Computer-Aided Diagnosis of Equine Pharyngeal Lymphoid Hyperplasia Using the Object Detection-Based Processing Technique of Digital Endoscopic Images
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Data Collection
Clinical Grading of PLH
2.3. Digital Data Collection
2.4. Statistical Analysis
2.5. Endoscopic Image Classification
3. Results
3.1. Clinical Data-Based Characteristics of PLH
3.2. Digital Data-Based Characteristics of PLH
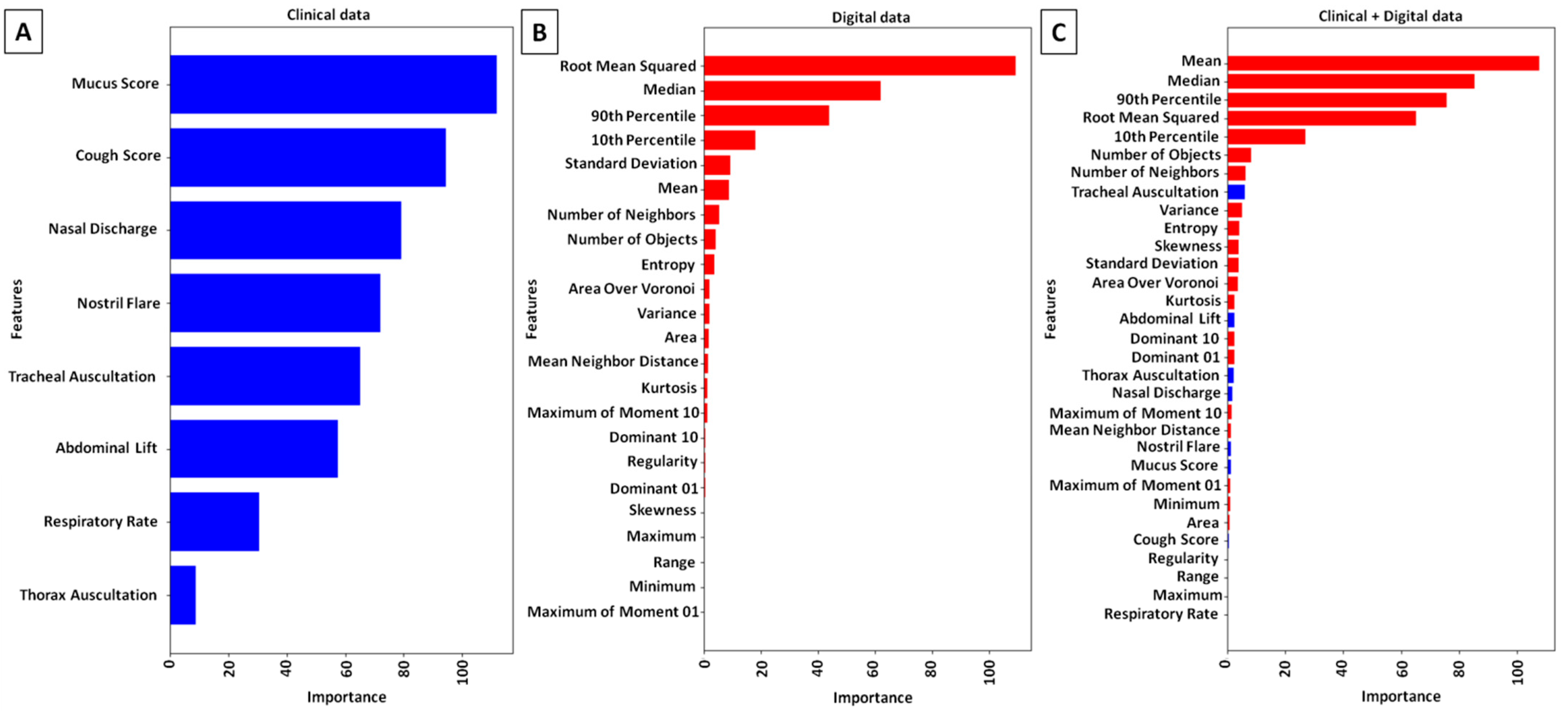
3.3. Computer-Aided Diagnosis of PLH
4. Discussion
Further Directions and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mukhtorov, D.; Rakhmonova, M.; Muksimova, S.; Cho, Y.-I. Endoscopic Image Classification Based on Explainable Deep Learning. Sensors 2023, 23, 3176. [Google Scholar] [CrossRef]
- Ahmad, J.; Muhammad, K.; Lee, M.Y.; Baik, S.W. Endoscopic Image Classification and Retrieval Using Clustered Convolutional Features. J. Med. Syst. 2017, 41, 196. [Google Scholar] [CrossRef]
- Yang, X.; Wei, Q.; Zhang, C.; Zhou, K.; Kong, L.; Jiang, W. Colon Polyp Detection and Segmentation Based on Improved MRCNN. IEEE Trans. Instrum. Meas. 2020, 70, 4501710. [Google Scholar] [CrossRef]
- Ragab, M.; Albukhari, A.; Alyami, J.; Mansour, R.F. Ensemble Deep-Learning-Enabled Clinical Decision Support System for Breast Cancer Diagnosis and Classification on Ultrasound Images. Biology 2022, 11, 439. [Google Scholar] [CrossRef]
- Ashwini, S.; Arunkumar, J.; Prabu, T.; Singh, N.; Singh, N. Diagnosis and Multi-Classification of Lung Diseases in CXR Images Using Optimized Deep Convolutional Neural Network. Soft Comput. 2024, 28, 6219–6233. [Google Scholar] [CrossRef]
- Ozsahin, I.; Sekeroglu, B.; Musa, M.S.; Mustapha, M.T.; Uzun Ozsahin, D. Review on Diagnosis of COVID-19 from Chest CT Images Using Artificial Intelligence. Comput. Math. Methods Med. 2020, 2020, 9756518. [Google Scholar] [CrossRef] [PubMed]
- Cheng, D.; Liu, M.; Fu, J.; Wang, Y. Classification of MR brain images by combination of multi-CNNs for AD diagnosis. In Proceedings of the 9th International Conference on Digital Image Processing (ICDIP 2017), Hong Kong, China, 19–22 May 2017; Volume 10420, p. 1042042. [Google Scholar]
- Han, Y.; Ma, Y.; Wu, Z.; Zhang, F.; Zheng, D.; Liu, X.; Tao, L.; Liang, Z.; Yang, Z.; Li, X.; et al. Histologic Subtype Classification of Non-Small Cell Lung Cancer Using PET/CT Images. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 350–360. [Google Scholar] [CrossRef]
- Kebir, S.; Weber, M.; Lazaridis, L.; Deuschl, C.; Schmidt, T.; Mönninghoff, C.; Keyvani, K.; Umutlu, L.; Pierscianek, D.; Forsting, M.; et al. Hybrid 11C-MET PET/MRI Combined with “Machine Learning” in Glioma Diagnosis According to the Revised Glioma WHO Classification 2016. Clin. Nucl. Med. 2019, 44, 214–220. [Google Scholar] [CrossRef]
