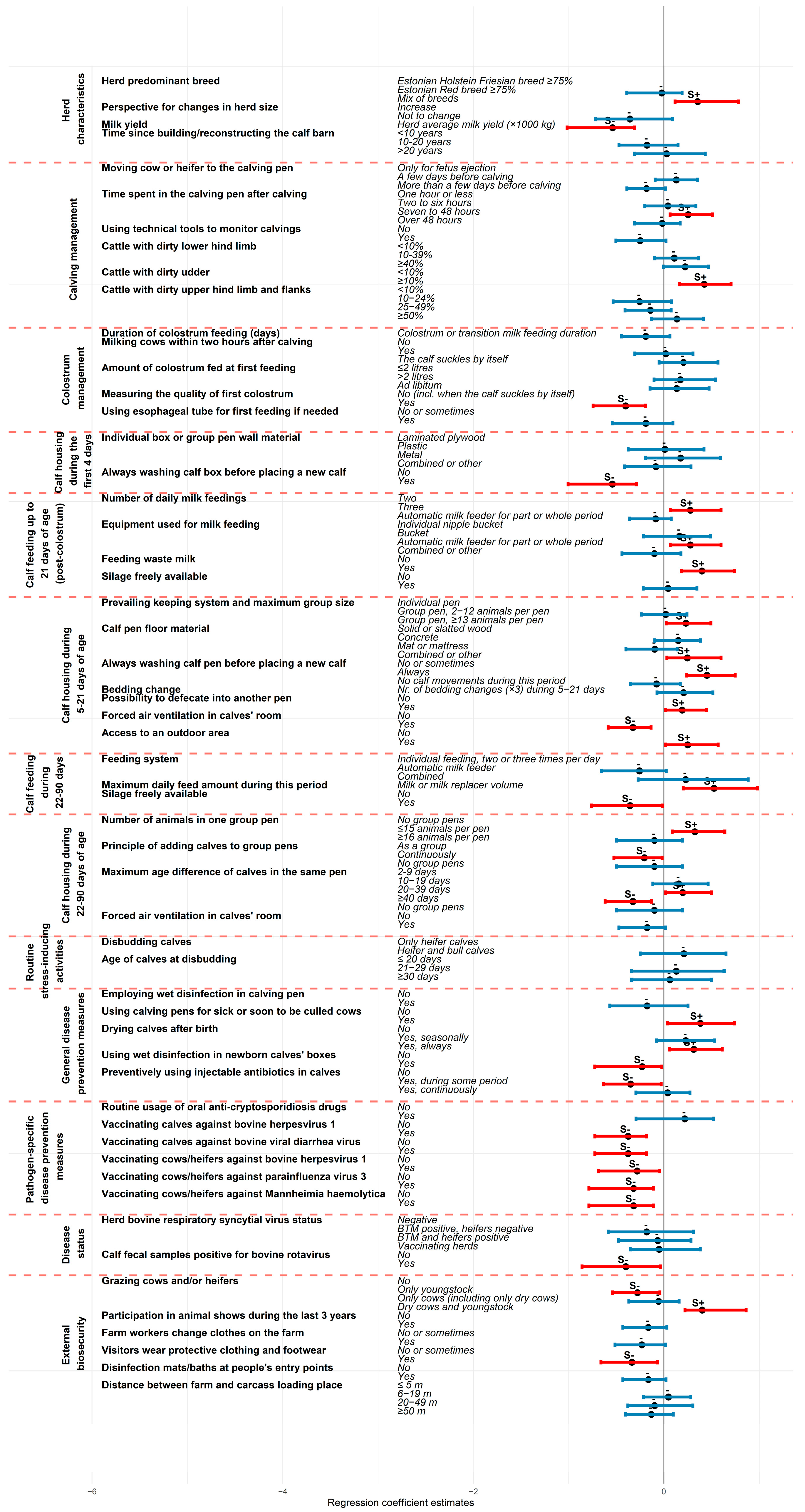
3.3. Risk Factors for Individual Calf Mortality Estimates
4. Discussion
4.1. Importance of Farming Areas and Individual Risk Factors in Explaining Preweaning Calf Losses
4.2. Risk Factors for Individual Calf Mortality Estimates
4.3. Study Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BHV-1 | bovine herpesvirus 1 |
| BlockImp | block importance index |
| BRSV | bovine respiratory syncytial virus |
| BTM | bulk tank milk |
| BVDV | bovine viral diarrhea virus |
| IgG | immunoglobulin G |
| IQR | interquartile range |
| mbPLS | multiblock partial least squares regression |
| MR21 | mortality risk (%) during the first 21 days of age |
| MR90 | mortality risk (%) during 22–90 days of age |
| OAG | older age group, calves aged 22–90 days |
| VarImp | variable importance index |
| YAG | younger age group, calves up to 21 days |
References
- Barkema, H.W.; von Keyserlingk, M.A.G.; Kastelic, J.P.; Lam, T.J.G.M.; Luby, C.; Roy, J.P.; LeBlanc, S.J.; Keefe, G.P.; Kelton, D.F. Invited Review: Changes in the Dairy Industry Affecting Dairy Cattle Health and Welfare. J. Dairy Sci. 2015, 98, 7426–7445. [Google Scholar] [CrossRef] [PubMed]
- Sischo, W.M.; Moore, D.A.; Pereira, R.; Warnick, L.; Moore, D.L.; Vanegas, J.; Kurtz, S.; Heaton, K.; Kinder, D.; Siler, J.; et al. Calf Care Personnel on Dairy Farms and Their Educational Opportunities. J. Dairy Sci. 2019, 102, 3501–3511. [Google Scholar] [CrossRef] [PubMed]
- Lava, M.; Pardon, B.; Schüpbach-Regula, G.; Keckeis, K.; Deprez, P.; Steiner, A.; Meylan, M. Effect of Calf Purchase and Other Herd-Level Risk Factors on Mortality, Unwanted Early Slaughter, and Use of Antimicrobial Group Treatments in Swiss Veal Calf Operations. Prev. Vet. Med. 2016, 126, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Reimus, K.; Alvåsen, K.; Emanuelson, U.; Viltrop, A.; Mõtus, K. Herd-Level Risk Factors for Cow and Calf on-Farm Mortality in Estonian Dairy Herds. Acta Vet. Scand. 2020, 62, 15. [Google Scholar] [CrossRef]
- Pannwitz, G. Standardized Analysis of German Cattle Mortality Using National Register Data. Prev. Vet. Med. 2015, 118, 260–270. [Google Scholar] [CrossRef]
- Raboisson, D.; Delor, F.; Cahuzac, E.; Gendre, C.; Sans, P.; Allaire, G. Perinatal, Neonatal, and Rearing Period Mortality of Dairy Calves and Replacement Heifers in France. J. Dairy Sci. 2013, 96, 2913–2924. [Google Scholar] [CrossRef]
- Bokma, J.; Boone, R.; Deprez, P.; Pardon, B. Short Communication: Herd-Level Analysis of Antimicrobial Use and Mortality in Veal Calves: Do Herds with Low Usage Face Higher Mortality? J. Dairy Sci. 2020, 103, 909–914. [Google Scholar] [CrossRef]
- Ortiz-Pelaez, A.; Pritchard, D.G.; Pfeiffer, D.U.; Jones, E.; Honeyman, P.; Mawdsley, J.J. Calf Mortality as a Welfare Indicator on British Cattle Farms. Vet. J. 2008, 176, 177–181. [Google Scholar] [CrossRef]
- Bauman, C.A.; Barkema, H.W.; Dubuc, J.; Keefe, G.P.; Kelton, D.F. Identifying Management and Disease Priorities of Canadian Dairy Industry Stakeholders. J. Dairy Sci. 2016, 99, 10194–10203. [Google Scholar] [CrossRef]
- Cardoso, C.S.; Hötzel, M.J.; Weary, D.M.; Robbins, J.A.; von Keyserlingk, M.A.G. Imagining the Ideal Dairy Farm. J. Dairy Sci. 2016, 99, 1663–1671. [Google Scholar] [CrossRef]
- Windeyer, M.C.; Leslie, K.; Godden, S.M.; Hodgins, D.C.; Lissemore, K.D.; LeBlanc, S.J. Factors Associated with Morbidity, Mortality, and Growth of Dairy Heifer Calves up to 3 Months of Age. Prev. Vet. Med. 2014, 113, 231–240. [Google Scholar] [CrossRef]
