Approved Ambiguities: An Analysis of Applications for the Ethical Review of Animal Research in Sweden—Focusing on Harm, Benefit, and the 3Rs
Simple Summary
Abstract
1. Introduction
1.1. Background
1.1.1. Directive 2010/63/EU and Its Implementation Across Member States
1.1.2. Ethical Review of Animal Research in Sweden
1.1.3. Our Research
1.2. Aims
- i.
- To map which written information is required in the applications for the ethical review of animal research based on relevant regulations of the ethical review of animal research (Directive 2010/63/EU and the L150). Also, to map when said information should include a justification by the applying researcher.
- ii.
- To analyze how well (a selection of) applications from 2020 live up to legal requirements (see aim (i)).
- iii.
- To gain an overview of how well the application form used by applying researchers in 2020 corresponds to legal requirements (see aim (i)).
1.3. Structure of the Article
2. Materials and Methods
2.1. Interpretation of Relevant Regulations
2.1.1. Applicable Regulations and Legal Expertise
2.1.2. Legal Methodology
2.2. Analysis of Applications
2.2.1. Data Collection
2.2.2. Data Analysis
3. Results
3.1. Regulatory Requirements
3.1.1. Content of Applications
3.1.2. Motivation of Content of Applications
3.2. Analysis of Applications for Ethical Review
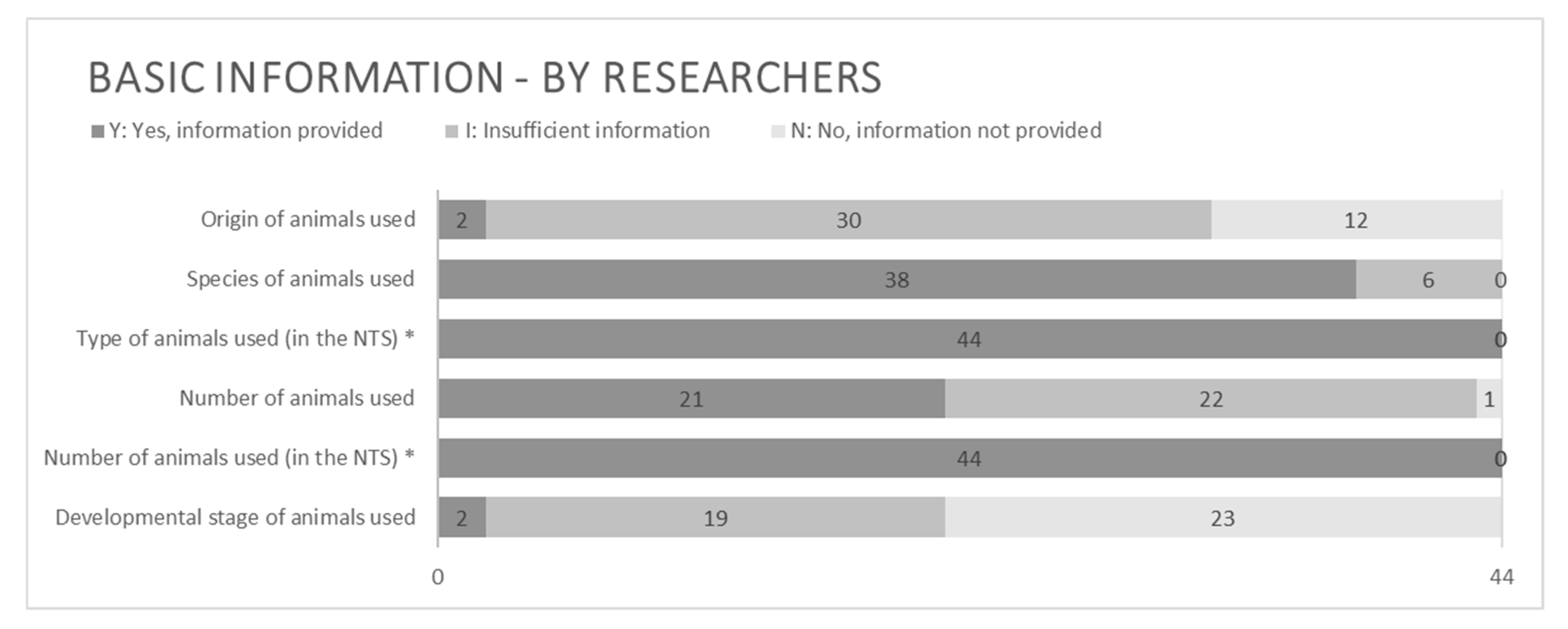
3.2.1. Basic Information
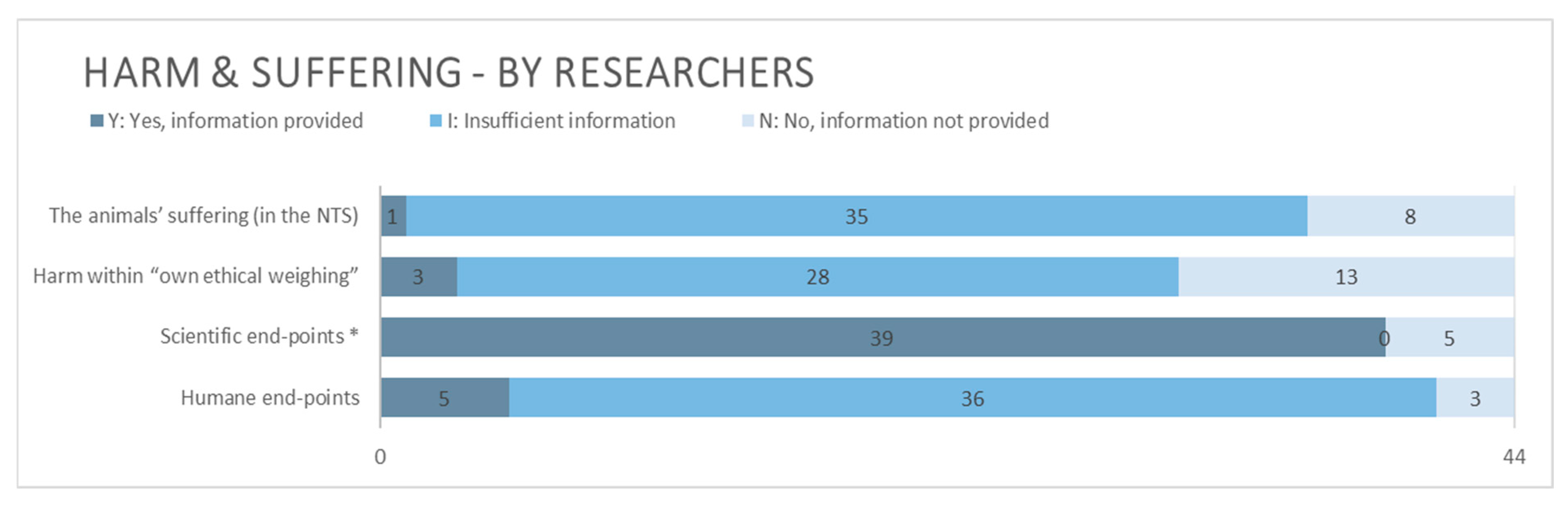
3.2.2. Harm and Suffering
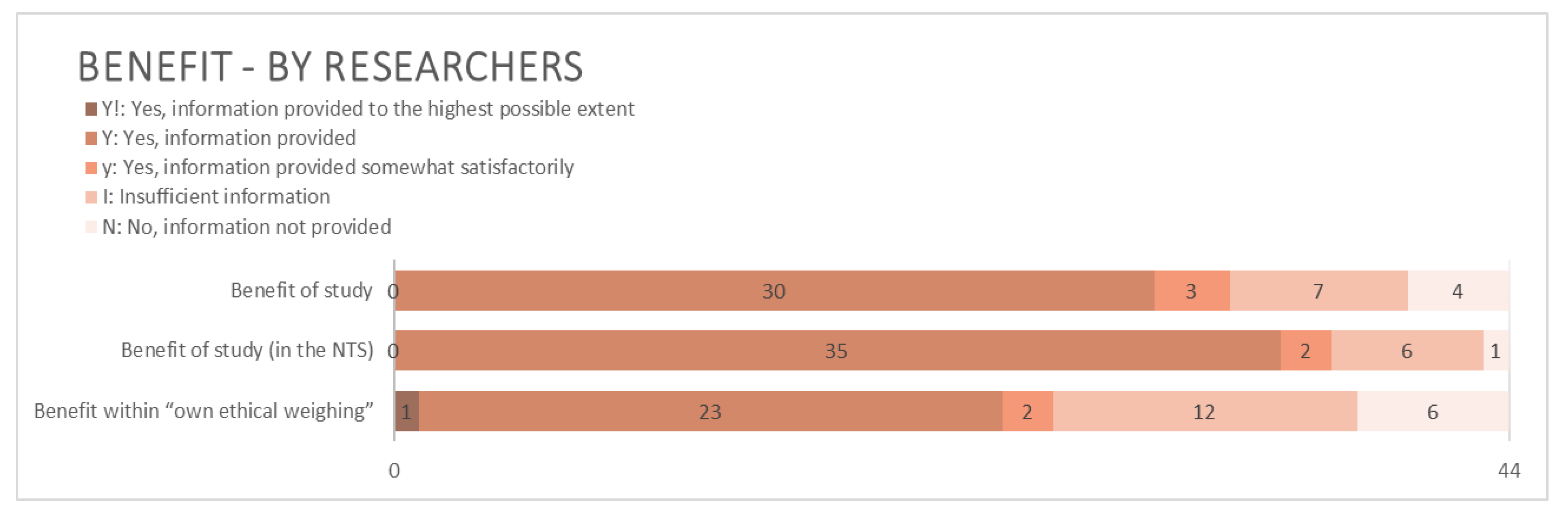
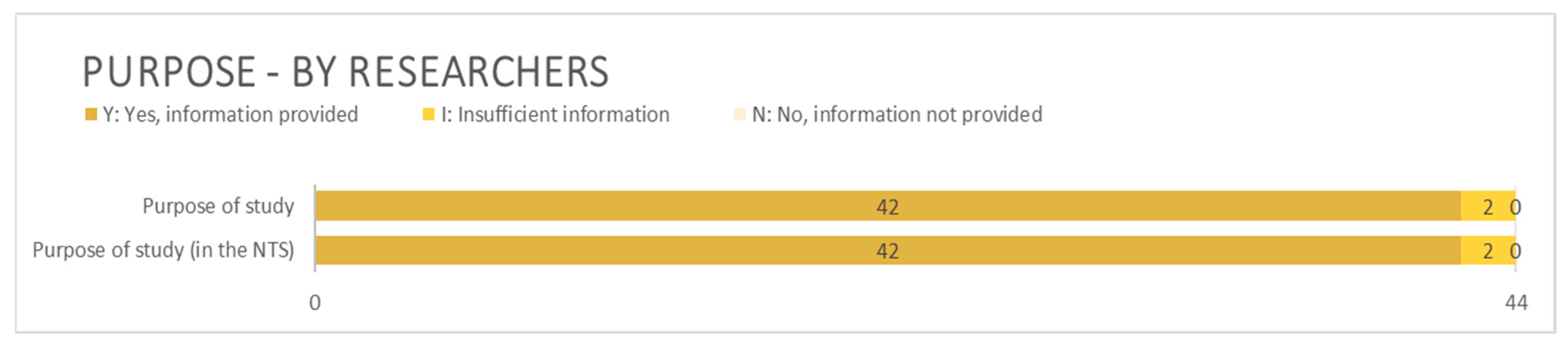
3.2.3. Benefit and Purpose
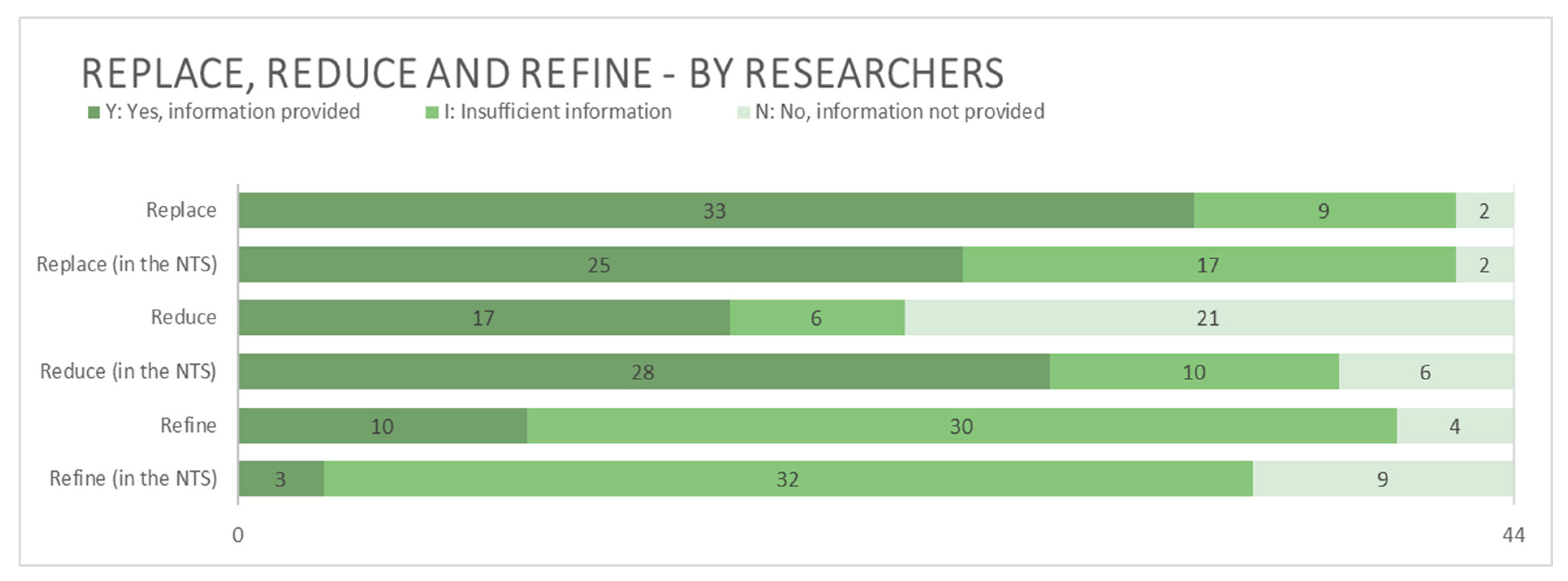
3.2.4. Replace, Reduce, and Refine (The 3Rs)
4. Discussion
4.1. Ambiguities Concerning Regulatory Requirements
4.2. Analysis of Applications by Researchers
4.2.1. Basic Information
4.2.2. Harm and Suffering
4.2.3. Benefit and Purpose
4.2.4. Replace, Reduce, and Refine
4.2.5. Limitations of the Study
5. Suggestions for Improvements