- Banik, D.; Roy, K.; Bhattacharjee, D.; Nasipuri, M.; Krejcar, O. Polyp-Net: A Multi-Model Fusion Network for Polyp Segmentation. IEEE Trans. Instrum. Meas. 2020, 70, 4000512. [Google Scholar] [CrossRef]
- Gaur, P.; Gupta, H.; Chowdhury, A.; Mc Creadie, K.; Pachori, R.B.; Wang, H. A Sliding Window Common Spatial Pattern for Enhancing Motor Imagery Classification in EEG-BCI. IEEE Trans. Instrum. Meas. 2021, 70, 4002709. [Google Scholar] [CrossRef]
- Yue, G.; Han, W.; Jiang, B.; Zhou, T.; Cong, R.; Wang, T. Boundary Constraint Network with Cross Layer Feature Integration for Polyp Segmentation. IEEE J. Biomed. Health Inform. 2022, 26, 4090–4099. [Google Scholar] [CrossRef]
- Yue, G.; Wei, P.; Liu, Y.; Luo, Y.; Du, J.; Wang, T. Automated Endoscopic Image Classification via Deep Neural Network with Class Imbalance Loss. IEEE Trans. Instrum. Meas. 2023, 72, 5010611. [Google Scholar] [CrossRef]
- Guo, L.; Yu, X.; Thair, A.; Rideout, A.; Collins, A.; Wang, Z.J.; Hore, M. Deep Learning Model Shows Promise for Detecting and Grading Sesamoiditis in Horse Radiographs. Am. J. Vet. Res. 2024, 85, ajvr.23.07.0173. [Google Scholar] [CrossRef]
- Turek, B.; Pawlikowski, M.; Jankowski, K.; Borowska, M.; Skierbiszewska, K.; Jasiński, T.; Domino, M. Selection of Density Standard and X-Ray Tube Settings for Computed Digital Absorptiometry in Horses Using the k-Means Clustering Algorithm. BMC Vet. Res. 2025, 21, 165. [Google Scholar] [CrossRef]
- Borowska, M. Wprowadzenie do Zastosowania Entropii w Analizie Sygnałów i Obrazów Biomedycznych Oraz Jej Aplikacje w Medycynie i Weterynarii; Oficyna Wydawnicza Politechniki Białostockiej: Białystok, Poland, 2023; pp. 1–284. [Google Scholar]
- Aszteborski, H.; Koneck, K.; Jasiński, T.; Borowska, M.; Lipowicz, P.; Turek, B.; Domino, M. AI-Driven Automatic 3D Segmentation of Equine Maxillary Sinuses—Integrating Convolutional Neural Networks in Veterinary Diagnostic Imaging. In Proceedings of the European Veterinary Diagnostic Imaging Conference (EVDI), Barcelona, Spain, 17–20 September 2025. [Google Scholar]
- Bakıcı, C.; Batur, B.; Akgün, R.; Kaya, U.; Ekim, O.; Oto, Ç.; Hazıroğlu, R. Computer-Aided Three Dimensional Morphometric Measurements of Cervical Vertebrae Variations Compared with Manual Measurements in Throughbred Horses. Eurasian J. Vet. Sci. 2022, 38, 41–49. [Google Scholar] [CrossRef]
- Borowska, M.; Lipowicz, P.; Daunoravičienė, K.; Turek, B.; Jasiński, T.; Pauk, J.; Domino, M. Three-Dimensional Segmentation of Equine Paranasal Sinuses in Multidetector Computed Tomography Datasets: Preliminary Morphometric Assessment Assisted with Clustering Analysis. Sensors 2024, 24, 3538. [Google Scholar] [CrossRef]
