Utilisation of Inorganic Phosphates in Standard Diets for Whiteleg Shrimp Litopenaeus vannamei (Boone, 1931)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Diets Formulation
2.2. Experimental Design and Facilities
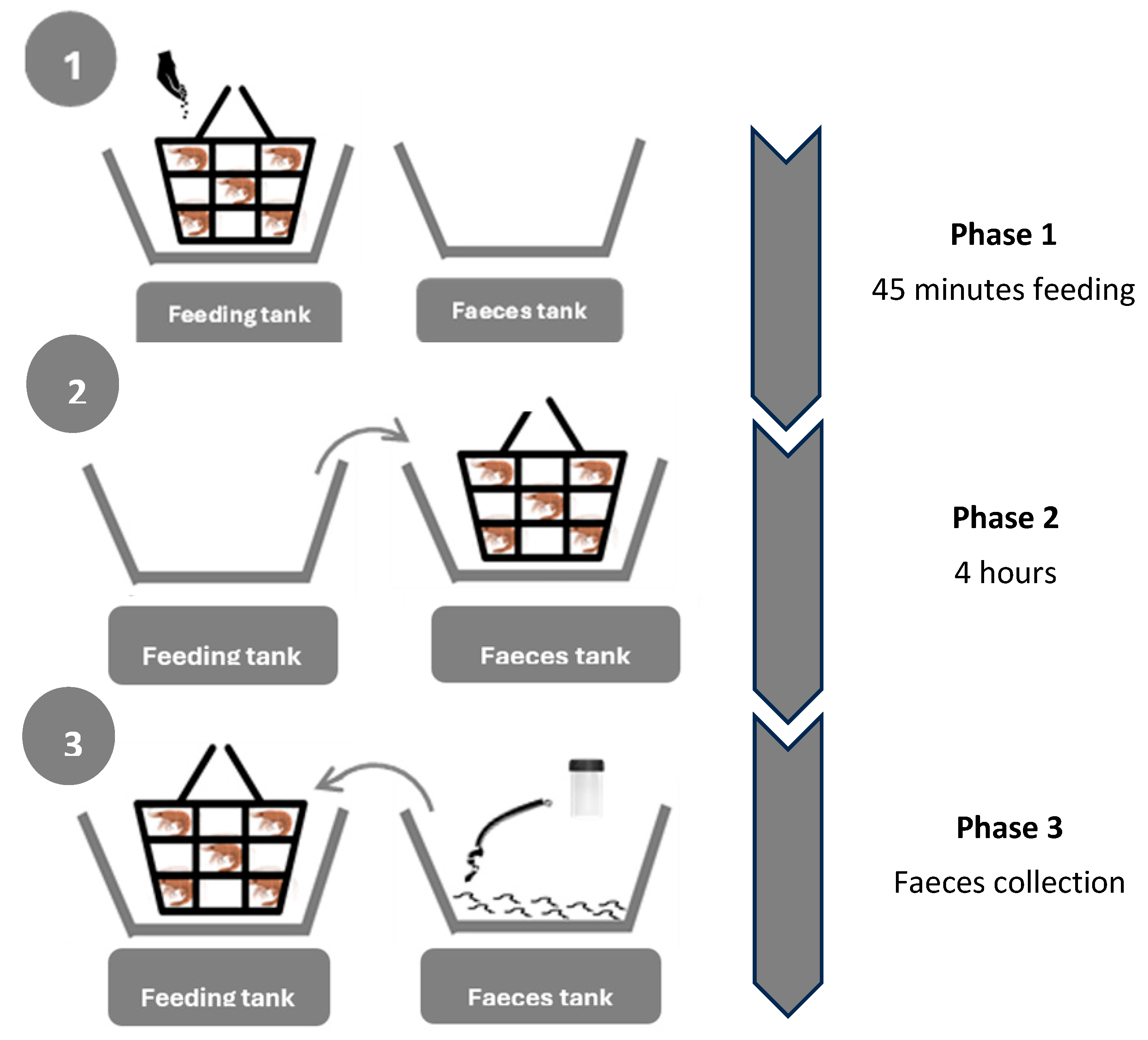
2.2.1. Digestibility Trial
2.2.2. Growth Trial
2.3. Water Quality Analysis
2.4. Estimation of N and P
2.5. Analysis and Calculations
2.6. Statistical Analysis
3. Results
3.1. Digestibility Coefficients
3.2. Growth and Nutritional Parameters
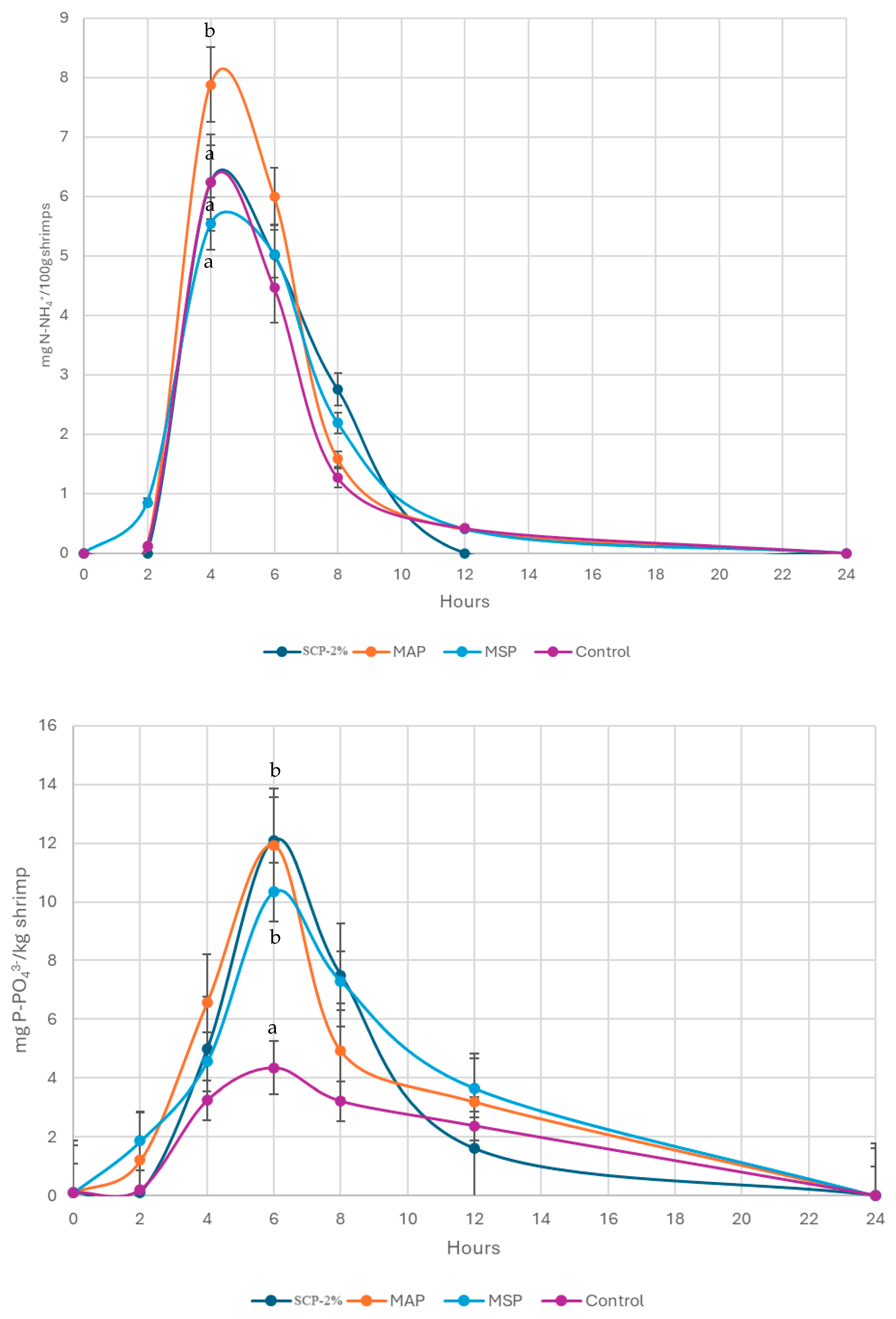
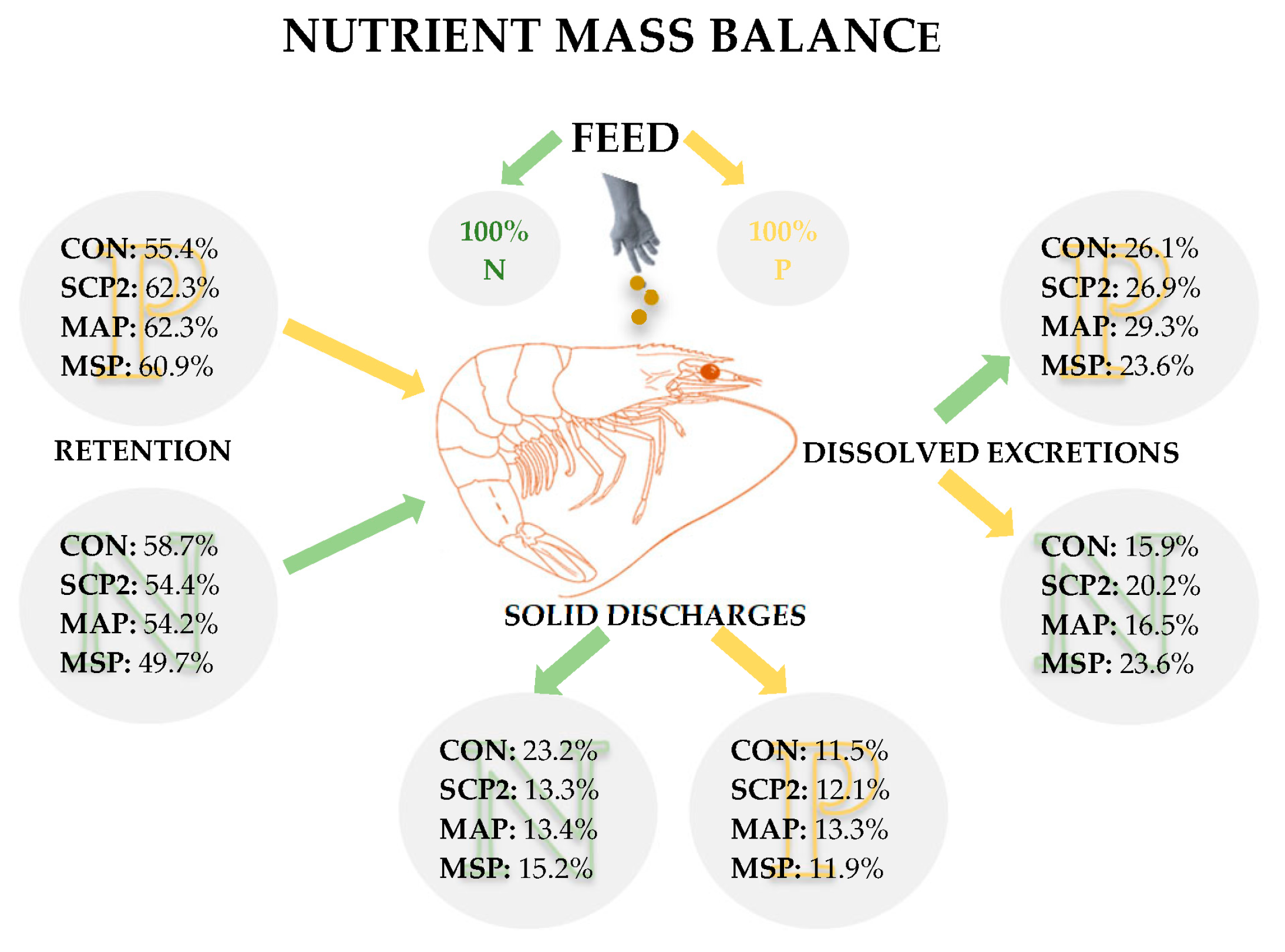
3.3. Phosphorus and Nitrogen Excretion Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miao, W.; Wang, W. Trends of Aquaculture Production and Trade: Carp, Tilapia, and Shrimp. Asian Fish. Sci. 2020, 33, 1–10. [Google Scholar] [CrossRef]
- Ayisi, C.L.; Hua, X.; Apraku, A.; Afriyie, G.; Kyei, B.A. Recent Studies Toward the Development of Practical Diets for Shrimp and Their Nutritional Requirements. Hayati 2017, 24, 109–117. [Google Scholar] [CrossRef]
- Truong, H.H.; Hines, B.M.; Emerenciano, M.G.; Blyth, D.; Berry, S.; Noble, T.H.; Bourne, N.A.; Wade, N.; Rombenso, A.N.; Simon, C.J. Mineral nutrition in penaeid shrimp. Rev. Aquac. 2023, 15, 1355–1373. [Google Scholar] [CrossRef]