- Gulliksen, S.M.; Lie, K.I.; Løken, T.; Østerås, O. Calf Mortality in Norwegian Dairy Herds. J. Dairy Sci. 2009, 92, 2782–2795. [Google Scholar] [CrossRef]
- Lombard, J.E.; Garry, F.B.; Urie, N.J.; McGuirk, S.M.; Godden, S.M.; Sterner, K.; Earleywine, T.J.; Catherman, D.; Maas, J. Proposed Dairy Calf Birth Certificate Data and Death Loss Categorization Scheme. J. Dairy Sci. 2019, 102, 4704–4712. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; LeBlanc, S.J.; Jones-Bitton, A.; DeVries, T.J.; Rushen, J.; Marie de Passillé, A.; Endres, M.I.; Haley, D.B. Associations between Management Practices and Within-Pen Prevalence of Calf Diarrhea and Respiratory Disease on Dairy Farms Using Automated Milk Feeders. J. Dairy Sci. 2018, 101, 2293–2308. [Google Scholar] [CrossRef] [PubMed]
- Ahmedin, U.M.; Assen, A.A. Calf Morbidity, Mortality, and Management Practices in Dairy Farms in Jimma City, Southwestern Ethiopia. BMC Vet. Res. 2023, 19, 249. [Google Scholar] [CrossRef] [PubMed]
- Mõtus, K.; Viltrop, A.; Emanuelson, U. Reasons and Risk Factors for Beef Calf and Youngstock On-Farm Mortality in Extensive Cow-Calf Herds. Animal 2018, 12, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Urie, N.J.; Lombard, J.E.; Shivley, C.B.; Kopral, C.A.; Adams, A.E.; Earleywine, T.J.; Olson, J.D.; Garry, F.B. Preweaned Heifer Management on US Dairy Operations: Part V. Factors Associated with Morbidity and Mortality in Preweaned Dairy Heifer Calves. J. Dairy Sci. 2018, 101, 9229–9244. [Google Scholar] [CrossRef]
- Sawa, A.; Jankowska, M.; Neja, W.; Kręzel-Czopek, S. Effect of Single and Multiple Pregnancies and Calf Sex on Parturition Process and Perinatal Mortality. Ann. Anim. Sci. 2014, 14, 851–858. [Google Scholar] [CrossRef]
- Bleul, U. Risk Factors and Rates of Perinatal and Postnatal Mortality in Cattle in Switzerland. Livest. Sci. 2011, 135, 257–264. [Google Scholar] [CrossRef]
- Misaka, M.; Uematsu, M.; Hashimoto, K.; Kitahara, G.; Osawa, T.; Sasaki, Y. Impact of Dystocia and Cow/Calf Characteristics on Mortality from 0 to 120 Days of Age in Japanese Black Calves in Commercial Cow-Calf Operations. Prev. Vet. Med. 2022, 207, 105716. [Google Scholar] [CrossRef]
- Tautenhahn, A.; Merle, R.; Müller, K.E. Factors Associated with Calf Mortality and Poor Growth of Dairy Heifer Calves in Northeast Germany. Prev. Vet. Med. 2020, 184, 105154. [Google Scholar] [CrossRef]
- Crannell, P.; Abuelo, A. Comparison of Calf Morbidity, Mortality, and Future Performance across Categories of Passive Immunity: A Retrospective Cohort Study in a Dairy Herd. J. Dairy Sci. 2023, 106, 2729–2738. [Google Scholar] [CrossRef]
- Raboisson, D.; Trillat, P.; Cahuzac, C. Failure of Passive Immune Transfer in Calves: A Meta-Analysis on the Consequences and Assessment of the Economic Impact. PLoS ONE 2016, 11, e0150452. [Google Scholar] [CrossRef]
- Sedó, S.G.U.; Renaud, D.L.; Molano, R.A.; Santschi, D.E.; Caswell, J.L.; Mee, J.F.; Winder, C.B. Exploring Herd-Level Perinatal Calf Mortality Risk Factors in Eastern Canadian Dairy Farms. J. Dairy Sci. 2024, 107, 3824–3835. [Google Scholar] [CrossRef] [PubMed]
- Hyde, R.M.; Green, M.J.; Sherwin, V.E.; Hudson, C.; Gibbons, J.; Forshaw, T.; Vickers, M.; Down, P.M. Quantitative Analysis of Calf Mortality in Great Britain. J. Dairy Sci. 2020, 103, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Perrin, J.B.; Ducrot, C.; Vinard, J.L.; Hendrikx, P.; Calavas, D. Analyse de La Mortalité Bovine En France de 2003 à 2009. Prod. Anim. 2011, 24, 235–244. [Google Scholar] [CrossRef]