- Raker, C.W.; Boles, C.L. Pharyngeal Lymphoid Hyperplasia in the Horse. J. Equine Med. Surg. 1978, 2, 202–207. [Google Scholar]
- Holcombe, S.; Ducharme, N. Disorders of the Nasopharynx and Soft Palate. In Equine Respiratory Medicine and Surgery, 1st ed.; McGorum, B., Dixon, P., Robinson, E., Schumacher, J., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2007; pp. 437–457. [Google Scholar]
- Domino, M.; Borowska, M.; Kozłowska, N.; Trojakowska, A.; Zdrojkowski, Ł.; Jasiński, T.; Smyth, G.; Maśko, M. Selection of Image Texture Analysis and Color Model in the Advanced Image Processing of Thermal Images of Horses Following Exercise. Animals 2022, 12, 444. [Google Scholar] [CrossRef]
- Górski, K.; Borowska, M.; Stefanik, E.; Polkowska, I.; Turek, B.; Bereznowski, A.; Domino, M. Selection of Filtering and Image Texture Analysis in the Radiographic Images Processing of Horses’ Incisor Teeth Affected by the EOTRH Syndrome. Sensors 2022, 22, 2920. [Google Scholar] [CrossRef]
- Toutain, M.; Lezoray, O.; Audigié, F.; Busoni, V.; Rossi, G.; Parillo, F.; Elmoataz, A. Analysis of Whole Slide Images of Equine Tendinopathy. In Proceedings of the ICIAR (International Conference on Image Analysis and Recognition), Aveiro, Portugal, 25–27 June 2012; Volume 7325, pp. 440–447. [Google Scholar]
- Mair, T.S.; Batten, E.H.; Stokes, C.R.; Bourne, F.J. The Histological Features of the Immune System of the Equine Respiratory Tract. J. Comp. Pathol. 1987, 97, 575–586. [Google Scholar] [CrossRef]
- McAllister, E.S.; Blakeslee, J.R. Clinical Observations of Pharyngitis in the Horse. J. Am. Vet. Med. Assoc. 1977, 170, 739–741. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Jackson, C.; Gerber, V.; Jefcoat, A.; Berney, C.; Eberhardt, S.; Robinson, N.E. Stabling Is Associated with Airway Inflammation in Young Arabian Horses. Equine Vet. J. 2001, 33, 244–249. [Google Scholar] [CrossRef]
- Galan, J.E.; Timoney, J.F. Mucosal Nasopharyngeal Immune Responses of Horses to Protein Antigens of Streptococcus equi. Infect. Immun. 1985, 47, 623–628. [Google Scholar] [CrossRef]
- Breathnach, C.C.; Yeargan, M.R.; Sheoran, A.S.; Allen, G.P. The Mucosal Humoral Immune Response of the Horse to Infective Challenge and Vaccination with Equine Herpesvirus-1 Antigens. Equine Vet. J. 2001, 33, 651–657. [Google Scholar] [CrossRef]
- Ahern, T.J. Tonsillitis and Tonsillar Hypertrophy Predisposing to Pharyngeal Dysfunction in the Horse. J. Equine Vet. Sci. 1997, 17, 232–234. [Google Scholar] [CrossRef]
- Holcombe, S.J. Epidemiology of Airway Inflammation and Mucus in Horses; AAEP Proceedings; American Association of Equine Practitioners: Lexington, KY, USA, 2005; Volume 51. [Google Scholar]
- Sweeney, C.R.; Maxson, A.D.; Soma, L.R. Endoscopic Findings in the Upper Respiratory Tract of 678 Thoroughbred Racehorses. J. Am. Vet. Med. Assoc. 1991, 198, 1037–1038. [Google Scholar] [CrossRef] [PubMed]
