Comparative Assessment of Sperm Morphology in Liquid-Preserved Boar Semen Using Cytological Stains
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Experimental Design and Procedures
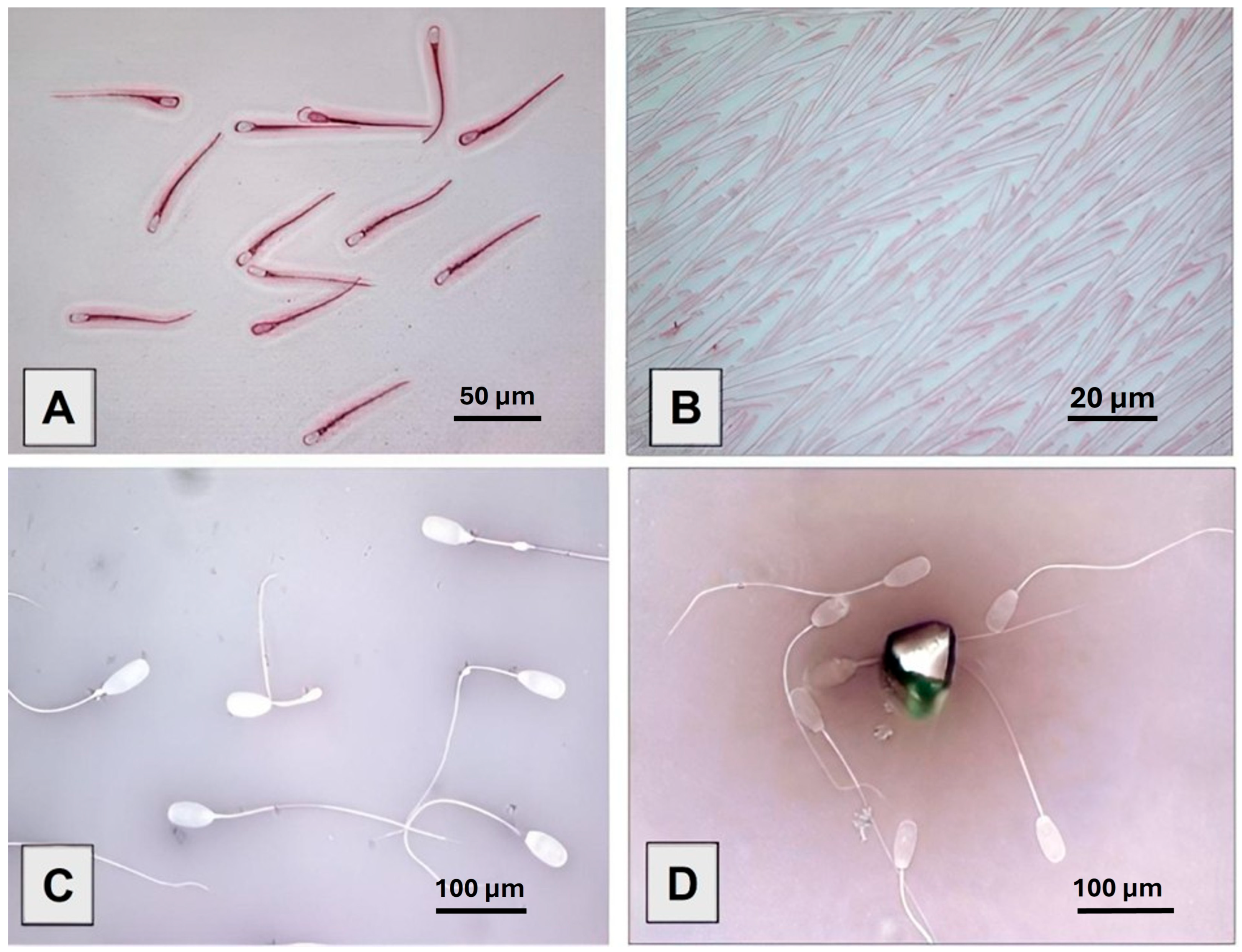
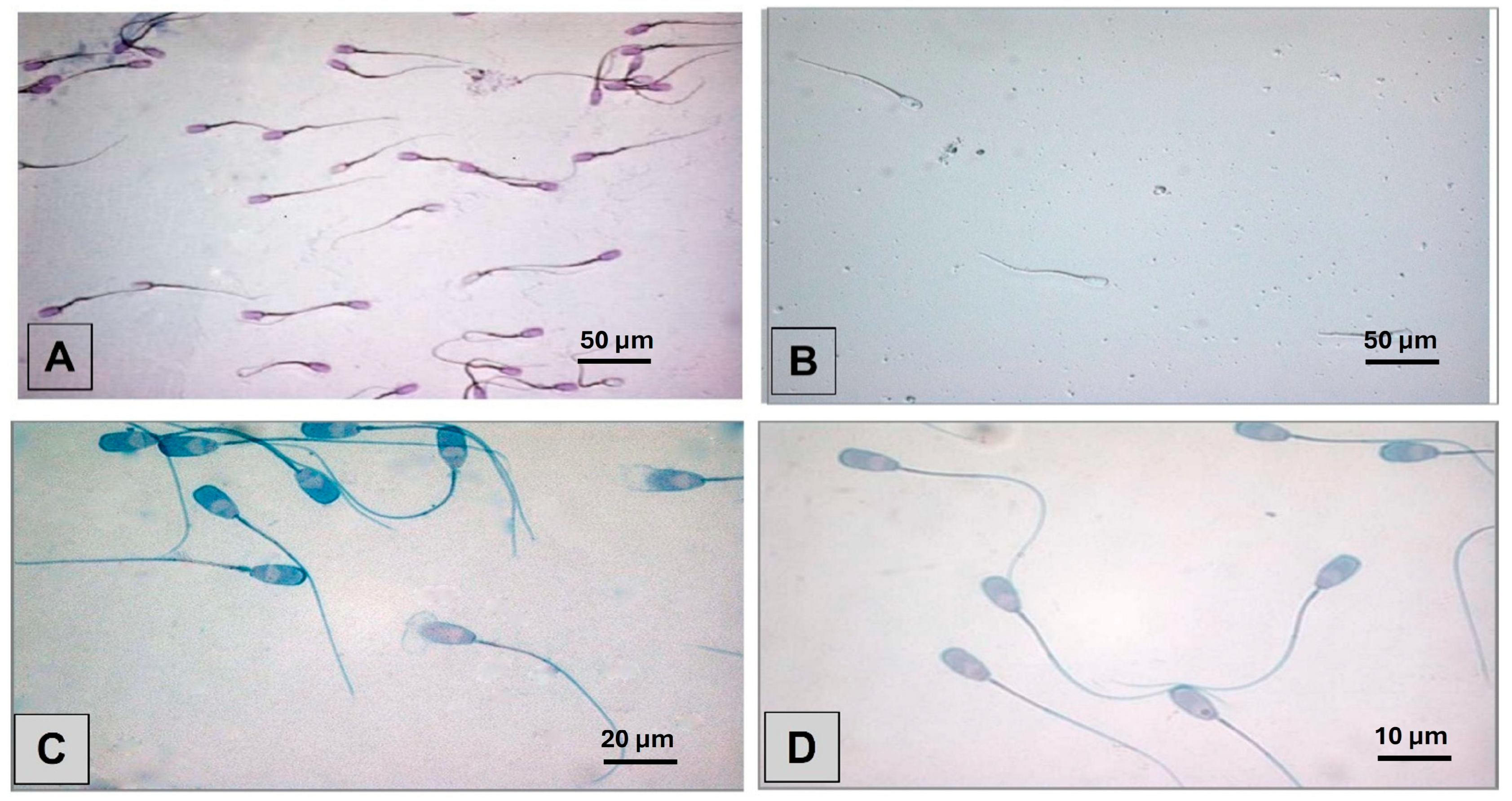
2.3. Staining Techniques
2.3.1. Eosin Staining
2.3.2. Eosin–Nigrosin Staining
2.3.3. Diff-Quick® Staining
2.3.4. Hemacolor® Staining
2.3.5. Sangodiff-G® Staining
2.3.6. Spermac® Staining
2.3.7. Formol–Citrate–Rose Bengal Staining
2.3.8. Testsimplets® Staining
2.3.9. Methyl Violet Staining
2.4. Statistical Methods
3. Results
3.1. Smear Quality of Staining Methods and Overall Morphology Results
3.2. Pathomorphological Differences Across Staining Methods and Storage Times
3.3. Time Consumption and Cost Analysis of Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farshad, A.; Diel, E.; Wehrend, A. Influence of antifreeze protein III on canine sperm cryopreservation. Theriogenology 2025, 235, 86–93. [Google Scholar] [CrossRef]
- Farshad, A.; Diel, E.; Wehrend, A. Evaluating the protective effects of MitoQ and antifreeze protein III on cryopreserved canine sperm. Animals 2025, 15, 270. [Google Scholar] [CrossRef]