- Todd, C.G.; McGee, M.; Tiernan, K.; Crosson, P.; O’Riordan, E.; McClure, J.; Lorenz, I.; Earley, B. An Observational Study on Passive Immunity in Irish Suckler Beef and Dairy Calves: Tests for Failure of Passive Transfer of Immunity and Associations with Health and Performance. Prev. Vet. Med. 2018, 159, 182–195. [Google Scholar] [CrossRef]
- Moreira, M.B.; Ferreira, F.C.; Campolina, J.P.; Coelho, S.G. Association of Passive Immunity and Genetic Composition, Health, and Performance of Tropical Dairy Calves. Res. Vet. Sci. 2024, 171, 105225. [Google Scholar] [CrossRef]
- Reimus, K.; Orro, T.; Emanuelson, U.; Viltrop, A.; Mõtus, K. Reasons and Risk Factors for On-Farm Mortality in Estonian Dairy Herds. Livest. Sci. 2017, 198, 1–9. [Google Scholar] [CrossRef][Green Version]
- Sandelin, A.; Hälli, O.; Härtel, H.; Herva, T.; Seppä-Lassila, L.; Tuunainen, E.; Rautala, H.; Soveri, T.; Simojoki, H. Effect of Farm and Animal-Level Factors on Youngstock Mortality and Growth on Calf Rearing Farms. Prev. Vet. Med. 2021, 193, 105416. [Google Scholar] [CrossRef]
- Raboisson, D.; Maigne, E.; Sans, P.; Allaire, G.; Cahuzac, E. Factors Influencing Dairy Calf and Replacement Heifer Mortality in France. J. Dairy Sci. 2014, 97, 202–211. [Google Scholar] [CrossRef]
- Jorgensen, M.W.; Adams-Progar, A.; de Passillé, A.M.; Rushen, J.; Salfer, J.A.; Endres, M.I. Mortality and Health Treatment Rates of Dairy Calves in Automated Milk Feeding Systems in the Upper Midwest of the United States. J. Dairy Sci. 2017, 100, 9186–9193. [Google Scholar] [CrossRef]
- Seppä-Lassila, L.; Sarjokari, K.; Hovinen, M.; Soveri, T.; Norring, M. Management Factors Associated with Mortality of Dairy Calves in Finland: A Cross Sectional Study. Vet. J. 2016, 216, 164–167. [Google Scholar] [CrossRef]
- Gates, M.C. Evaluating the Reproductive Performance of British Beef and Dairy Herds Using National Cattle Movement Records. Vet. Rec. 2013, 173, 499. [Google Scholar] [CrossRef] [PubMed]
- McAloon, C.G.; Tratalos, J.A.; O’Grady, L.; Green, M.J.; Gavey, L.; Graham, D.; More, S.J.; McGrath, G.; Mee, J.F. An Observational Study of Ear-Tagged Calf Mortality (1 to 100 Days) on Irish Dairy Farms and Associations between Biosecurity Practices and Calf Mortality on Farms Participating in a Johne’s Disease Control Program. J. Dairy Sci. 2023, 106, 4966–4977. [Google Scholar] [CrossRef] [PubMed]
- Zablotski, Y.; Voigt, K.; Hoedemaker, M.; Müller, K.E.; Kellermann, L.; Arndt, H.; Volkmann, M.; Dachrodt, L.; Stock, A. Perinatal Mortality in German Dairy Cattle: Unveiling the Importance of Cow-Level Risk Factors and Their Interactions Using a Multifaceted Modelling Approach. PLoS ONE 2024, 19, e0302004. [Google Scholar] [CrossRef]
- Silva Del Río, N.; Stewart, S.; Rapnicki, P.; Chang, Y.M.; Fricke, P.M. An Observational Analysis of Twin Births, Calf Sex Ratio, and Calf Mortality in Holstein Dairy Cattle. J. Dairy Sci. 2007, 90, 1255–1264. [Google Scholar] [CrossRef]
- Voljč, M.; Čepon, M.; Malovrh, Š.; Žgur, S. The Effect of Dam Breed on Calf Mortality in the First Month of Life in Slovenia. Agric. Conspec. Sci. 2017, 82, 69–73. [Google Scholar]
- Zucali, M.; Bava, L.; Tamburini, A.; Guerci, M.; Sandrucci, A. Management Risk Factors for Calf Mortality in Intensive Italian Dairy Farms. Ital. J. Anim. Sci. 2013, 12, 162–166. [Google Scholar] [CrossRef]
- Santman-Berends, I.M.G.A.; Buddiger, M.; Smolenaars, A.J.G.; Steuten, C.D.M.; Roos, C.A.J.; Van Erp, A.J.M.; Van Schaik, G. A Multidisciplinary Approach to Determine Factors Associated with Calf Rearing Practices and Calf Mortality in Dairy Herds. Prev. Vet. Med. 2014, 117, 375–387. [Google Scholar] [CrossRef]