- Gajarlwar, O.S.; Suryawanshi, R.V.; Ulemale, A.H.; Rangnekar, M.N.; Khambatta, P. Prevalence of Upper Respiratory Tract Diseases in Thoroughbred Racehorses. Haryana Vet. 2020, 59, 14–19. [Google Scholar]
- King, D.S.; Tulleners, E.; Martin, B.B.; Parente, E.J.; Boston, R. Clinical Experiences with Axial Deviation of the Aryepiglottic Folds in 52 Racehorses. Vet. Surg. 2001, 30, 151–160. [Google Scholar] [CrossRef]
- Courouce-Malblanc, A.; Deniau, V.; Rossignol, F.; Corde, R.; Leleu, C.; Maillard, K.; Pitel, P.-H.; Pronost, S.; Fortier, G. Physiological Measurements and Prevalence of Lower Airway Diseases in Trotters with Dorsal Displacement of the Soft Palate. Equine Vet. J. 2010, 42, 246–255. [Google Scholar] [CrossRef] [PubMed]
- Kaiseler, P.H.; Dzyekanski, B.; Schiefelbein, R.; Silveira, R.G.; Pimpão, C.T.; Michelotto, P.V., Jr. Upper Airway Evaluations of Thoroughbred Racehorses in a Private Clinic in Curitiba, Brazil—Resitng Endoscopic Findings in 587 Horses. Arch. Vet. Sci. 2012, 17, 1–9. [Google Scholar] [CrossRef]
- Kozłowska, N.; Wierzbicka, M.; Jasiński, T.; Domino, M. Co-Occurrence of Equine Asthma and Pharyngeal Lymphoid Hyperplasia in Pleasure Horses. Agriculture 2024, 14, 1157. [Google Scholar] [CrossRef]
- Wilkins, P.A. Lower Airway Diseases of the Adult Horse. Vet. Clin. Equine Pract. 2003, 19, 101–121. [Google Scholar] [CrossRef]
- Grossman, J. One Airway, One Disease. Chest 1997, 111, 11S–16S. [Google Scholar] [CrossRef]
- Giovannini-Chami, L.; Paquet, A.; Sanfiorenzo, C.; Pons, N.; Cazareth, J.; Magnone, V.; Lebrigand, K.; Chevalier, B.; Vallauri, A.; Julia, V.; et al. The “One Airway, One Disease” Concept in Light of Th2 Inflammation. Eur. Respir. J. 2018, 52, 1800437. [Google Scholar] [CrossRef]
- Holcombe, S. Upper Airway Function of Normal Horses during Exercise. In Equine Exercise Physiology: The Science of Exercise in the Athletic Horse, 1st ed.; Hinchcliff, K.W., Geor, R.J., Kaneps, A.J., Eds.; Elsevier: Saunder, UK; St. Louis, MO, USA, 2008; pp. 170–192. [Google Scholar]
- Auer, D.E.; Wilson, R.G.; Groenendyk, S. Pharyngeal Lymphoid Hyperplasia in Thoroughbred Racehorses in Training. Aust. Vet. J. 1985, 62, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.F.; Madelin, T.M.; Allpress, R.G. The Relationship of Air Hygiene in Stables to Lower Airway Disease and Pharyngeal Lymphoid Hyperplasia in Two Groups of Thoroughbred Horses. Equine Vet. J. 1987, 19, 524–530. [Google Scholar] [CrossRef]
- Holcombe, S.J.; Robinson, N.E.; Derksen, F.J.; Bertold, B.; Genovese, R.; Miller, R.; de Feiter Rupp, H.; Carr, E.A.; Eberhart, S.W.; Boruta, D.; et al. Effect of Tracheal Mucus and Tracheal Cytology on Racing Performance in Thoroughbred Racehorses. Equine Vet. J. 2006, 38, 300–304. [Google Scholar] [CrossRef]
