Molecular and Serological Detection of Leishmania spp. in Mediterranean Wild Carnivores and Feral Cats: Implications for Wildlife Health and One Health Surveillance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
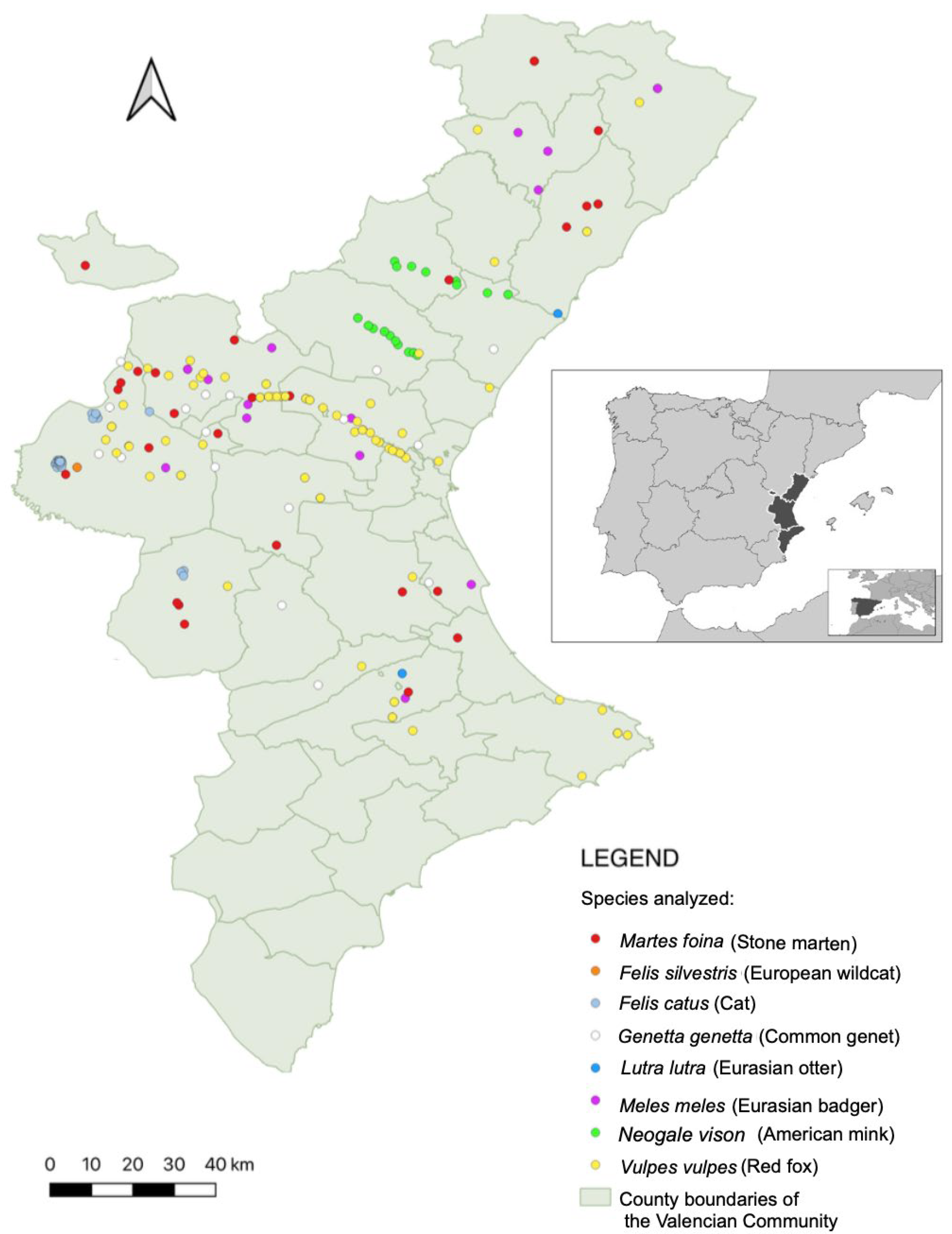
2.1. Study Area
2.2. Sample Collection and Passive Surveillance Strategy
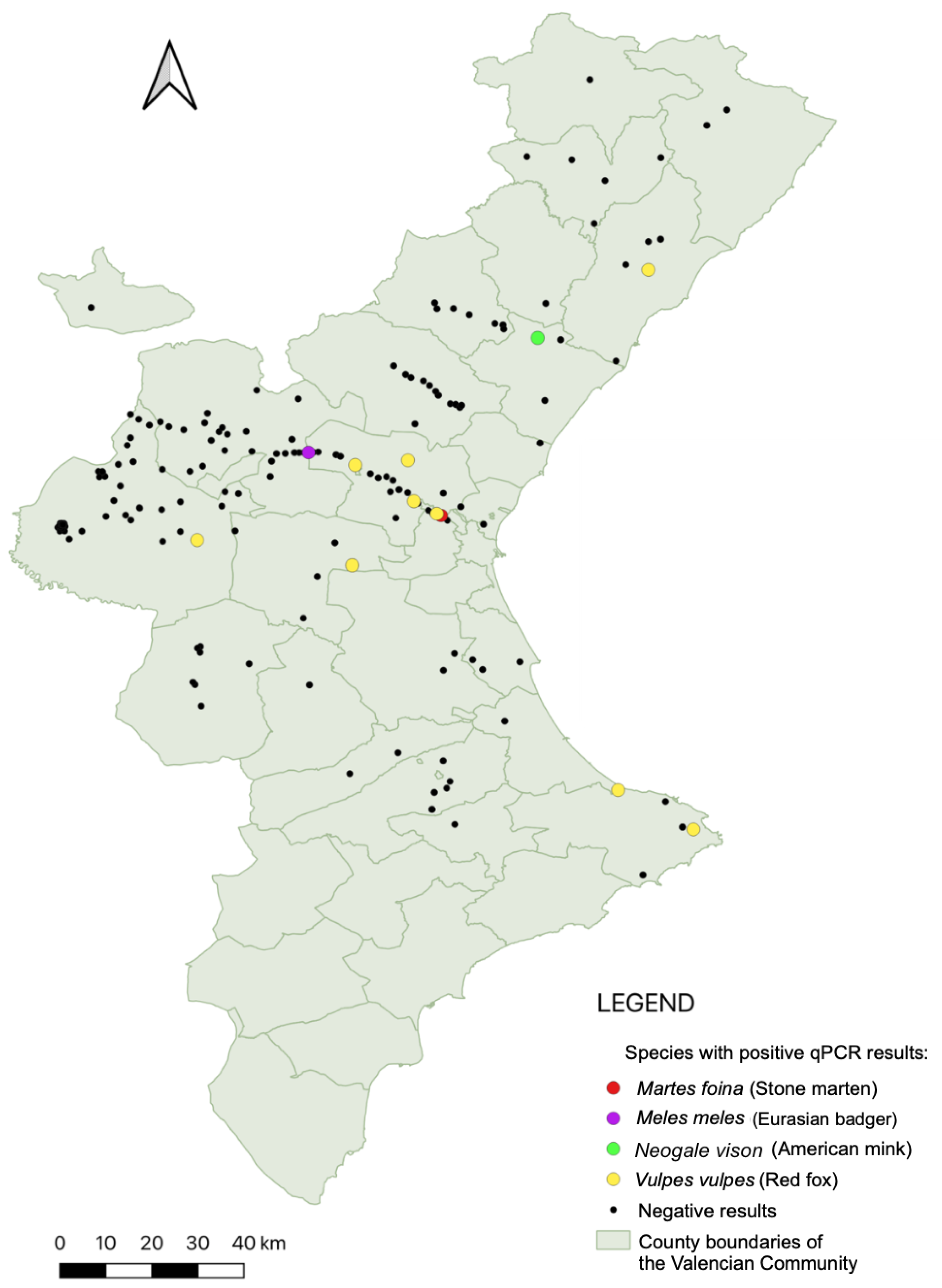
2.3. Molecular Detection of Leishmania spp.
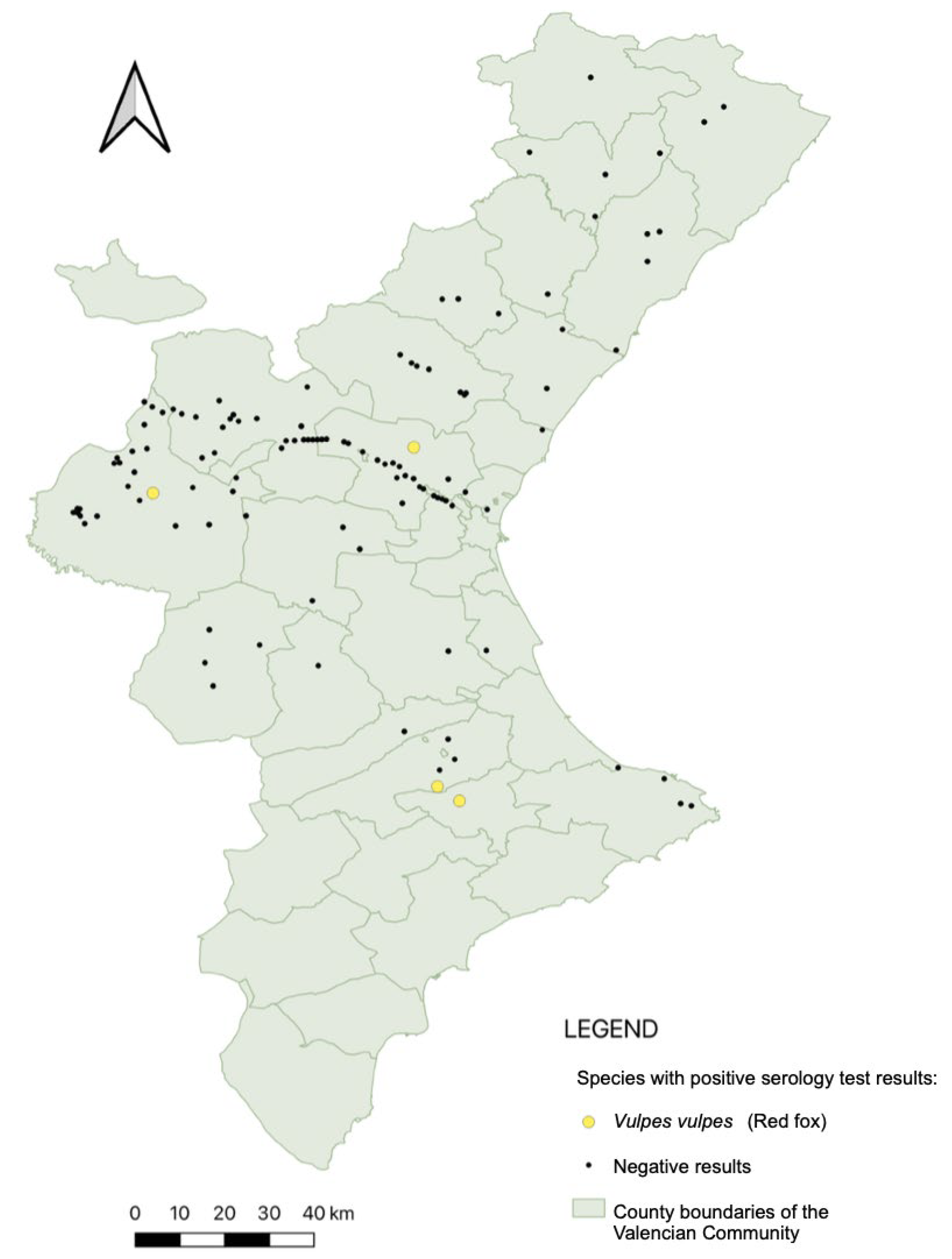
2.4. Serological Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| qPCR | Quantitative Polymerase Chain Reaction |
| Ct | Cycle threshold |
| Cq | quantification Cycle |
| ELISA | Enzyme-Linked Immunosorbent Assay |
References
- Ortuño, M.; Latrofa, M.S.; Iborra, M.A.; Pérez-Cutillas, P.; Bernal, L.J.; Risueño, J.; Muñoz, C.; Bernal, A.; Sánchez-Lopez, P.F.; Segovia, M.; et al. Genetic diversity and phylogenetic relationships between Leishmania infantum from dogs, humans and wildlife in south-east Spain. Zoonoses Public Health 2019, 66, 961–973. [Google Scholar] [CrossRef]
- Palatnik-de-Sousa, C.B.; Day, M.J. One Health: The global challenge of epidemic and endemic leishmaniasis. Parasites Vectors 2011, 4, 197. [Google Scholar] [CrossRef]
- World Health Organization. Leishmaniasis: Background Information. Available online: http://www.who.int/leishmaniasis/en/ (accessed on 25 March 2025).