- Torsein, M.; Lindberg, A.; Sandgren, C.H.; Waller, K.P.; Törnquist, M.; Svensson, C. Risk Factors for Calf Mortality in Large Swedish Dairy Herds. Prev. Vet. Med. 2011, 99, 136–147. [Google Scholar] [CrossRef]
- Cuttance, E.L.; Mason, W.A.; McDermott, J.; Laven, R.A.; McDougall, S.; Phyn, C.V.C. Calf and Replacement Heifer Mortality from Birth until Weaning in Pasture-Based Dairy Herds in New Zealand. J. Dairy Sci. 2017, 100, 8347–8357. [Google Scholar] [CrossRef]
- Johnsen, J.F.; Holm⊘y, I.H.; Mejdell, C.M.; Ellingsen-Dalskau, K.; Østerås, O.; Døsen, A.; Skjerve, E.; Nødtvedt, A. A Cross-Sectional Study of Associations between Herd-Level Calf Mortality Rates, Compliance with Legislation on Calf Welfare, and Milk Feeding Management in Norwegian Dairy Herds. J. Dairy Sci. 2021, 104, 839–848. [Google Scholar] [CrossRef]
- Falkenberg, U.; Krömker, V.; Konow, M.; Flor, J.; Sanftleben, P.; Losand, B. Management of Calves in Commercial Dairy Farms in Mecklenburg-Western Pomerania, Germany and Its Impact on Calf Mortality and Prevalence of Rotavirus and Cryptosporidium Parvum Infections in Pre-Weaned Calves. Vet. Anim. Sci. 2022, 16, 100243. [Google Scholar] [CrossRef]
- Barry, J.; Bokkers, E.A.M.; Berry, D.P.; de Boer, I.J.M.; McClure, J.; Kennedy, E. Associations between Colostrum Management, Passive Immunity, Calf-Related Hygiene Practices, and Rates of Mortality in Preweaning Dairy Calves. J. Dairy Sci. 2019, 102, 10266–10276. [Google Scholar] [CrossRef]
- Heinemann, C.; Leubner, C.D.; Hayer, J.J.; Steinhoff-Wagner, J. Hygiene Management in Newborn Individually Housed Dairy Calves Focusing on Housing and Feeding Practices. J. Anim. Sci. 2021, 99, skaa391. [Google Scholar] [CrossRef]
- Lora, I.; Barberio, A.; Contiero, B.; Paparella, P.; Bonfanti, L.; Brscic, M.; Stefani, A.L.; Gottardo, F. Factors Associated with Passive Immunity Transfer in Dairy Calves: Combined Effect of Delivery Time, Amount and Quality of the First Colostrum Meal. Animal 2018, 12, 1041–1049. [Google Scholar] [CrossRef] [PubMed]
- Barry, J.; Bokkers, E.A.M.; de Boer, I.J.M.; Kennedy, E. Pre-Weaning Management of Calves on Commercial Dairy Farms and Its Influence on Calf Welfare and Mortality. Animal 2020, 14, 2580–2587. [Google Scholar] [CrossRef] [PubMed]
- Abuelo, A.; Havrlant, P.; Wood, N.; Hernandez-Jover, M. An Investigation of Dairy Calf Management Practices, Colostrum Quality, Failure of Transfer of Passive Immunity, and Occurrence of Enteropathogens among Australian Dairy Farms. J. Dairy Sci. 2019, 102, 8352–8366. [Google Scholar] [CrossRef] [PubMed]
- Mellado, M.; Lopez, E.; Veliz, F.G.; De Santiago, M.A.; Macias-Cruz, U.; Avendaño-Reyes, L.; Garcia, J.E. Factors Associated with Neonatal Dairy Calf Mortality in a Hot-Arid Environment. Livest. Sci. 2014, 159, 149–155. [Google Scholar] [CrossRef]
- Azizzadeh, M.; Shooroki, H.F.; Kamalabadi, A.S.; Stevenson, M.A. Factors Affecting Calf Mortality in Iranian Holstein Dairy Herds. Prev. Vet. Med. 2012, 104, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Svensson, C.; Linder, A.; Olsson, S.-O. Mortality in Swedish Dairy Calves and Replacement Heifers. J. Dairy Sci. 2006, 89, 4769–4777. [Google Scholar] [CrossRef] [PubMed]
- United States Department of Agriculture. Morbidity and Mortality in U.S. Preweaned Dairy Heifer Calves, NAHMS Dairy 2014 Study Calf Component. 2021. Available online: https://www.aphis.usda.gov/animal_health/nahms/dairy/downloads/dairy17/morb-mort-us-prewean-dairy-heifer-nahms-2014.pdf (accessed on 25 April 2022).