- Coloso, R.M.; Basantes, S.; King, K.; Hendrix, M.; Fletcher, J.; Weis, P.; Ferraris, R. Effect of dietary phosphorus and vitamin D3 on phosphorus levels in effluent from the experimental culture of rainbow trout (Oncorhynchus mykiss). Aquaculture 2001, 202, 145–161. [Google Scholar] [CrossRef]
- Amaya, E.A.; Davis, D.A.; Rouse, D.B. Replacement of fish meal in practical diets for the Pacific white shrimp (Litopenaeus vannamei) reared under pond conditions. Aquaculture 2007, 262, 393–401. [Google Scholar] [CrossRef]
- Chamberlain, G. Frontiers in shrimp nutrition research. In Swimming Through Troubled Water; The World Aquaculture Society: Sorrento, LA, USA, 1995. [Google Scholar]
- Cheng, K.M.; Hu, C.Q.; Liu, Y.N.; Zheng, S.X.; Qi, X.J. Effects of dietary calcium, phosphorus and calcium/phosphorus ratio on the growth and tissue mineralization of Litopenaeus vannamei reared in low-salinity water. Aquaculture 2006, 251, 472–483. [Google Scholar] [CrossRef]
- Zheng, C.; Cao, J.; Chi, S.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Xie, S.; Tan, B. Dietary phosphorus supplementation in the diet of Pacific white shrimp (Litopenaeus vannamei) alleviated the adverse impacts caused by high Clostridium autoethanogenum protein. Fish Shellfish. Immunol 2022, 131, 137–149. [Google Scholar] [CrossRef] [PubMed]