- Saulez, M.N.; Gummow, B. Prevalence of Pharyngeal, Laryngeal and Tracheal Disorders in Thoroughbred Racehorses, and Effect on Performance. Vet. Rec. 2009, 165, 431–435. [Google Scholar] [CrossRef]
- Bagshaw, J.; Sanz, M.; Wang, Y.; Shoemaker, S.; Bayly, W. Severity and Effects of Pharyngeal Lymphoid Hyperplasia Vary with Age and Racetrack Location in Thoroughbred Racehorses. Comp. Exerc. Physiol. 2023, 19, 449–460. [Google Scholar] [CrossRef]
- Kozłowska, N.; Wierzbicka, M.; Pawliński, B.; Domino, M. Co-Occurrence of Severe Equine Asthma and Palatal Disorders in Privately Owned Pleasure Horses. Animals 2023, 13, 1962. [Google Scholar] [CrossRef]
- Fjeldborg, J.; Lindegaard, C.; van Erck-Westergren, E.; Strand, E.; Hackett, E. Endoscopic Examination of the Respiratory Tract. In Equine Respiratory Endoscopy; CABI Books: Wallingford, UK, 2024; pp. 35–62. [Google Scholar]
- Hunter, R.S. Photoelectric Color Difference Meter. J. Opt. Soc. Am. 1958, 48, 985–995. [Google Scholar] [CrossRef]
- Zuiderveld, K. Contrast Limited Adaptive Histogram Equalization. In Graphics Gems IV; Academic Press Professional, Inc.: Cambridge, MA, USA, 1994; pp. 474–485. [Google Scholar]
- Dobrin, A. A Review of Properties and Variations of Voroni Diagrams; Whitman College: Walla Walla, WA, USA, 2005; pp. 1–43. [Google Scholar]
- Huang, Z.; Huang, G.; Cheng, L. Medical Image Segmentation of Blood Vessels Based on Clifford Algebra and Voronoi Diagram. J. Softw. 2018, 13, 360–373. [Google Scholar] [CrossRef]
- Sudbø, J.; Marcelpoil, R.; Reith, A. New Algorithms Based on the Voronoi Diagram Applied in a Pilot Study on Normal Mucosa and Carcinomas. Anal. Cell. Pathol. 2000, 21, 389361. [Google Scholar] [CrossRef]
- Lau, C.; Kalantari, B.; Batts, K.P.; Ferrell, L.D.; Nyberg, S.L.; Graham, R.P.; Moreira, R.K. The Voronoi Theory of the Normal Liver Lobular Architecture and Its Applicability in Hepatic Zonation. Sci. Rep. 2021, 11, 9343. [Google Scholar] [CrossRef]
- Lin, W.; Gao, Q.; Du, M.; Chen, W.; Tong, T. Multiclass Diagnosis of Stages of Alzheimer’s Disease Using Linear Discriminant Analysis Scoring for Multimodal Data. Comput. Biol. Med. 2021, 134, 104478. [Google Scholar] [CrossRef]
- Adebiyi, M.O.; Arowolo, M.O.; Mshelia, M.D.; Olugbara, O.O. A Linear Discriminant Analysis and Classification Model for Breast Cancer Diagnosis. Appl. Sci. 2022, 12, 11455. [Google Scholar] [CrossRef]
- Rezaei, Z. A Review on Image-Based Approaches for Breast Cancer Detection, Segmentation, and Classification. Expert Syst. Appl. 2021, 182, 115204. [Google Scholar] [CrossRef]