- Bourdeau, P.; Saridomichelakis, M.N.; Oliveira, A.; Oliva, G.; Kotnik, T.; Gálvez, R.; Foglia Manzillo, V.; Koutinas, A.F.; Pereira da Fonseca, I.; Miró, G. Management of canine leishmaniosis in endemic SW European regions: A questionnaire-based multinational survey. Parasites Vectors 2014, 7, 110. [Google Scholar] [CrossRef]
- Risueño, J.; Ortuño, M.; Pérez-Cutillas, P.; Goyena, E.; Maia, C.; Cortes, S.; Campino, L.; Bernal, L.J.; Muñoz, C.; Arcenillas, I.; et al. Epidemiological and genetic studies suggest a common Leishmania infantum transmission cycle in wildlife, dogs and humans associated to vector abundance in Southeast Spain. Vet. Parasitol. 2018, 259, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Torres, M.; López, M.C.; Tasker, S.; Lappin, M.R.; Blasi-Brugué, C.; Roura, X. Review and statistical analysis of clinical management of feline leishmaniosis caused by Leishmania infantum. Parasites Vectors 2022, 15, 253–256. [Google Scholar] [CrossRef]
- Beck, A.; Beck, R.; Kusak, J.; Gudan, A.; Martinković, F.; Artuković, B.; Hohšteter, M.; Huber, Đ.; Marinculić, A.; Grabarević, Ž. A Case of Visceral Leishmaniosis in a Gray Wolf (Canis lupus) from Croatia. J. Wildl. Dis. 2008, 44, 451–456. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, L.; Schallig, H.; Persichetti, M.F.; Pennisi, M.G. New Epidemiological Aspects of Animal Leishmaniosis in Europe: The Role of Vertebrate Hosts Other Than Dogs. Pathogens 2021, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Tsakmakidis, I.; Lefkaditis, M.; Zaralis, K.; Arsenos, G. Alternative hosts of Leishmania infantum: A neglected parasite in Europe. Trop. Anim. Health Prod. 2024, 56, 128. [Google Scholar] [CrossRef]
- Ashford, R.W.; Bettini, S. Ecology and Epidemiology: Old World; Academic Press: London, UK, 1987. [Google Scholar]
- Duscher, G.G.; Fuehrer, H.; Kübber-Heiss, A. Fox on the run—Molecular surveillance of fox blood and tissue for the occurrence of tick-borne pathogens in Austria. Parasites Vectors 2014, 7, 521. [Google Scholar] [CrossRef][Green Version]
- Lima, C.M.; Santarém, N.; Neves, N.C.; Sarmento, P.; Carrapato, C.; de Sousa, R.; Cardoso, L.; Cordeiro-da-Silva, A. Serological and Molecular Survey of Leishmania infantum in a Population of Iberian Lynxes (Lynx pardinus). Microorganisms 2022, 10, 2447. [Google Scholar] [CrossRef]
- Kilpatrick, A.M.; Randolph, S.E. Drivers, dynamics, and control of emerging vector-borne zoonotic diseases. Lancet 2012, 380, 1946–1955. [Google Scholar] [CrossRef]
- Del Río, L.; Chitimia, L.; Cubas, A.; Victoriano, I.; De la Rúa, P.; Gerrikagoitia, X.; Barral, M.; Muñoz-García, C.I.; Goyena, E.; García-Martínez, D.; et al. Evidence for widespread Leishmania infantum infection among wild carnivores in L. infantum periendemic northern Spain. Prev. Vet. Med. 2014, 113, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Sobrino, J.A. Surface urban heat island analysis based on local climate zones using ECOSTRESS and Landsat data: A case study of Valencia city (Spain). Int. J. Appl. Earth Obs. Geoinf. 2024, 130, 103875. [Google Scholar] [CrossRef]
- Talbi, F.Z.; Najy, M.; Fadil, M.; Nouayti, N.; Lalami, A.E.O. Diversity and Seasonal Occurrence of Sand Flies and the Impact of Climatic Change in Aichoune Locality, Central Morocco. Lect. Notes Netw. Syst. 2021, 183, 583–593. [Google Scholar] [CrossRef]