- Dinesh Kumar, S.; Santhanam, P.; Krishnaveni, N.; Raju, P.; Begum, A.; Ahmed, S.; Perumal, P.; Pragnya, M.; Dhanalakshmi, B.; Kim, M.-K. Baseline assessment of water quality and ecological indicators in Penaeus vannamei farm wastewater along the Southeast coast of India. Mar. Pollut. Bull. 2020, 160, 111579. [Google Scholar] [CrossRef]
- Kibria, G.; Nugegoda, D.; Lam, P.; Fairclough, R. Aspects of phosphorus pollution from aquaculture. NAGA 1996, 19, 20–24. [Google Scholar]
- Qiu, X.; Davis, D.A. Effects of dietary phytase supplementation on growth performance and apparent digestibility coefficients of Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2017, 23, 942–951. [Google Scholar] [CrossRef]
- Manikandan, K.; Felix, N. Growth performance, apparent digestibility coefficients and digestive enzyme activities of Pacific white shrimp, Penaeus vannamei fed low fishmeal diets supplemented with DL-methionine and phytase. Aquac. Nutr. 2021, 27, 2240–2250. [Google Scholar] [CrossRef]
- Yúfera, M.; Moyano, F.J.; Astola, A.; Pousão-Ferreira, P.; Martínez-Rodríguez, G. Acidic digestion in a teleost: Postprandial and circadian pattern of gastric pH, pepsin activity, and pepsinogen and proton pump mRNAs expression. PLoS ONE 2012, 7, e33687. [Google Scholar] [CrossRef]