- Singh, D.D.; Kumar, M.; Choudhury, P.K.; Singh, H.N. Neonatal Calf Mortality—An Overview. Intas Polivet 2009, 10, 165–169. [Google Scholar]
- Bougeard, S.; Lupo, C.; Le Bouquin, S.; Chauvin, C.; Qannari, E.M. Multiblock Modelling to Assess the Overall Risk Factors for a Composite Outcome. Epidemiol. Infect. 2012, 140, 337–347. [Google Scholar] [CrossRef]
- Wold, S. Three PLS Algorithms According to SW. In Symposium MULDAST (Multivariate Analysis in Science and Technology); Umea University: Umea, Sweden, 1984; pp. 26–30. [Google Scholar]
- Wangen, L.E.; Kowalski, B.R. A Multiblock Partial Least Squares Algorithm for Investigating Complex Chemical Systems. J. Chemom. 1989, 3, 3–20. [Google Scholar] [CrossRef]
- Cook, N.B.; Reinemann, D.J. A Tool Box for Assessing Cow, Udder and Teat Hygiene. In Proceedings of the NMC Annual Meeting Paper, Washington, DC, USA, 4–7 November 2007. [Google Scholar]
- Mõtus, K.; Rilanto, T.; Viidu, D.A.; Orro, T.; Viltrop, A. Seroprevalence of Selected Endemic Infectious Diseases in Large-Scale Estonian Dairy Herds and Their Associations with Cow Longevity and Culling Rates. Prev. Vet. Med. 2021, 192, 105389. [Google Scholar] [CrossRef]
- Maaeluminister. Procedure for Marking and Registering Farm Animals and Issuing Cattle Passports, Procedure for Notifying the Slaughter, Death and Disposal of Farm Animals, and Requirements for Removing and Replacing Identification Devices. 2021. Available online: https://www.riigiteataja.ee/akt/127032025026 (accessed on 19 April 2022).
- LimeSurvey GmbH, Hamburg, G. LimeSurvey: An Open Source Survey Tool. Available online: http://www.limesurvey.org (accessed on 5 June 2019).
- Dohoo, I.R.; Martin, S.W.; Stryhn, H. Veterinary Epidemiologic Research; VER, Inc.: Charlotte, NC, USA, 2009; ISBN 97809190136050919013600. [Google Scholar]
- Bougeard, S.; Dray, S. Supervised Multiblock Analysis in R with the Ade4 Package. J. Stat. Softw. 2018, 86, 1–17. [Google Scholar] [CrossRef]
- Collineau, L.; Bougeard, S.; Backhans, A.; Dewulf, J.; Emanuelson, U.; Grosse Beilage, E.; Lehébel, A.; Lösken, S.; Postma, M.; Sjölund, M.; et al. Application of Multiblock Modelling to Identify Key Drivers for Antimicrobial Use in Pig Production in Four European Countries. Epidemiol. Infect. 2018, 146, 1003–1014. [Google Scholar] [CrossRef]
- Mõtus, K.; Viidu, D.A.; Rilanto, T.; Niine, T.; Orro, T.; Viltrop, A.; Bougeard, S. Application of Multiblock Analysis to Identify Key Areas and Risk Factors for Dairy Cow Persistence. Prev. Vet. Med. 2024, 222, 106081. [Google Scholar] [CrossRef]
- R Core Team R: A Language and Environment for Statistical Computing 2019, R Foundation for Statistical Computing, Vienna, Austria. Available online: https://www.R-project.org/ (accessed on 25 March 2020).