- Bonde, J.P.; Ernst, E.; Jensen, T.K.; Hjollund, N.H.; Kolstad, H.; Henriksen, T.B.; Scheike, T.; Giwercman, A.; Olsen, J.; Skakkebaek, N.E. Relation between semen quality and fertility: A population-based study of 430 first-pregnancy planners. Lancet 1998, 352, 1172–1177. [Google Scholar] [CrossRef] [PubMed]
- Chaiya, J.; Vinayanuvattikhun, N.; Tanprasertkul, C.; Chaidarun, T.; Mebuathong, T.; Kaset, C. Effect of staining methods on human sperm morphometrics using HT CASA II. J. Gynecol. Obstet. Hum. Reprod. 2022, 51, 102322. [Google Scholar] [CrossRef]
- Slama, R.; Eustache, F.; Ducot, B.; Jensen, T.K.; Jørgensen, N.; Horte, A.; Irvine, S.; Suominen, J.; Andersen, A.G.; Auger, J.; et al. Time to pregnancy and semen parameters: A cross-sectional study among fertile couples from four European cities. Hum. Reprod. 2002, 17, 503–515. [Google Scholar] [CrossRef]
- Farias, L.B.; da Cunha Barreto-Vianna, A.R.; de Mello, M.D.; Dos Santos, A.L.; da Fonte Ramos, C.; Fontoura, P. Comparison of Diff-Quick and spermac staining methods for sperm morphology evaluation. J. Reprod. Infertil. 2023, 24, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Gatimel, N.; Moreau, J.; Parinaud, J.; Léandri, R.D. Sperm morphology: Assessment, pathophysiology, clinical relevance, and state of the art in 2017. Andrology 2017, 5, 845–862. [Google Scholar] [CrossRef] [PubMed]