- Lemos, D.; Coelho, R.; Zwart, S.; Tacon, A.G.J. Performance and digestibility of inorganic phosphates in diets for juvenile shrimp (Litopenaeus vannamei): Dicalcium phosphate, monocalcium phosphate, and monoammonium phosphate. Aquac. Int. 2021, 29, 681–695. [Google Scholar] [CrossRef]
- Hossain, M.S.; Chance, A.B.; El Kertaoui, N.; Wattiez, X.; Houndji, A.; Mandiki, S.N.; Kestemont, P. Dietary inorganic monophosphates in high plant ingredient-based diets influence nutrient digestibility, postprandial macro-mineral status and immune functions of juvenile rainbow trout, Oncorhynchus mykiss. Aquac. Nutr. 2020, 26, 2178–2194. [Google Scholar] [CrossRef]
- Milián-Sorribes, M.C.; Tomás-Vidal, A.; Peñaranda, D.S.; Carpintero, L.; Mesa, J.S.; Dupuy, J.; Donadeu, A.; Macías-Vidal, J.; Martínez-Llorens, S. Estimation of Phosphorus and Nitrogen Waste in Rainbow Trout (Oncorhynchus mykiss, Walbaum, 1792) Diets Including Different Inorganic Phosphorus Sources. Animals 2021, 11, 1700. [Google Scholar] [CrossRef]
- Carpintero, L.; Donadeu, A.; Dupuy, J.; Macías-Vidal, J. EPCD Index: Predictive Equations for Digestibility Comparison Among Different Sources of Commercial Phosphates in Poultry. 2020. Available online: https://globalfeed.es/en/news/147-indice-epcd (accessed on 15 September 2025).