- Bechelli, S.; Delhommelle, J. Machine Learning and Deep Learning Algorithms for Skin Cancer Classification from Dermoscopic Images. Bioengineering 2022, 9, 97. [Google Scholar] [CrossRef] [PubMed]
- Pedregosa, F.; Varoquaux, G.; Gramfort, A.; Michel, V.; Thirion, B.; Grisel, O.; Blondel, M.; Prettenhofer, P.; Weiss, R.; Dubourg, V.; et al. Scikit-Learn: Machine Learning in Python. J. Mach. Learn. Res. 2011, 12, 2825–2830. [Google Scholar]
- Babenko, V.; Nastenko, I.; Pavlov, V.; Horodetska, O.; Dykan, I.; Tarasyuk, B.; Lazoryshinets, V. Classification of Pathologies on Medical Images Using the Algorithm of Random Forest of Optimal-Complexity Trees. Cybern. Syst. Anal. 2023, 59, 346–358. [Google Scholar] [CrossRef]
- Jasti, V.D.P.; Zamani, A.S.; Arumugam, K.; Naved, M.; Pallathadka, H.; Sammy, F.; Raghuvanshi, A.; Kaliyaperumal, K. Computational Technique Based on Machine Learning and Image Processing for Medical Image Analysis of Breast Cancer Diagnosis. Secur. Commun. Netw. 2022, 2022, 1918379. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Wichtel, M.; Gomez, D.; Burton, S.; Wichtel, J.; Hoffman, A. Relationships between Equine Airway Reactivity Measured by Flowmetric Plethysmography and Specific Indicators of Airway Inflammation in Horses with Suspected Inflammatory Airway Disease. Equine Vet. J. 2016, 48, 466–471. [Google Scholar] [CrossRef]
- Robinson, N.E.; Berney, C.; Behan, A.; Derksen, F.J. Fluticasone Propionate Aerosol Is More Effective for Prevention than Treatment of Recurrent Airway Obstruction. J. Vet. Intern. Med. 2009, 23, 1247–1253. [Google Scholar] [CrossRef]
- Couetil, L.L.; Rosenthal, F.S.; DeNicola, D.B.; Chilcoat, C.D. Clinical Signs, Evaluation of Bronchoalveolar Lavage Fluid, and Assessment of Pulmonary Function in Horses with Inflammatory Respiratory Disease. Am. J. Vet. Res. 2001, 62, 538–546. [Google Scholar] [CrossRef]
- Robinson, N.E.; Karmaus, W.; Holcombe, S.J.; Carr, E.A.; Derksen, F.J. Airway Inflammation in Michigan Pleasure Horses: Prevalence and Risk Factors. Equine Vet. J. 2006, 38, 293–299. [Google Scholar] [CrossRef]
- Couëtil, L.L.; Hoffman, A.M.; Hodgson, J.; Buechner-Maxwell, V.; Viel, L.; Wood, J.L.N.; Lavoie, J.-P. Inflammatory Airway Disease of Horses. J. Vet. Intern. Med. 2007, 21, 356–361. [Google Scholar] [CrossRef]
- Kannegieter, N.J.; Dore, M.L. Endoscopy of the Upper Respiratory Tract during Treadmill Exercise: A Clinical Study of 100 Horses. Aust. Vet. J. 1995, 72, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Jia, X.; Meng, M.-H.Q. Bleeding Detection in Wireless Capsule Endoscopy Image Video Using Superpixel-Color Histogram and a Subspace KNN Classifier. In Proceedings of the 1st International Conference on Advances in Science, Engineering and Robotics Technology 2019, Dhaka, Bangladesh, 3–5 May 2019; Institute of Electrical and Electronics Engineers Inc.: New York, NY, USA, 2019. [Google Scholar]