- Díaz-Sáez, V.; Corpas-López, V.; Merino-Espinosa, G.; Morillas-Mancilla, M.; Abattouy, N.; Martín-Sánchez, J. Seasonal dynamics of phlebotomine sand flies and autochthonous transmission of Leishmania infantum in high-altitude ecosystems in southern Spain. Acta Trop. 2021, 213, 105749. [Google Scholar] [CrossRef]
- Gálvez, R.; Miró, G.; Descalzo, M.A.; Nieto, J.; Dado, D.; Martín, O.; Cubero, E.; Molina, R. Emerging trends in the seroprevalence of canine leishmaniosis in the Madrid region (central Spain). Vet. Parasitol. 2010, 169, 327–334. [Google Scholar] [CrossRef]
- Laguna, E.; Gallego, P.P.F. Global environmental changes in a unique Flora: Endangered plant communities in the Valencia region. Metode 2016, 6, 36–45. [Google Scholar] [CrossRef]
- García-Leal, J.; Carrero-Sarmiento, D.; Hoyos-López, R. Diversity of the genus Lutzomyia (Diptera: Psychodidae) in municipalities of the department of Córdoba—Colombia. Acta Biol. Colomb. 2022, 27, 394–402. [Google Scholar] [CrossRef]
- Roth-Damas, P.; Sempere-Manuel, M.; Mialaret-Lahiguera, A.; Fernández-García, C.; Gil-Tomás, J.J.; Colomina-Rodríguez, J.; Palop-Larrea, V. Brote comunitario de leishmaniasis cutánea en la comarca de La Ribera: A propósito de las medidas de Salud Pública. Enfermedades Infecc. Microbiol. Clínica 2017, 35, 338–343. [Google Scholar] [CrossRef]
- Gimeno, M.P.; Navas, L.S.; Solaz, S.R.; Claros, D.B. Leishmaniasis cutaneous-visceral, suspect it to diagnose it. Pediatr. Aten. Primaria 2020, 22, 49–53. [Google Scholar]
- Medenica, S.; Miladinović-Tasić, N.; Stojanović, N.M.; Lakićević, N.; Rakočević, B. Climate Variables Related to the Incidence of Human Leishmaniosis in Montenegro in Southeastern Europe during Seven Decades (1945–2014). Int. J. Environ. Res. Public Health 2023, 20, 1656. [Google Scholar] [CrossRef]
- Pérez-Cutillas, P.; Goyena, E.; Chitimia, L.; De la Rúa, P.; Bernal, L.J.; Fisa, R.; Riera, C.; Iborra, A.; Murcia, L.; Segovia, M.; et al. Spatial distribution of human asymptomatic Leishmania infantum infection in southeast Spain: A study of environmental, demographic and social risk factors. Acta Trop. 2015, 146, 127–134. [Google Scholar] [CrossRef]
- Martín-Ezquerra, G.; Fisa, R.; Riera, C.; Rocamora, V.; Fernández-Casado, A.; Barranco, C.; Serra, T.; Baró, T.; Pujol, R.M. Role of Leishmania spp. infestation in nondiagnostic cutaneous granulomatous lesions: Report of a series of patients from a Western Mediterranean area. Br. J. Dermatol. 2009, 161, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Alcover, M.M.; Ribas, A.; Guillén, M.C.; Berenguer, D.; Tomás-Pérez, M.; Riera, C.; Fisa, R. Wild mammals as potential silent reservoirs of Leishmania infantum in a Mediterranean area. Prev. Vet. Med. 2020, 175, 104874. [Google Scholar] [CrossRef] [PubMed]
- Castelli, G.; Bruno, F.; Reale, S.; Catanzaro, S.; Valenza, V.; Vitale, F. Molecular Diagnosis of Leishmaniasis: Quantification of Parasite Load by a Real-Time PCR Assay with High Sensitivity. Pathogens 2021, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Reiczigel, J.; Marozzi, M.; Fábián, I.; Rózsa, L. Biostatistics for parasitologists—A primer to Quantitative Parasitology. Trends Parasitol. 2019, 35, 277–281. [Google Scholar] [CrossRef]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2009. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing, Version 4.3.1; R Foundation for Statistical Computing: Vienna, Austria, 2023. Available online: https://www.R-project.org/ (accessed on 25 August 2025).