5.1. Creation of Comprehensive Guidelines Clarifying Regulatory Requirements:
5.2. Revision and Improvement of Application Form(s):
5.3. Revision of AEC Structure and Competence:
5.4. Mandatory Education and Training:
5.5. Support and Resources:
5.6. Empower AECs to Reject or Postpone Inadequate Applications:
5.7. Assess Compliance Through Random Audits:
5.8. EU Commission Involvement:
5.9. Qualitative Research on Perception and Implementation:
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Available online: https://eur-lex.europa.eu/eli/dir/2010/63/oj/eng (accessed on 12 March 2024).
- Olsson, I.A.S.; da Silva, S.P.; Townend, D.; Sandøe, P. Protecting Animals and Enabling Research in the European Union: An Overview of Development and Implementation of Directive 2010/63/EU. ILAR J. 2016, 57, 347–357. [Google Scholar] [CrossRef]
- Guillén, J.; Robinson, S.; Decelle, T.; Exner, C.; van Vlissingen, M.F. Approaches to animal research project evaluation in Europe after implementation of Directive 2010/63/EU. Lab. Anim. 2015, 44, 23–31. [Google Scholar] [CrossRef]
- Hajosi, D.; Grimm, H. Mission impossible accomplished? A European cross-national comparative study on the integration of the harm-benefit analysis into law and policy documents. PLoS ONE 2024, 19, e0297375. [Google Scholar] [CrossRef]
- SJVFS 2019:9. The Swedish Board of Agriculture’s Regulations And General Advice on Laboratory Animals (Case No L150). 2020. Available online: https://lagen.nu/sjvfs/2019:9#page001-text (accessed on 12 November 2024).
- SFS 2018:1192. Djurskyddslag [Swedish Animal Welfare Act]. Available online: https://www.riksdagen.se/sv/dokument-lagar/dokument/svensk-forfattningssamling/djurskyddslag-20181192_sfs-2018-1192 (accessed on 3 April 2024).
- SFS 2019:66. Djurskyddsförordning [Swedish Animal Welfare Ordinance]. Available online: https://www.riksdagen.se/sv/dokument-och-lagar/dokument/svensk-forfattningssamling/djurskyddsforordning-201966_sfs-2019-66/ (accessed on 3 April 2024).