- Bębas, E.; Pauk, K.; Pauk, J.; Daunoravičienė, K.; Mojsak, M.; Hładuński, M.; Domino, M.; Borowska, M. Application of Fractal Radiomics and Machine Learning for Differentiation of Non-Small Cell Lung Cancer Subtypes on PET/MR Images. J. Clin. Med. 2025, 14, 5776. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Meng, M.Q.-H. Tumor Recognition in Wireless Capsule Endoscopy Images Using Textural Features and SVM-Based Feature Selection. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Bębas, E.; Borowska, M.; Derlatka, M.; Oczeretko, E.; Hładuński, M.; Szumowski, P.; Mojsak, M. Machine-Learning-Based Classification of the Histological Subtype of Non-Small-Cell Lung Cancer Using MRI Texture Analysis. Biomed. Signal Process. Control 2021, 66, 102446. [Google Scholar] [CrossRef]
- Hossain, M.R.I.; Ahmed, I.; Kabir, M. Automatic Lung Tumor Detection Based on GLCM Features. In Computer Vision, Proceedings of the ACCV 2014 Workshops, Singapore, 1–2 November 2014; Jawahar, C.V., Shan, S., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 109–121. [Google Scholar]
- Adi, K.; Widodo, C.E.; Widodo, A.P.; Gernowo, R.; Pamungkas, A.; Syifa, R.A. Detection Lung Cancer Using Gray Level Co-Occurrence Matrix (GLCM) and Back Propagation Neural Network Classification. J. Eng. Sci. Technol. Rev. 2018, 11, 8–12. [Google Scholar] [CrossRef]
- Domino, M.; Borowska, M.; Zdrojkowski, Ł.; Jasiński, T.; Sikorska, U.; Skibniewski, M.; Maśko, M. Application of the Two-Dimensional Entropy Measures in the Infrared Thermography-Based Detection of Rider: Horse Bodyweight Ratio in Horseback Riding. Sensors 2022, 22, 6052. [Google Scholar] [CrossRef] [PubMed]

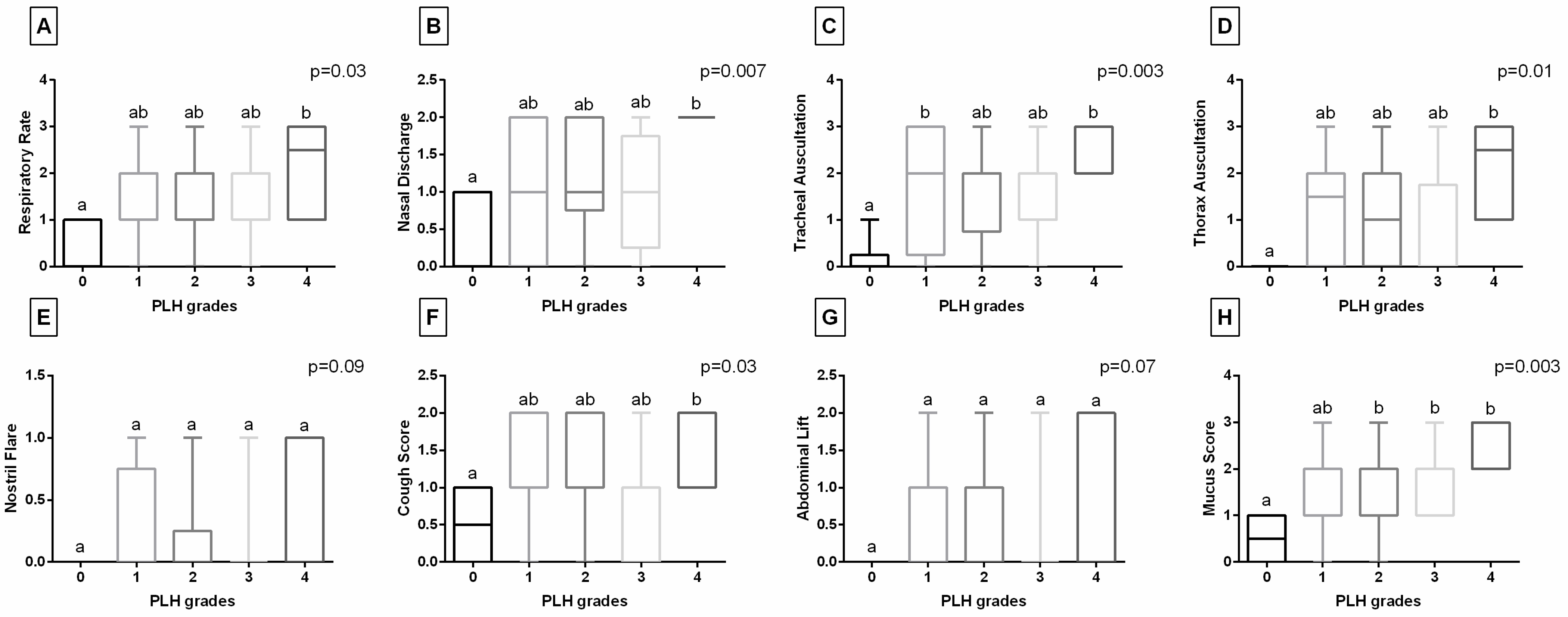
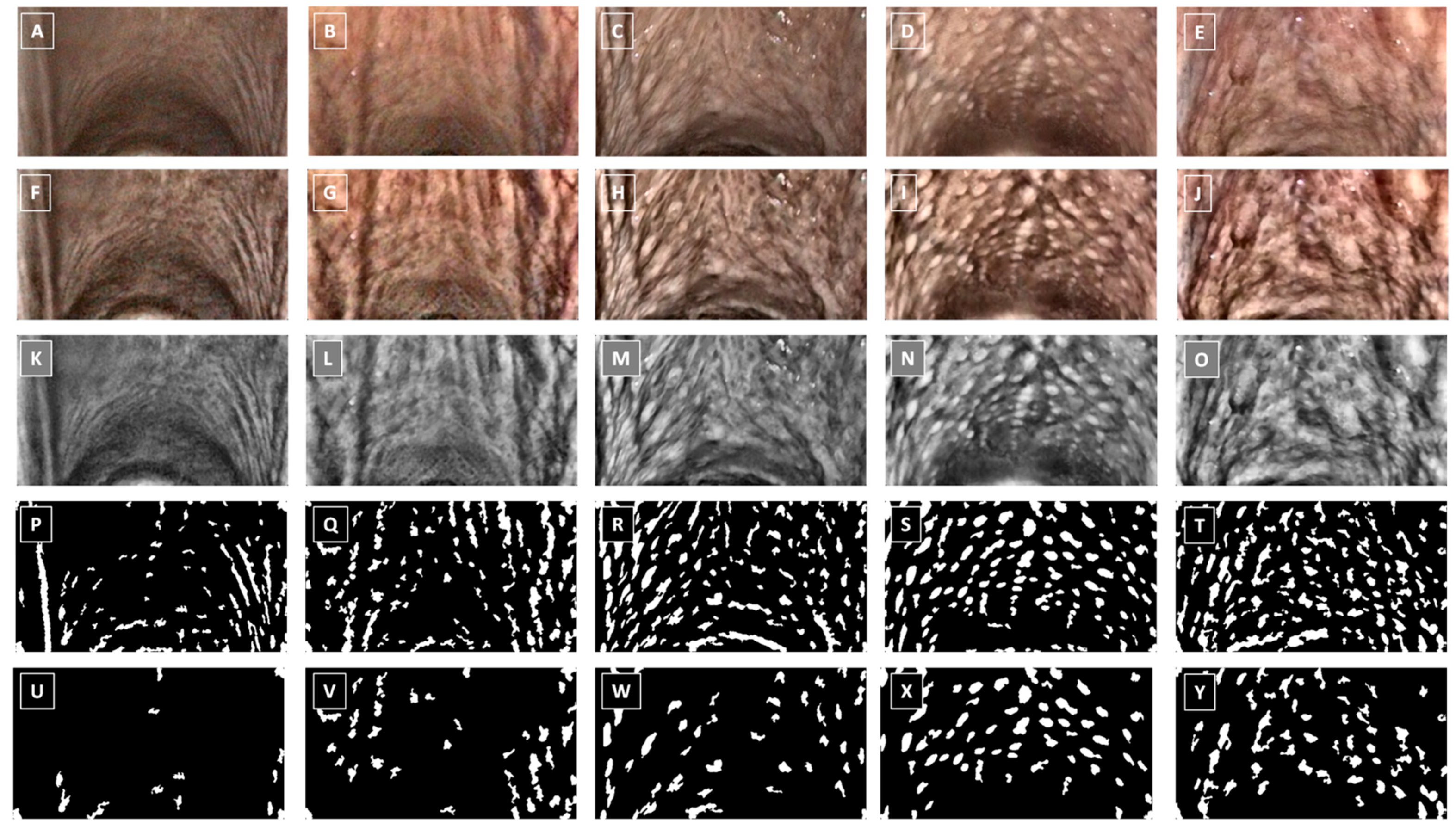
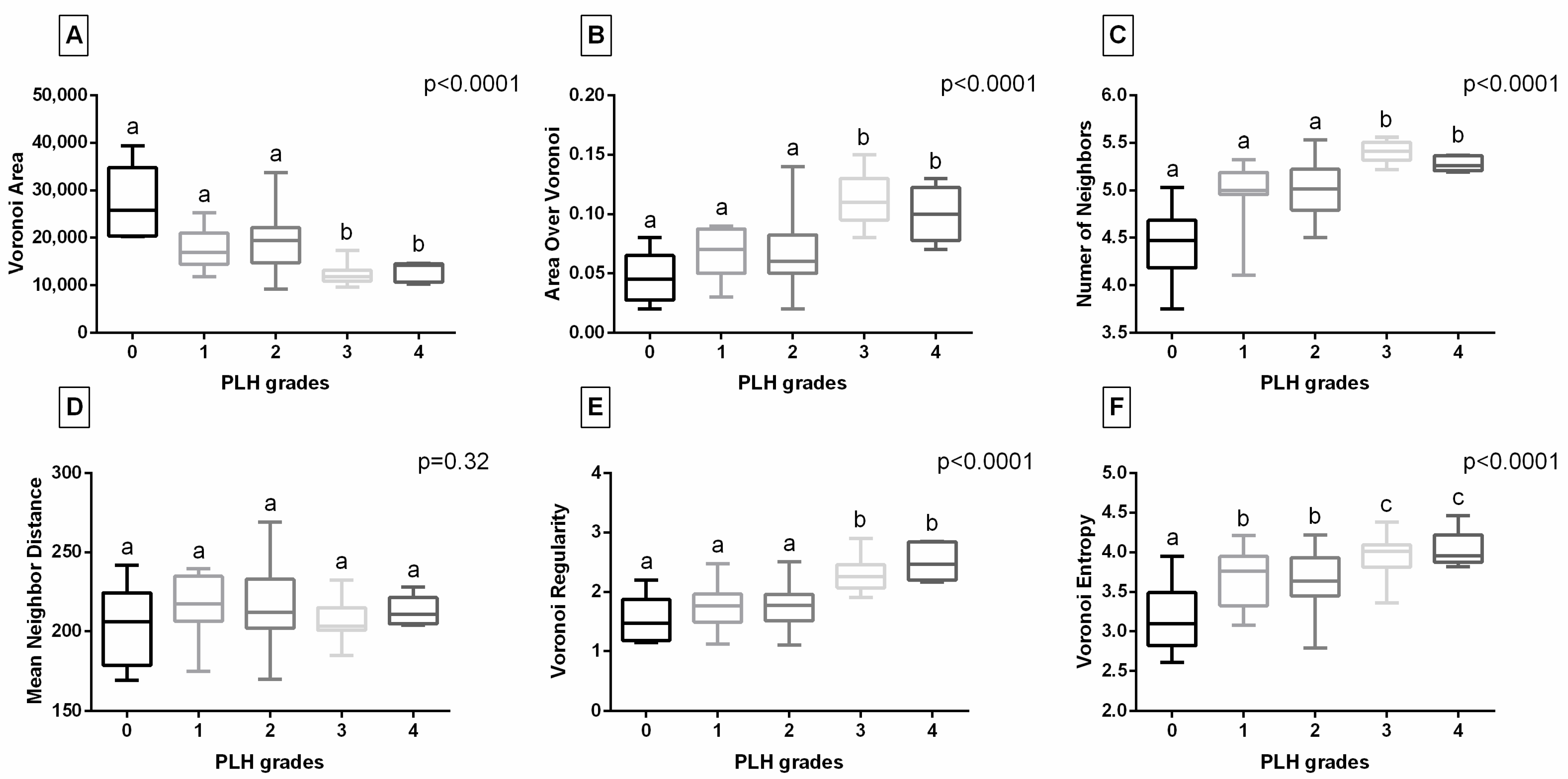
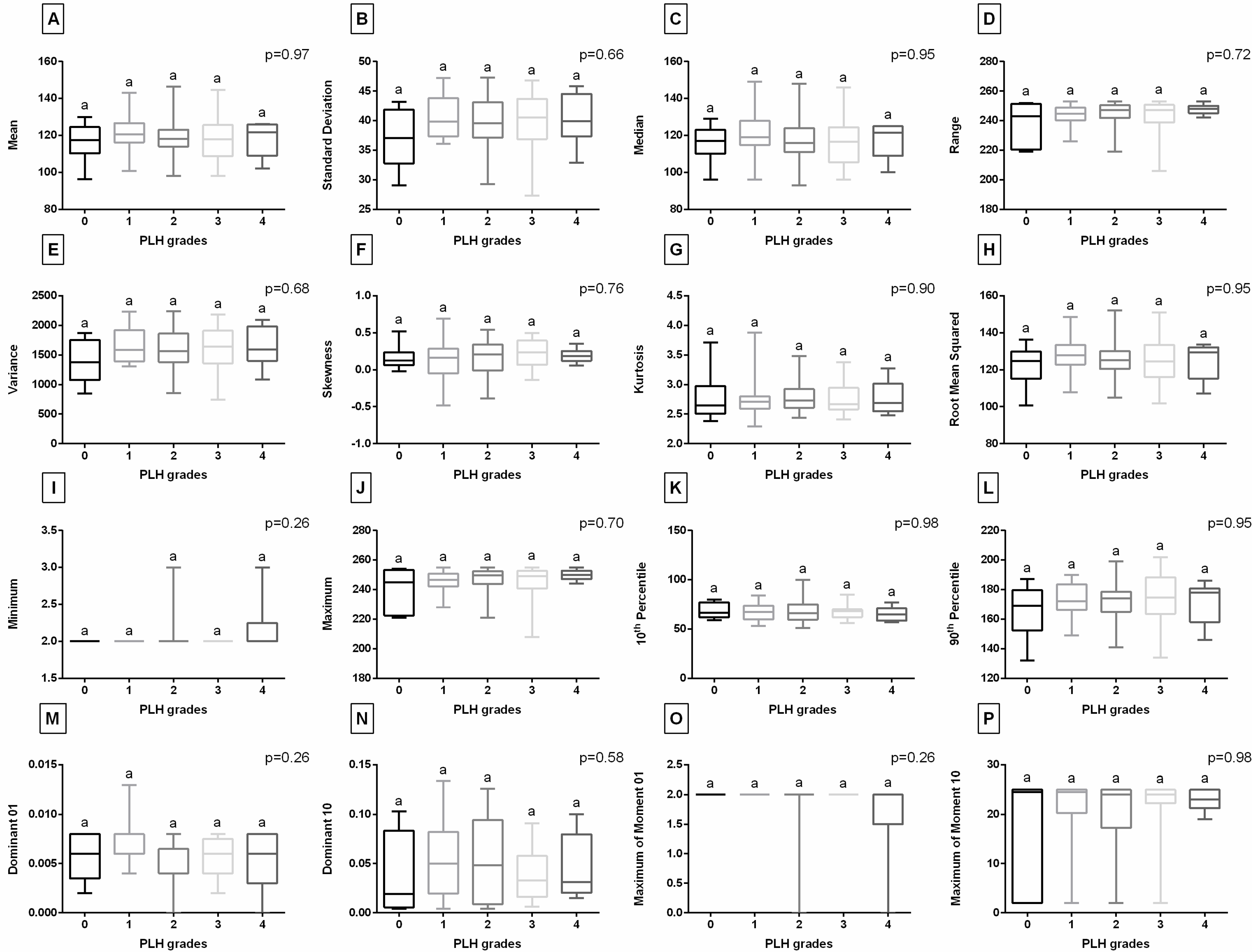

| Clinical Symptoms | Descriptor | Score |
|---|---|---|
| Respiratory Rate | <16 | 0 |
| 17–20 | 1 | |
| 21–30 | 2 | |
| >30 | 3 | |
| Nasal Discharge | none | 0 |
| serous | 1 | |
| mucopurulent/epistaxis | 2 | |
| Tracheal Auscultation | normal tracheal sounds | 0 |
| slight increase | 1 | |
| clearly audible increased | 2 | |
| crackles and wheezing present | 3 | |
| Thorax Auscultation | normal pulmonary sounds | 0 |
| slight increased pulmonary sounds | 1 | |
| clearly audible increased pulmonary sounds | 2 | |
| crackles and wheezing present | 3 | |
| Nostril Flare | none | 0 |
| present | 1 | |
| Cough Score | none | 0 |
| coughs at specific times of day (feeding/exercising/making beds) | 1 | |
| frequent cough | 2 | |
| Abdominal Lift | none | 0 |
| slight flattening of ventral flank | 1 | |
| obvious abdominal lift and “heave line” | 2 |