- Cardoso, L.; Gilad, M.; Cortes, H.C.E.; Nachum-Biala, Y.; Lopes, A.P.; Vila-Viçosa, M.J.; Simões, M.; Rodrigues, P.A.; Baneth, G. First report of Anaplasma platys infection in red foxes (Vulpes vulpes) and molecular detection of Ehrlichia canis and Leishmania infantum in foxes from Portugal. Parasites Vectors 2015, 8, 144. [Google Scholar] [CrossRef]
- Millán, J.; Zanet, S.; Gomis, M.; Trisciuoglio, A.; Negre, N.; Ferroglio, E. An investigation into alternative reservoirs of canine leishmaniasis on the endemic island of Mallorca (Spain). Transbound. Emerg. Dis. 2011, 58, 352–357. [Google Scholar] [CrossRef]
- Verin, R.; Poli, A.; Ariti, G.; Nardoni, S.; Fanucchi, M.; Mancianti, F. Detection of Leishmania infantum DNA in tissues of free-ranging red foxes (Vulpes vulpes) in Central Italy. Eur. J. Wildl. Res. 2010, 56, 689–692. [Google Scholar] [CrossRef]
- Abbate, J.M.; Arfuso, F.; Napoli, E.; Gaglio, G.; Giannetto, S.; Latrofa, M.S.; Otranto, D.; Brianti, E. Leishmania infantum in wild animals in endemic areas of southern Italy. Comp. Immunol. Microbiol. Infect. Dis. 2019, 67, 101374. [Google Scholar] [CrossRef]
- Tomassone, L.; Berriatua, E.; De Sousa, R.; Duscher, G.G.; Mihalca, A.D.; Silaghi, C.; Sprong, H.; Zintl, A. Neglected vector-borne zoonoses in Europe: Into the wild. Vet. Parasitol. 2018, 251, 17–26. [Google Scholar] [CrossRef]
- Quinnell, R.J.; Courtenay, O. Transmission, reservoir hosts and control of zoonotic visceral leishmaniasis. Parasitology 2009, 136, 1915–1934. [Google Scholar] [CrossRef]
- Molina, R.; Jiménez, M.I.; Cruz, I.; Iriso, A.; Martín-Martín, I.; Sevillano, O.; Melero, S.; Bernal, J. The hare (Lepus granatensis) as potential sylvatic reservoir of Leishmania infantum in Spain. Vet. Parasitol. 2012, 190, 268–271. [Google Scholar] [CrossRef]
- Jiménez, M.; González, E.; Iriso, A.; Marco, E.; Alegret, A.; Fúster, F.; Molina, R. Detection of Leishmania infantum and identification of blood meals in Phlebotomus perniciosus from a focus of human leishmaniasis in Madrid, Spain. Parasitol. Res. 2013, 112, 2453–2459. [Google Scholar] [CrossRef]
- Oleaga, A.; Zanet, S.; Espí, A.; Pegoraro de Macedo, M.R.; Gortázar, C.; Ferroglio, E. Leishmania in wolves in northern Spain: A spreading zoonosis evidenced by wildlife sanitary surveillance. Vet. Parasitol. 2018, 255, 26–31. [Google Scholar] [CrossRef]