- Kai, H.; Wu, W.; Chunhua, L. Requirement of essential amino acids for Penaeus vannamei. Shuichan Xuebao 2003, 27, 456–461. [Google Scholar]
- Kureshy, N.; Allen Davis, D. Protein requirement for maintenance and maximum weight gain for the Pacific white shrimp, Litopenaeus vannamei. Aquaculture 2002, 204, 125–143. [Google Scholar] [CrossRef]
- McGoogan, B.B.; Gatlin, D.M. Dietary manipulations affecting growth and nitrogenous waste production of red drum, Sciaenops ocellatus I. Effects of dietary protein and energy levels. Aquaculture 1999, 178, 333–348. [Google Scholar] [CrossRef]
- AOAC. Official Analysis Chemists International; AOAC International. In Association of Official Analysis Chemists International; AOAC International: Gaithersburg, MD, USA, 2005. [Google Scholar]
- Cho, C.Y.; Kaushik, S.J. Nutritional energetics in fish: Energy and protein utilization in rainbow trout (Salmo gairdneri). World Rev. Nutr. Diet. 1990, 61, 132–172. [Google Scholar] [CrossRef]
- Grasshoff, K.; Kremling, K.; Ehrhardt, M. Methods of Seawater Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Baumgarten, M.G.Z.; Wallner-Kersanach, M.; Niencheski, L.F.H. Manual de Análises em Oceanografia Química; Editora da FURG: Rio Grande, Brazil, 2010; 163p. [Google Scholar]
- Morales, G.A.; Azcuy, R.L.; Casaretto, M.E.; Márquez, L.; Hernández, A.J.; Gómez, F.; Koppe, W.; Mereu, A. Effect of different inorganic phosphorus sources on growth performance, digestibility, retention efficiency and discharge of nutrients in rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 495, 568–574. [Google Scholar] [CrossRef]
- Coelho, R.; Reynaud, J.G.; Biard, C.; Ribeiro, B.; Lemos, D. Inorganic Phosphorus Supplementation in Diets for Farmed Shrimp: Performance and Digestibility of Juvenile Litopenaeus vannamei Fed Monosodium Phosphate, Monoammonium Phosphate, Magnesium Phosphate, and Monocalcium Phosphate. Aquac. Res. 2024, 2024, 8810430. [Google Scholar] [CrossRef]
- European Union Commission. European Union Commission Regulation (EU) 2017/1017 of 15 June 2017, amending Regulation (EU) No 68/2013 on the Catalogue of Feed Materials. Off. J. Eur. Union 2017, 159, 48–119. [Google Scholar]
- Davis, D.A.; Lawrence, A.L.; Gatlin, D.M. Response of Penaeus vannamei to Dietary Calcium, Phosphorus and Calcium: Phosphorus Ratio. J. World Aquac. Soc. 1993, 24, 504–515. [Google Scholar] [CrossRef]
- Davis, D.A.; Arnold, C.R. Estimation of apparent phosphorus availability from inorganic phosphorus sources for Penaeus vannamei. Aquaculture 1994, 127, 245–254. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Rothlisberg, P.C. Aspects of penaeid biology and ecology of relevance to aquaculture: A review. Aquaculture 1998, 164, 49–65. [Google Scholar] [CrossRef]