- Ljung, P.E.; Udén, E.; Van Den Weghe, J.; Bornestaf, C. Jordbruksverkets Rapport: Användning av försöksdjur i Sverige under 2018. In Report by Swedish Board of Agriculture; Swedish Board of Agriculture: Jönköping, Sweden, 2021; Dnr: 5.2.17-17593/2019; Available online: https://jordbruksverket.se/djur/ovriga-djur/forsoksdjur-och-djurforsok/verksamhet-med-forsoksdjur#forsoksdjursstatistik (accessed on 3 August 2025).
- The European Commission Expert Working Group. Commission Staff Working Document Accompanying the Document Report from the Commission to the European Parliament, The Council, The European Economic and Social Committee and the Committee of the Regions in Accordance with Article 58 of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes; CORRIGENDUM SWD/2017/0353 final/2; The European Commission Expert Working Group: Brussels, Belgium, 2017; Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52017SC0353R%2801%29&qid=1747027410789 (accessed on 3 August 2025).
- Grimm, H.; Eggel, M.; Deplazes-Zemp, A.; Biller-Andorno, N. The Road to Hell Is Paved with Good Intentions: Why Harm-Benefit Analysis and Its Emphasis on Practical Benefit Jeopardizes the Credibility of Research. Animals 2017, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Grimm, H.; Olsson, I.A.S.; Sandøe, P. Harm–benefit analysis—What is the added value? A review of alternative strategies for weighing harms and benefits as part of the assessment of animal research. Lab. Anim. 2019, 53, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Gutfreund, Y. Harm-Benefit Analysis May Not Be the Best Approach to Ensure Minimal Harms and Maximal Benefits of Animal Research-Alternatives Should Be Explored. Animals 2020, 10, 291. [Google Scholar] [CrossRef]
- Niemi, S.M. Harm-Benefit Analyses Can Be Harmful. ILAR J. 2021, 60, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Ringblom, J.; Törnqvist, E.; Hansson, S.O.; Rudén, C.; Öberg, M. Assigning ethical weights to clinical signs observed during toxicity testing. ALTEX 2017, 34, 148–156. [Google Scholar] [CrossRef]
- Cojocaru, M.D. Beyond Plausibility Checks: A Case for Moral Doubt in Review Processes of Animal Experimentation. In Animal Experimentation: Working Towards a Paradigm Change; Brill: Leiden, The Netherlands, 2019; pp. 289–304. [Google Scholar]
- Rawle, F. The Role of Review and Regulatory Approvals Processes for Animal Research in Supporting Implementation of the 3Rs; National Centre for the Replacement Refinement & Reduction of Animals in Research (NC3Rs): London, UK, 2023; Available online: https://nc3rs.org.uk/role-review-and-regulatory-approvals-processes-animal-research-supporting-implementation-3rs-2023 (accessed on 1 July 2025).
- Sena, E.S.; Currie, G.L. How our approaches to assessing benefits and harms can be improved. Anim. Welf. 2019, 28, 107–115. [Google Scholar] [CrossRef]
- Lindsjö, J.; Berg, C.; Olsson, U.; Törnqvist, E. The 3Rs in animal welfare bodies at Swedish universities—Knowledge, attitudes, implementation. ALTEX 2021, 38, 477–489. [Google Scholar] [CrossRef]
- Jörgensen, S.; Lindsjö, J.; Weber, E.M.; Röcklinsberg, H. Reviewing the Review: A Pilot Study of the Ethical Review Process of Animal Research in Sweden. Animals 2021, 11, 708. [Google Scholar] [CrossRef]
- Sandgren, C. Rättsvetenskap för Uppsatsförfattare—Ämne, Material, Metod, Argumentation och Språk; Norstedts Juridik: Stockholm, Sweden, 2021; Volume 5. [Google Scholar]
- Elo, S.; Kyngäs, H. The qualitative content analysis process. J. Adv. Nurs. 2008, 62, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, H.F.; Shannon, S.E. Three approaches to qualitative content analysis. Qual. Health Res. 2005, 15, 1277–1288. [Google Scholar] [CrossRef]
- Krippendorff, K. Content Analysis: An Introduction to Its Methodology, 4th ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Silverman, D. Interpreting Qualitative Data, 5th ed.; SAGE Publications, Inc.: Thousand Oaks, CA, USA, 2015. [Google Scholar]
- The European Commission Expert Working Group. National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Working Document on Project Evaluation and Retrospective Assessment; The European Commission Expert Working Group: Brussels, Belgium, 2013. [Google Scholar]