- Hulbert, L.E.; Moisá, S.J. Stress, Immunity, and the Management of Calves1. J. Dairy Sci. 2016, 99, 3199–3216. [Google Scholar] [CrossRef]
- Hassig, M.; Stadler, T.; Lutz, H. Transition from Maternal to Endogenous Antibodies in Newborn Calves. Vet. Rec. 2007, 160, 234–235. [Google Scholar] [CrossRef] [PubMed]
- Allen, K.A.; Coetzee, J.F.; Edwards-Callaway, L.N.; Glynn, H.; Dockweiler, J.; KuKanich, B.; Lin, H.; Wang, C.; Fraccaro, E.; Jones, M.; et al. The Effect of Timing of Oral Meloxicam Administration on Physiological Responses in Calves after Cautery Dehorning with Local Anesthesia. J. Dairy Sci. 2013, 96, 5194–5205. [Google Scholar] [CrossRef] [PubMed]
- Robles, I.; Zambelis, A.; Kelton, D.F.; Barkema, H.W.; Keefe, G.P.; Roy, J.P.; von Keyserlingk, M.A.G.; DeVries, T.J. Associations of Freestall Design and Cleanliness with Cow Lying Behavior, Hygiene, Lameness, and Risk of High Somatic Cell Count. J. Dairy Sci. 2021, 104, 2231–2242. [Google Scholar] [CrossRef]
- Sweeney, R.W.; Collins, M.T.; Koets, A.P.; McGuirk, S.M.; Roussel, A.J. Paratuberculosis (Johne’s Disease) in Cattle and Other Susceptible Species. J. Vet. Intern. Med. 2012, 26, 1239–1250. [Google Scholar] [CrossRef]
- Lee, H.H.; Lee, J.Y.; Liu, S.S.; Chen, C.C.; Hsu, H.Y. Cryptosporidium Parvum Infection and Management-Based Risk Factors of Dairy Calves in Taiwan. J. Vet. Med. Sci. 2021, 83, 1838–1844. [Google Scholar] [CrossRef]
- Schnyder, P.; Schönecker, L.; Schüpbach-Regula, G.; Meylan, M. Effects of Management Practices, Animal Transport and Barn Climate on Animal Health and Antimicrobial Use in Swiss Veal Calf Operations. Prev. Vet. Med. 2019, 167, 146–157. [Google Scholar] [CrossRef]
- Svensson, C.; Lundborg, K.; Emanuelson, U.; Olsson, S.O. Morbidity in Swedish Dairy Calves from Birth to 90 Days of Age and Individual Calf-Level Risk Factors for Infectious Diseases. Prev. Vet. Med. 2003, 58, 179–197. [Google Scholar] [CrossRef]
- Torsein, M.; Jansson-Mörk, M.; Lindberg, A.; Hallén-Sandgren, C.; Berg, C. Associations between Calf Mortality during Days 1 to 90 and Herd-Level Cow and Production Variables in Large Swedish Dairy Herds. J. Dairy Sci. 2014, 97, 6613–6621. [Google Scholar] [CrossRef]
- Bonizzi, S.; Gislon, G.; Brasca, M.; Morandi, S.; Sandrucci, A.; Zucali, M. Air Quality, Management Practices and Calf Health in Italian Dairy Cattle FarmsDairy Cattle Farms. Animals 2022, 12, 2286. [Google Scholar] [CrossRef]
- Smith, T.; Little, R.B. The Significance of Colostrum to the New-Born Calf. J. Exp. Med. 1922, 36, 181–198. [Google Scholar] [CrossRef]
- Acres, S.D.; Isaacson, R.E.; Babiuk, L.A.; Kapitany, R.A. Immunization of Calves against Enterotoxigenic Colibacillosis by Vaccinating Dams with Purified K99 Antigen and Whole Cell Bacterins. Infect. Immun. 1979, 25, 121–126. [Google Scholar] [CrossRef]
- Viidu, D.A.; Mõtus, K. Implementation of a Pre-Calving Vaccination Programme against Rotavirus, Coronavirus and Enterotoxigenic Escherichia Coli (F5) and Association with Dairy Calf Survival. BMC Vet. Res. 2022, 18, 59. [Google Scholar] [CrossRef] [PubMed]
- Lora, I.; Gottardo, F.; Bonfanti, L.; Stefani, A.L.; Soranzo, E.; Dall’Ava, B.; Capello, K.; Martini, M.; Barberio, A. Transfer of Passive Immunity in Dairy Calves: The Effectiveness of Providing a Supplementary Colostrum Meal in Addition to Nursing from the Dam. Animal 2019, 13, 2621–2629. [Google Scholar] [CrossRef] [PubMed]
- Brignole, T.J.; Stott, G.H. Effect of Suckling Followed by Bottle Feeding Colostrum on Immunoglobulin Absorption and Calf Survival. J. Dairy Sci. 1980, 63, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Johnsen, J.F.; Viljugrein, H.; Bøe, K.E.; Gulliksen, S.M.; Beaver, A.; Grøndahl, A.M.; Sivertsen, T.; Mejdell, C.M. A Cross-Sectional Study of Suckling Calves’ Passive Immunity and Associations with Management Routines to Ensure Colostrum Intake on Organic Dairy Farms. Acta Vet. Scand. 2019, 61, 7. [Google Scholar] [CrossRef]
- Besser, T.E.; Gay, C.C.; Pritchett, L. Comparison of Three Methods of Feeding Colostrum to Dairy Calves. J. Am. Vet. Med. Assoc. 1991, 198, 419–422. [Google Scholar] [CrossRef]
- Murray, C.F.; Fick, L.J.; Pajor, E.A.; Barkema, H.W.; Jelinski, M.D.; Windeyer, M.C. Calf Management Practices and Associations with Herd-Level Morbidity and Mortality on Beef Cow-Calf Operations. Animal 2015, 10, 468–477. [Google Scholar] [CrossRef]