- Chang, E.S.; Mykles, D.L. Regulation of Crustacean Molting: A Review and Our Perspectives. Gen. Comp. Endocrinol. 2011, 172, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Cheng, W.; Liu, C.H.; Yan, D.F.; Chen, J.C. Hemolymph Oxyhemocyanin, Protein, Osmolality and Electrolyte Levels of Whiteleg Shrimp Litopenaeus vannamei in Relation to Size and Molt Stage. Aquaculture 2002, 211, 325–339. [Google Scholar] [CrossRef]
- Niu, J.; Liu, Y.-J.; Tian, L.-X.; Mai, K.-S.; Yang, H.-J.; Ye, C.-X.; Gao, W. Effect of dietary phosphorus sources and varying levels of supplemental phosphorus on survival, growth and body composition of postlarval shrimp (Litopenaeus vannamei). Aquac. Nutr. 2008, 14, 472–479. [Google Scholar] [CrossRef]
- Briggs, M.R.P.; Fvnge-Smith, S.J. A nutrient budget of some intensive marine shrimp ponds in Thailand. Aquac. Res. 1994, 25, 789–811. [Google Scholar] [CrossRef]


| INGREDIENTS (G/KG) | CONTROL | MAP | SCP-2% | MSP |
|---|---|---|---|---|
| FISHMEAL A | 100 | 100 | 100 | 100 |
| WHEAT MEAL B | 399.0 | 369.0 | 365.0 | 366.0 |
| SOYBEAN MEAL C | 304.0 | 315.1 | 316.6 | 316.2 |
| GLUTEN MEAL D | 80 | 80 | 80 | 80 |
| FISH OIL | 20 | 20 | 20 | 20 |
| SOYBEAN OIL | 27 | 27 | 27 | 27 |
| MALTODEXTRIN | 50 | 50 | 50 | 50 |
| MAP E | 18.9 | |||
| SCP-2% F | 21.4 | |||
| MSP G | 20.8 | |||
| VITAMIN PREMIX H | 10 | 10 | 10 | 10 |
| SOYBEAN LECITHIN I | 10 | 10 | 10 | 10 |
| THEORICAL COMPOSITION (% DM) ** | ||||
| CP (%) | 35.0 | 35.0 | 35.0 | 35.0 |
| CL (%) | 10.0 | 10.0 | 10.0 | 10.0 |
| CHO (%) | 48.9 | 47.0 | 46.7 | 46.8 |
| ASH (%) | 6.2 | 8.1 | 8.4 | 8.3 |
| CA (%) | 0.52 | 0.52 | 0.78 | 0.52 |
| P (%) | 0.66 | 1.16 | 1.16 | 1.16 |
| CA/P | 0.79 | 0.45 | 0.67 | 0.45 |
| DIET | Ca (%) | P (%) | Ca/P | Na (%) | N (%) | CP ** (%) | Dm (%) |
|---|---|---|---|---|---|---|---|
| CONTROL | 0.55 | 0.66 | 0.81 | 0.22 | 5.56 | 34.73 | 95.0 |
| MAP | 0.65 | 1.16 | 0.58 | 0.25 | 6.06 | 37.96 | 93.0 |
| SCP-2% | 0.80 | 1.16 | 0.72 | 0.27 | 5.68 | 35.52 | 92.9 |
| MSP | 0.56 | 1.16 | 0.49 | 0.55 | 5.60 | 35.03 | 94.2 |
| Diets | ||||||||
|---|---|---|---|---|---|---|---|---|
| ADC (%) | Control | SE | MAP | SE | SCP-2% | SE | MSP | SE |
| Dry matter | 75.0 | 2.7 | 77.2 | 1.4 | 74.7 | 1.4 | 75.3 | 2.0 |
| Protein | 67.2 | 1.0 | 67.1 | 2.3 | 66.6 | 1.1 | 66.3 | 1.9 |
| P | 75.4 a | 1.2 | 84.3 b | 0.3 | 84.3 b | 0.4 | 86.0 b | 0.4 |
| Ca | 34 a | 3.3 | 40.3 ab | 0.9 | 55.2 c | 0.6 | 42.2 b | 4.1 |
| P source digestibility | ||||||||
| P | 96.1 a | 0.6 | 96.1 a | 1.0 | 100.0 b | 1.0 | ||
| Ca | 93.1 | 0.8 | ||||||
| DIETS | CONTROL | SE | MAP | SE | SCP-2% | SE | MSP | SE |
|---|---|---|---|---|---|---|---|---|
| INITIAL WEIGHT (G) | 0.55 | 0.03 | 0.52 | 0.03 | 0.50 | 0.04 | 0.55 | 0.05 |