- Schindler, S. The animal’s dignity in Swiss Animal Welfare Legislation—Challenges and opportunities. Eur. J. Pharm. Biopharm. 2013, 84, 251–254. [Google Scholar] [CrossRef]
- Viksten, S.M.; Visser, E.K.; Blokhuis, H.J. A comparative study of the application of two horse welfare assessment protocols. Acta Agric. Scand. Sect. A—Anim. Sci. 2016, 66, 56–65. [Google Scholar] [CrossRef]
- The European Commission. Commission Staff Working Document Fitness Check of the EU Animal Welfare Legislation; The European Commission: Brussels, Belgium, 2022. [Google Scholar]
- Lundmark, F.; Röcklinsberg, H.; Berg, C. ‘Unnecessary suffering’ as a concept in animal welfare legislation and standards. In The Ethics of Consumption—The Citizen, the Market and the Law; Röcklinsberg, H., Sandin, P., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2013; pp. 114–119. [Google Scholar]
- Lundmark, F.; Helena, R.; Birgitta, W.; Berg, C. Content and structure of Swedish animal welfare legislation and private standards for dairy cattle. Acta Agric. Scand. Sect. A—Anim. Sci. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Lindsjö, J.; Cvek, K.; Spangenberg, E.M.F.; Olsson, J.N.G.; Stéen, M. The Dividing Line Between Wildlife Research and Management—Implications for Animal Welfare. Front. Vet. Sci. 2019, 6, 13. [Google Scholar] [CrossRef]
- Vogt, L.; Reichlin, T.S.; Nathues, C.; Würbel, H. Authorization of Animal Experiments Is Based on Confidence Rather than Evidence of Scientific Rigor. PLoS Biol. 2016, 14, e2000598. [Google Scholar] [CrossRef]
- Lindsjö, J.; Fahlman, A.; Tornqvist, E. Animal Welfare from Mouse to Moose—Implementing the Principles of the 3Rs in Wildlife Research. J. Wildl. Dis. 2016, 52, S65–S77. [Google Scholar] [CrossRef] [PubMed]
- Butler, R.K.; Finn, D.P. Stress-induced analgesia. Prog. Neurobiol. 2009, 88, 184–202. [Google Scholar] [CrossRef] [PubMed]
- Braithwaite, V.A.; Ebbesson, L.O. Pain and stress responses in farmed fish. Sci. Tech. Rev. 2014, 33, 245–253. [Google Scholar] [CrossRef]
- Grandin, T.; Shivley, C. How Farm Animals React and Perceive Stressful Situations Such As Handling, Restraint, and Transport. Animals 2015, 5, 1233–1251. [Google Scholar] [CrossRef]
- Walters, E.T.; Williams, A.C. de C. Evolution of mechanisms and behaviour important for pain. Phil. Trans. R. Soc. B 2019, 374, 20190275. [Google Scholar] [CrossRef] [PubMed]
- Arndt, S.S.; Goerlich, V.C.; van der Staay, F.J. A dynamic concept of animal welfare: The role of appetitive and adverse internal and external factors and the animal’s ability to adapt to them. Front. Anim. Sci. 2022, 3, 908513. [Google Scholar] [CrossRef]
- Roelofs, S.; Boleij, H.; Nordquist, R.E.; van der Staay, F.J. Making Decisions under Ambiguity: Judgment Bias Tasks for Assessing Emotional State in Animals. Front. Behav. Neurosci. 2016, 10, 119. [Google Scholar] [CrossRef]
- The European Commission Expert Working Group. National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Scientific Purposes. Working Document on a Severity Assessment Framework; The European Commission Expert Working Group: Brussels, Belgium, 2012. [Google Scholar]
- Imbe, H.; Iwai-Liao, Y.; Senba, E. Stress-induced hyperalgesia: Animal models and putative mechanisms. Front. Biosci. 2006, 11, 2179–2192. [Google Scholar] [CrossRef]
- Sadler, K.E.; Mogil, J.S.; Stucky, C.L. Innovations and advances in modelling and measuring pain in animals. Nat. Rev. Neurosci. 2022, 23, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Brønstad, A.; Newcomer, C.E.; Decelle, T.; Everitt, J.I.; Guillen, J.; Laber, K. Current concepts of harm–benefit analysis of animal experiments—Report from the AALAS–FELASA working group on harm–benefit analysis—Part 1. Lab. Anim. 2016, 50, 1–20. [Google Scholar] [CrossRef]
- The European Commission Expert Working Group. National Competent Authorities for the Implementation of Directive 2010/63/EU on the Protection of Animals Used for Sci-entific Purposes. Working Document on Non-Technical Project Summaries; The European Commission Expert Working Group: Brussels, Belgium, 2013. [Google Scholar]
- The European Commission. Statistics and Non-Technical Project Summaries. Available online: https://environment.ec.europa.eu/topics/chemicals/animals-science/statistics-and-non-technical-project-summaries_en (accessed on 23 May 2025).