- Penati, M.; Sala, G.; Biscarini, F.; Boccardo, A.; Bronzo, V.; Castiglioni, B.; Cremonesi, P.; Moroni, P.; Pravettoni, D.; Addis, M.F. Feeding Pre-Weaned Calves with Waste Milk Containing Antibiotic Residues Is Related to a Higher Incidence of Diarrhea and Alterations in the Fecal Microbiota. Front. Vet. Sci. 2021, 8, 650150. [Google Scholar] [CrossRef]
- Aust, V.; Knappstein, K.; Kunz, H.J.; Kaspar, H.; Wallmann, J.; Kaske, M. Feeding Untreated and Pasteurized Waste Milk and Bulk Milk to Calves: Effects on Calf Performance, Health Status and Antibiotic Resistance of Faecal Bacteria. J. Anim. Physiol. Anim. Nutr. 2013, 97, 1091–1103. [Google Scholar] [CrossRef]
- Brunton, L.A.; Reeves, H.E.; Snow, L.C.; Jones, J.R. A Longitudinal Field Trial Assesing the Impact of Feeding Waste Milk Containing Antibiotic Residues on the Prevalence of ESBL-Producing Escherichia Coli in Calves. Prev. Vet. Med. 2014, 117, 403–412. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, T.; Cheng, C.; Lv, J.; Bai, H.; Jiang, X.; Zhang, Y.; Xin, H. Effects of Waste Milk on Growth Performance, Immunity, and Gut Health of Dairy Calves. Anim. Feed Sci. Technol. 2022, 285, 115241. [Google Scholar] [CrossRef]
- Li, J.H.; Yousif, M.H.; Li, Z.Q.; Wu, Z.H.; Li, S.L.; Yang, H.J.; Wang, Y.J.; Cao, Z.J. Effects of Antibiotic Residues in Milk on Growth, Ruminal Fermentation, and Microbial Community of Preweaning Dairy Calves. J. Dairy Sci. 2019, 102, 2298–2307. [Google Scholar] [CrossRef] [PubMed]
- Foutz, C.A.; Godden, S.M.; Bender, J.B.; Diez-Gonzalez, F.; Akhtar, M.; Vatulin, A. Exposure to Antimicrobials through the Milk Diet or Systemic Therapy Is Associated with a Transient Increase in Antimicrobial Resistance in Fecal Escherichia Coli of Dairy Calves. J. Dairy Sci. 2018, 101, 10126–10141. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Rushen, J.; de Passillé, A.M. Dairy Calves’ Adaptation to Group Housing with Automated Feeders. Appl. Anim. Behav. Sci. 2014, 158, 1–7. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; LeBlanc, S.J.; DeVries, T.J.; Jones-Bitton, A.; Rushen, J.; Marie de Passillé, A.; Endres, M.I.; Haley, D.B. Effect of Age of Introduction to an Automated Milk Feeder on Calf Learning and Performance and Labor Requirements. J. Dairy Sci. 2018, 101, 9371–9384. [Google Scholar] [CrossRef]
- Perttu, R.K.; Peiter, M.; Bresolin, T.; Dórea, J.R.R.; Endres, M.I. Feeding Behaviors Collected from Automated Milk Feeders Were Associated with Disease in Group-Housed Dairy Calves in the Upper Midwest United States. J. Dairy Sci. 2023, 106, 1206–1217. [Google Scholar] [CrossRef]
- Medrano-Galarza, C.; LeBlanc, S.J.; DeVries, T.J.; Jones-Bitton, A.; Rushen, J.; Marie de Passillé, A.; Haley, D.B. A Survey of Dairy Calf Management Practices among Farms Using Manual and Automated Milk Feeding Systems in Canada. J. Dairy Sci. 2017, 100, 6872–6884. [Google Scholar] [CrossRef]
- Gulliksen, S.M.; Jor, E.; Lie, K.I.; Løken, T.; Åkerstedt, J.; Østerås, O. Respiratory Infections in Norwegian Dairy Calves. J. Dairy Sci. 2009, 92, 5139–5146. [Google Scholar] [CrossRef]
- Svensson, C.; Liberg, P. The Effect of Group Size on Health and Growth Rate of Swedish Dairy Calves Housed in Pens with Automatic Milk-Feeders. Prev. Vet. Med. 2006, 73, 43–53. [Google Scholar] [CrossRef]
- Brscic, M.; Leruste, H.; Heutinck, L.F.M.; Bokkers, E.A.M.; Wolthuis-Fillerup, M.; Stockhofe, N.; Gottardo, F.; Lensink, B.J.; Cozzi, G.; Van Reenen, C.G. Prevalence of Respiratory Disorders in Veal Calves and Potential Risk Factors. J. Dairy Sci. 2012, 95, 2753–2764. [Google Scholar] [CrossRef]
- Meek, K.; Strain, S.; O’Connell, N.E.; Grant, I.R. Analysis of the Veterinary Risk Assessment and Management Plan Questionnaire Responses for Dairy Herds Enrolled in the Northern Ireland Johne’s Disease Control Programme. Vet. Rec. Open 2023, 10, e71. [Google Scholar] [CrossRef]
- Windeyer, M.C.; Leslie, K.E.; Godden, S.M.; Hodgins, D.C.; Lissemore, K.D.; LeBlanc, S.J. The Effects of Viral Vaccination of Dairy Heifer Calves on the Incidence of Respiratory Disease, Mortality, and Growth. J. Dairy Sci. 2012, 95, 6731–6739. [Google Scholar] [CrossRef]
- Sandelin, A.; Härtel, H.; Seppä-Lassila, L.; Kaartinen, L.; Rautala, H.; Soveri, T.; Simojoki, H. Field Trial to Evaluate the Effect of an Intranasal Respiratory Vaccine Protocol on Bovine Respiratory Disease Incidence and Growth in a Commercial Calf Rearing Unit. BMC Vet. Res. 2020, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Makoschey, B.; Bielsa, J.M.; Oliviero, L.; Roy, O.; Pillet, F.; Dufe, D.; Valla, G.; Cavirani, S. Field Efficacy of Combination Vaccines against Bovine Respiratory Pathogens in Calves. Acta Vet. Hung. 2008, 56, 485–493. [Google Scholar] [CrossRef] [PubMed]