| FINAL WEIGHT (G) | 13.4 | 0.7 | 13.0 | 1.0 | 12.9 | 0.92 | 13.6 | 0.5 |
| SGR (%/DAY) 1 | 3.33 | 0.05 | 3.35 | 0.06 | 3.38 | 0.07 | 3.35 | 0.06 |
| SURVIVAL (%) | 79.02 | 6.00 | 89.10 | 4.05 | 82.11 | 2.05 | 84.00 | 2.00 |
| FIR 2 | 4.95 | 0.22 | 4.70 | 0.17 | 4.60 | 0.3 | 4.59 | 0.08 |
| FCR 3 | 2.63 | 0.14 | 2.48 | 0.11 | 2.44 | 0.12 | 2.43 | 0.08 |
| Initial | Control | SE | MAP | SE | SCP-2% | SE | MSP | SE | |
|---|---|---|---|---|---|---|---|---|---|
| Dry Matter (%) | 20.2 | 23.0 | 0.9 | 24.6 | 1.12 | 21.9 | 0.9 | 23.0 | 0.7 |
| Crude Protein (%) | 15.2 | 17.0 | 0.7 | 18.5 | 0.9 | 16.1 | 0.7 | 17.4 | 0.7 |
| Ash (%) | 3.11 | 3.36 | 0.18 | 3.38 | 0.13 | 3.32 | 0.11 | 3.45 | 0.15 |
| P (%) | 0.23 | 0.28 | 0.01 | 0.32 | 0.01 | 0.28 | 0.01 | 0.29 | 0.01 |
| Ca (%) | 0.64 | 0.74 | 0.02 | 0.73 | 0.03 | 0.71 | 0.02 | 0.70 | 0.02 |
| NPV (%) 1 | 20.4 | 1.3 | 21.2 | 1.7 | 19.8 | 1.4 | 23.4 | 1.4 | |
| CaPV (%) 2 | 55.2 b | 2.48 | 49.1 a | 2.00 | 40.1 a | 2.05 | 54.2 b | 2.02 | |
| PPV (%) 3 | 17.1 b | 0.9 | 12.7 a | 1.12 | 11.4 a | 0.9 | 11.3 a | 0.9 |
| N-NAT | SEM | P-PO43− | SEM | |
|---|---|---|---|---|
| CONTROL | 31.62 | 5.80 | 5.72 | 2.29 |
| MAP | 39.15 | 2.63 | 9.74 | 2.82 |
| SCP-2% | 33.34 | 2.30 | 9.71 | 1.17 |
| MSP | 36.40 | 6.11 | 11.53 | 3.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Candela-Maldonado, Y.; Serrano, R.; Tomás-Vidal, A.; Peñaranda, D.S.; Jauralde, I.; Carpintero, L.; Mesa, J.S.; Limón, J.L.; Dupuy, J.; Donadeu, A.; et al. Utilisation of Inorganic Phosphates in Standard Diets for Whiteleg Shrimp Litopenaeus vannamei (Boone, 1931). Animals 2025, 15, 2769. https://doi.org/10.3390/ani15192769
Candela-Maldonado Y, Serrano R, Tomás-Vidal A, Peñaranda DS, Jauralde I, Carpintero L, Mesa JS, Limón JL, Dupuy J, Donadeu A, et al. Utilisation of Inorganic Phosphates in Standard Diets for Whiteleg Shrimp Litopenaeus vannamei (Boone, 1931). Animals. 2025; 15(19):2769. https://doi.org/10.3390/ani15192769
Chicago/Turabian StyleCandela-Maldonado, Yosu, Raquel Serrano, Ana Tomás-Vidal, David S. Peñaranda, Ignacio Jauralde, Laura Carpintero, Juan S. Mesa, José L. Limón, Javier Dupuy, Andrés Donadeu, and et al. 2025. "Utilisation of Inorganic Phosphates in Standard Diets for Whiteleg Shrimp Litopenaeus vannamei (Boone, 1931)" Animals 15, no. 19: 2769. https://doi.org/10.3390/ani15192769
APA StyleCandela-Maldonado, Y., Serrano, R., Tomás-Vidal, A., Peñaranda, D. S., Jauralde, I., Carpintero, L., Mesa, J. S., Limón, J. L., Dupuy, J., Donadeu, A., Grindlay, G., Macías-Vidal, J., & Martínez-Llorens, S. (2025). Utilisation of Inorganic Phosphates in Standard Diets for Whiteleg Shrimp Litopenaeus vannamei (Boone, 1931). Animals, 15(19), 2769. https://doi.org/10.3390/ani15192769