- Bayne, K.; Turner, P.V. Chapter 7—Animal Environments and Their Impact on Laboratory Animal Welfare. In Laboratory Animal Welfare; Bayne, K., Turner, P.V., Eds.; Academic Press: Boston, MA, USA, 2014; pp. 77–93. [Google Scholar]
- Poole, T. Happy animals make good science. Lab. Anim. 1997, 31, 116–124. [Google Scholar] [CrossRef]
- Mazhary, H.; Hawkins, P. Applying the 3Rs: A Case Study on Evidence and Perceptions Relating to Rat Cage Height in the UK. Animals 2019, 9, 1104. [Google Scholar] [CrossRef]
- Cait, J.; Cait, A.; Scott, R.W.; Winder, C.B.; Mason, G.J. Conventional laboratory housing increases morbidity and mortality in research rodents: Results of a meta-analysis. BMC Biol. 2022, 20, 15. [Google Scholar] [CrossRef]
- Sandgren, R.; Grims, C.; Waters, J.; Hurst, J.L. Using cage ladders as a handling device reduces aversion and anxiety in laboratory mice, similar to tunnel handling. Scand. J. Lab. Anim. Sci. 2021, 47, 31–41. [Google Scholar] [CrossRef]
- Novak, J.; Jaric, I.; Rosso, M.; Rufener, R.; Touma, C.; Würbel, H. Handling method affects measures of anxiety, but not chronic stress in mice. Sci. Rep. 2022, 12, 20938. [Google Scholar] [CrossRef] [PubMed]
- Gouveia, K.; Hurst, J.L. Improving the practicality of using non-aversive handling methods to reduce background stress and anxiety in laboratory mice. Sci. Rep. 2019, 9, 20305. [Google Scholar] [CrossRef]
- Lidster, K.; Owen, K.; Browne, W.J.; Prescott, M.J. Cage aggression in group-housed laboratory male mice: An international data crowdsourcing project. Sci. Rep. 2019, 9, 15211. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Zidar, J.; Ewaldsson, B.; Askevik, K.; Udén, E.; Svensk, E.; Törnqvist, E. Aggression in Group-Housed Male Mice: A Systematic Review. Animals 2022, 13, 143. [Google Scholar] [CrossRef]
- Varga, O. Critical Analysis of Assessment Studies of the Animal Ethics Review Process. Animals 2013, 3, 907–922. [Google Scholar] [CrossRef]
- Voipio, H.-M.; Kaliste, E.; Hirsjärvi, P.; Nevalainen, T.; Ritskes-Hoitinga, M. Nordic-European Workshop on Ethical Evaluation of Animal Experiments. Workshop Report on the Cost Benefit Principle. Scand. J. Lab. Anim. Sci. 2004, 34, 2151–2267. [Google Scholar]
- Vieira de Castro, A.C.; Olsson, I.A.S. Does the Goal Justify the Methods? Harm and Benefit in Neuroscience Research Using Animals. In Ethical Issues in Behavioral Neuroscience. Current Topics in Behavioral Neurosciences; Lee, G., Illes, J., Ohl, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 19, pp. 47–78. [Google Scholar]
- Binder, R. Balancing Competing Values in a Legal Setting: Evaluating Harm and Benefit of Proposed Animal Experiments. ALTEX Proc. 2015, 4, 8–11. [Google Scholar]
- Maisack, C. Harm-Benefit Analysis According to Directive 2010/63/EU, Article 38: What Does It Mean and How To Realize It. ALTEX Proc. 2015, 4, 24–27. [Google Scholar]
- Bateson, P. Ethics and behavioral biology. Adv. Study Behav. 2005, 35, 211–233. [Google Scholar] [CrossRef]
- Röcklinsberg, H.; Bratbo Sørensen, D.; Kornum, A.; Gjerris, M. The ethical assessment process. In Biotech Animals in Research: Ethical and Regulatory Aspects, 1st ed.; Gjerris, M., Kornum, A., Röcklinsberg, H., Sørensen, D.B., Eds.; CRC Press: Boca Raton, FL, USA, 2023; pp. 173–189. [Google Scholar]
- Centrala Djurförsöksetiska Nämnden (CDFN). Datasammanställning av Utvärderingar i Efterhand och Överklaganden; Centrala Djurförsöksetiska Nämnden: Jönköping, Sweden, 2022. [Google Scholar]