- Hancox, L.R.; Le Bon, M.; Dodd, C.E.R.; Mellits, K.H. Inclusion of Detergent in a Cleaning Regime and Effect on Microbial Load in Livestock Housing. Vet. Rec. 2013, 173, 167. [Google Scholar] [CrossRef] [PubMed]
- Santman-Berends, I.M.G.A.; Schukken, Y.H.; van Schaik, G. Quantifying Calf Mortality on Dairy Farms: Challenges and Solutions. J. Dairy Sci. 2019, 102, 6404–6417. [Google Scholar] [CrossRef]
- Reiten, M.; Rousing, T.; Thomsen, P.T.; Otten, N.D.; Forkman, B.; Houe, H.; Sørensen, J.T.; Kirchner, M.K. Mortality, Diarrhea and Respiratory Disease in Danish Dairy Heifer Calves: Effect of Production System and Season. Prev. Vet. Med. 2018, 155, 21–26. [Google Scholar] [CrossRef]
- Diéguez, F.J.; Yus, E.; Vilar, M.J.; Sanjuán, M.L.; Arnaiz, I. Effect of the Bovine Viral Diarrhoea Virus (BVDV) Infection on Dairy Calf Rearing. Res. Vet. Sci. 2009, 87, 39–40. [Google Scholar] [CrossRef]
- Pardon, B.; Buczinski, S. Bovine Respiratory Disease Diagnosis: What Progress Has Been Made in Infectious Diagnosis? Vet. Clin. N. Am. Food Anim. Pract. 2020, 36, 425–444. [Google Scholar] [CrossRef]
- Andersson, A.M.; Aspán, A.; Wisselink, H.J.; Smid, B.; Ridley, A.; Pelkonen, S.; Autio, T.; Lauritsen, K.T.; Kensø, J.; Gaurivaud, P.; et al. A European inter-laboratory trial to evaluate the performance of three serological methods for diagnosis of Mycoplasma bovis infection in cattle using latent class analysis. BMC Vet. Res. 2019, 15, 369. [Google Scholar] [CrossRef]
- Hanon, J.B.; De Baere, M.; De la Ferté, C.; Roelandt, S.; Van der Stede, Y.; Cay, B. Evaluation of 16 commercial antibody ELISAs for the detection of bovine viral diarrhea virus–specific antibodies in serum and milk using well-characterized sample panels. J. Vet. Diagn. Investig. 2017, 29, 833–843. [Google Scholar] [CrossRef]
- Kramps, J.A.; Banks, M.; Beer, M.; Kerkhofs, P.; Perrin, M.; Wellenberg, G.J.; Oirschot, J.T.V. Evaluation of tests for antibodies against bovine herpesvirus 1 performed in national reference laboratories in Europe. Vet. Microbiol. 2004, 102, 169–181. [Google Scholar] [CrossRef]
- Nielsen, L.R.; Ersbøll, A.K. Age-stratified validation of an indirect Salmonella Dublin serum enzyme-linked immunosorbent assay for individual diagnosis in cattle. J. Vet. Diagn. Investig. 2004, 16, 212–218. [Google Scholar] [CrossRef]

| Block Name | Variables in the Block (n) | Preselected Variables 1 (n) | |
|---|---|---|---|
| MR21 | MR90 | ||
| Herd characteristics | 7 | 4 | 3 |
| Calving management | 11 | 4 | 8 |
| Colostrum management | 13 | 7 | 3 |
| Calf housing during the first four days of life | 10 | 2 | 4 |
| Calf feeding up to 21 days of age (post-colostrum) | 11 | 2 | 3 |
| Calf housing during 5–21 days of age | 13 | 2 | 9 |
| Calf feeding during 22–90 days of age | 10 | NA 2 | 3 |
| Calf housing during 22–90 days of age | 13 | NA 2 | 8 |
| Routine stress-inducing activities | 4 | 3 | 0 |
| General disease prevention measures | 15 | 2 | 5 |
| Pathogen-specific disease prevention measures | 17 | 4 | 9 |
| Disease status | 10 | 1 | 2 |
| External biosecurity | 13 | 2 | 6 |
| TOTAL | 147 | 33 | 62 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Viidu, D.-A.; Rilanto, T.; Bougeard, S.; Kaart, T.; Mõtus, K. Multiblock Analysis of Risk Factors and Management Areas of Calf Mortality in Large-Scale Dairy Herds. Animals 2025, 15, 2780. https://doi.org/10.3390/ani15192780
Viidu D-A, Rilanto T, Bougeard S, Kaart T, Mõtus K. Multiblock Analysis of Risk Factors and Management Areas of Calf Mortality in Large-Scale Dairy Herds. Animals. 2025; 15(19):2780. https://doi.org/10.3390/ani15192780
Chicago/Turabian StyleViidu, Dagni-Alice, Triin Rilanto, Stéphanie Bougeard, Tanel Kaart, and Kerli Mõtus. 2025. "Multiblock Analysis of Risk Factors and Management Areas of Calf Mortality in Large-Scale Dairy Herds" Animals 15, no. 19: 2780. https://doi.org/10.3390/ani15192780
APA StyleViidu, D.-A., Rilanto, T., Bougeard, S., Kaart, T., & Mõtus, K. (2025). Multiblock Analysis of Risk Factors and Management Areas of Calf Mortality in Large-Scale Dairy Herds. Animals, 15(19), 2780. https://doi.org/10.3390/ani15192780