- de Andrade, H.M.; Reis, A.B.; dos Santos, S.L.; Volpini, Â.C.; Marques, M.J.; Romanha, A.J. Use of PCR–RFLP to identify Leishmania species in naturally-infected dogs. Vet. Parasitol. 2006, 140, 231–238. [Google Scholar] [CrossRef]
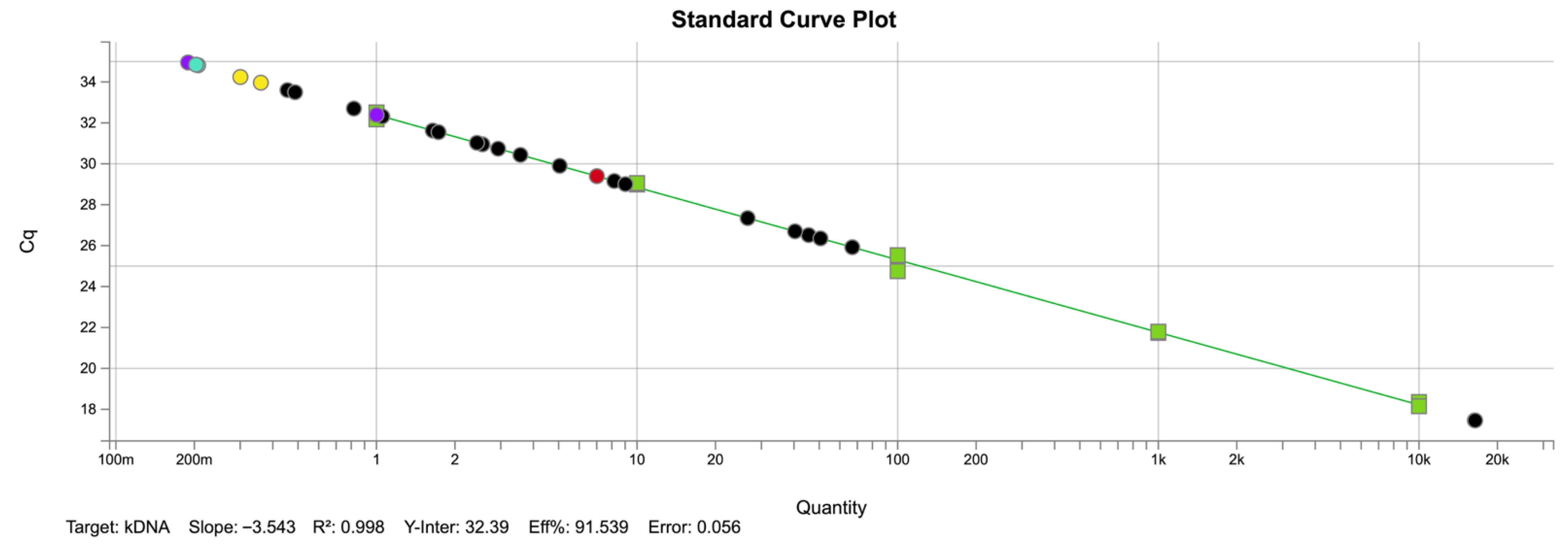
| Sample | Ct Means | Estimated Concentration (Parasites/µL) |
|---|---|---|
| Fc23055 | 34.8295 | 0.20495 |
| Mf25005 | 34.1015 | 0.3302 |
| Mm21005 | 29.394 | 7.009 |
| Nv21007 | 33.671 | 0.59515 |
| Vv19004 | 28.2505 | 17.3735 |
| Vv21001 | 26.2185 | 56.25 |
| Vv21023 | 29.8715 | 5.976 |
| Vv22002 | 30.4615 | 3.7385 |
| Vv24002 | 31.586 | 1.6871 |
| Vv24012 | 30.692 | 3.0585 |
| Vv24017 | 32.5095 | 0.9355 |
| Vv25004 | 26.527 | 45.461 |
| Vv25016 | 17.4575 | 16,395.0 |
| Vv25019 | 33.5485 | 0.47135 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suita, F.; Lizana, V.; Aguiló-Gisbert, J.; López-Ramon, J.; Torres Da Silva, J.; Díaz, E.A.; Cardells, J. Molecular and Serological Detection of Leishmania spp. in Mediterranean Wild Carnivores and Feral Cats: Implications for Wildlife Health and One Health Surveillance. Animals 2025, 15, 2751. https://doi.org/10.3390/ani15182751
Suita F, Lizana V, Aguiló-Gisbert J, López-Ramon J, Torres Da Silva J, Díaz EA, Cardells J. Molecular and Serological Detection of Leishmania spp. in Mediterranean Wild Carnivores and Feral Cats: Implications for Wildlife Health and One Health Surveillance. Animals. 2025; 15(18):2751. https://doi.org/10.3390/ani15182751
Chicago/Turabian StyleSuita, Francesca, Víctor Lizana, Jordi Aguiló-Gisbert, Jordi López-Ramon, João Torres Da Silva, Eduardo A. Díaz, and Jesús Cardells. 2025. "Molecular and Serological Detection of Leishmania spp. in Mediterranean Wild Carnivores and Feral Cats: Implications for Wildlife Health and One Health Surveillance" Animals 15, no. 18: 2751. https://doi.org/10.3390/ani15182751
APA StyleSuita, F., Lizana, V., Aguiló-Gisbert, J., López-Ramon, J., Torres Da Silva, J., Díaz, E. A., & Cardells, J. (2025). Molecular and Serological Detection of Leishmania spp. in Mediterranean Wild Carnivores and Feral Cats: Implications for Wildlife Health and One Health Surveillance. Animals, 15(18), 2751. https://doi.org/10.3390/ani15182751