- Schuppli, C.A.; Fraser, D. Factors influencing the effectiveness of research ethics committees. J. Med. Ethics 2007, 33, 294–301. [Google Scholar] [CrossRef]
- Balls, M. It’s Time to Include Harm to Humans in Harm-Benefit Analysis—But How to Do It, That is the Question. Altern. Lab. Anim. 2021, 49, 182–196. [Google Scholar] [CrossRef]
- Bout, H.J.; Fentener van Vlissingen, J.M.; Karssing, E.D. Evaluating the ethical acceptability of animal research. Lab. Anim. 2014, 43, 411–414. [Google Scholar] [CrossRef]
- Würbel, H. More than 3Rs: The importance of scientific validity for harm-benefit analysis of animal research. Lab. Anim. 2017, 46, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Eggel, M.; Würbel, H. Internal consistency and compatibility of the 3Rs and 3Vs principles for project evaluation of animal research. Lab. Anim. 2021, 55, 233–243. [Google Scholar] [CrossRef]
- Eggel, M.; Grimm, H. Necessary, but Not Sufficient. The Benefit Concept in the Project Evaluation of Animal Research in the Context of Directive 2010/63/EU. Animals 2018, 8, 34. [Google Scholar] [CrossRef]
- Houde, L.; Dumas, C.; Leroux, T. Ethics: Views from IACUC Members. Altern. Lab. Anim. 2009, 37, 291–296. [Google Scholar] [CrossRef]
- Schuppli, C.A.; Fraser, D. The Interpretation and Application of the Three Rs by Animal Ethics Committee Members. Altern. Lab. Anim. 2005, 33, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Nøhr, R.; Lund, T.B.; Lassen, J. The Danish 3R Survey: Knowledge, Attitudes and Experiences with the 3Rs Among Researchers Involved in Animal Experiments in Denmark; IFRO Report No. 249; Department of Food and Resource Economics, University of Copenhagen: Frederiksberg, Denmark, 2016; p. 57. [Google Scholar]
- National Centre for the Replacement Refinement & Reduction of Animals in Research (NC3Rs). Views on the 3Rs—Survey Report; National Centre for the Replacement Refinement & Reduction of Animals in Research (NC3Rs): London, UK, 2008. [Google Scholar]
- Tadich, T.; Tarazona, A.M. Replacement, Reduction and Refinement: Ethical Considerations in the Current Applications of the 3Rs. In Handbook of Bioethical Decisions; Collaborative Bioethics 2; Valdés, E., Lecaros, J.A., Eds.; Springer: Cham, Switzerland, 2023; Volume I, pp. 667–683. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jörgensen, S.; Weber, E.M.; Lindsjö, J.; Lundmark Hedman, F.; Röcklinsberg, H. Approved Ambiguities: An Analysis of Applications for the Ethical Review of Animal Research in Sweden—Focusing on Harm, Benefit, and the 3Rs. Animals 2025, 15, 2771. https://doi.org/10.3390/ani15192771
Jörgensen S, Weber EM, Lindsjö J, Lundmark Hedman F, Röcklinsberg H. Approved Ambiguities: An Analysis of Applications for the Ethical Review of Animal Research in Sweden—Focusing on Harm, Benefit, and the 3Rs. Animals. 2025; 15(19):2771. https://doi.org/10.3390/ani15192771
Chicago/Turabian StyleJörgensen, Svea, Elin M. Weber, Johan Lindsjö, Frida Lundmark Hedman, and Helena Röcklinsberg. 2025. "Approved Ambiguities: An Analysis of Applications for the Ethical Review of Animal Research in Sweden—Focusing on Harm, Benefit, and the 3Rs" Animals 15, no. 19: 2771. https://doi.org/10.3390/ani15192771
APA StyleJörgensen, S., Weber, E. M., Lindsjö, J., Lundmark Hedman, F., & Röcklinsberg, H. (2025). Approved Ambiguities: An Analysis of Applications for the Ethical Review of Animal Research in Sweden—Focusing on Harm, Benefit, and the 3Rs. Animals, 15(19), 2771. https://doi.org/10.3390/ani15192771