| PLH Grade | Endoscopic Signs of the Pharyngeal Mucosa |
|---|---|
| Grade 0 | No visible lymphoid tissue |
| Grade 1 | A few small white lymphoid follicles on the dorsal wall of the pharynx |
| Grade 2 | Numerous small lymphoid follicles on the dorsal and lateral walls of the pharynx |
| Grade 3 | Numerous large hyperemic lymphoid follicles on the entire dorsal and lateral walls of the pharynx |
| Grade 4 | Numerous large edematous hyperemic lymphoid follicles coalesce broad-based polypoid aggregate on the entire dorsal and lateral walls of the pharynx |
| Demographic Data | PLH Grades | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Sex | |||||
| Mare | 1 | 5 | 12 | 6 | 5 |
| Gelding | 2 | 9 | 14 | 10 | 1 |
| Stallion | 3 | 2 | 0 | 0 | 0 |
| In total | 6 | 16 | 26 | 16 | 6 |
| Age | |||||
| Median | 16 a years | 12 ab years | 12 b years | 10 b years | 9 b years |
| Range (Min; Max) | (13; 19) | (6; 24) | (6; 18) | (6; 16) | (6; 14) |
| p value | p = 0.009 | ||||
| Number of Objects | PLH Grades | ||||
|---|---|---|---|---|---|
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | |
| Median | 18.0 a | 33.0 a | 30.5 a | 52.5 b | 50.5 b |
| Range (Min; Max) | (12.0; 34.0) | (19.0; 44.0) | (15.0; 69.0) | (32.0; 75.0) | (37.0; 56.0) |
| p value | p < 0.0001 | ||||
| Classification Metrics | Clinical Data | Digital Data | Clinical + Digital Data |
|---|---|---|---|
| Accuracy | 0.43 | 0.73 | 0.76 |
| Precision | 0.44 | 0.70 | 0.83 |
| Recall | 0.44 | 0.69 | 0.78 |
| F1 | 0.41 | 0.65 | 0.76 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozłowska, N.; Borowska, M.; Jasiński, T.; Wierzbicka, M.; Domino, M. Computer-Aided Diagnosis of Equine Pharyngeal Lymphoid Hyperplasia Using the Object Detection-Based Processing Technique of Digital Endoscopic Images. Animals 2025, 15, 2758. https://doi.org/10.3390/ani15182758
Kozłowska N, Borowska M, Jasiński T, Wierzbicka M, Domino M. Computer-Aided Diagnosis of Equine Pharyngeal Lymphoid Hyperplasia Using the Object Detection-Based Processing Technique of Digital Endoscopic Images. Animals. 2025; 15(18):2758. https://doi.org/10.3390/ani15182758
Chicago/Turabian StyleKozłowska, Natalia, Marta Borowska, Tomasz Jasiński, Małgorzata Wierzbicka, and Małgorzata Domino. 2025. "Computer-Aided Diagnosis of Equine Pharyngeal Lymphoid Hyperplasia Using the Object Detection-Based Processing Technique of Digital Endoscopic Images" Animals 15, no. 18: 2758. https://doi.org/10.3390/ani15182758
APA StyleKozłowska, N., Borowska, M., Jasiński, T., Wierzbicka, M., & Domino, M. (2025). Computer-Aided Diagnosis of Equine Pharyngeal Lymphoid Hyperplasia Using the Object Detection-Based Processing Technique of Digital Endoscopic Images. Animals, 15(18), 2758. https://doi.org/10.3390/ani15182758