- Gacem, S.; Catalán, J.; Yánez-Ortiz, I.; Soler, C.; Miró, J. New sperm morphology analysis in equids: Trumorph® vs eosin-nigrosin stain. Vet. Sci. 2021, 8, 79. [Google Scholar] [CrossRef]
- Lu, J.C. Analysis of sperm morphology: Yes or no? Zhonghua Nan Ke Xue 2013, 19, 291–295. [Google Scholar]
- Xu, Y.H.; Lu, J.C.; Tang, S.S. Effects of six kinds of sperm staining methods on human sperm size and evaluation of their staining effects. J. Clin. Lab. Anal. 2022, 36, e24794. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Soderlund, I.; Kvist, U. Evaluation of the one-step eosin-nigrosin staining technique for human sperm vitality assessment. Hum. Reprod. 2003, 18, 813–816. [Google Scholar] [CrossRef] [PubMed]
- Lasley, J.F.; Easley, G.T.; McKenzie, F.F. A staining method for the differentiation of live and dead spermatozoa. Anat. Rec. 1942, 82, 167–172. [Google Scholar] [CrossRef]
- Hancock, J.L. The morphology of boar spermatozoa. J. R. Microsc. Soc. 1956, 76, 84–97. [Google Scholar] [CrossRef]
- Kruger, T.F.; Ackerman, S.B.; Simmons, K.F.; Swanson, R.J.; Brugo, S.S.; Acosta, A.A. A quick, reliable staining technique for human sperm morphology. Arch. Androl. 1987, 18, 275–277. [Google Scholar] [CrossRef] [PubMed]
- Garcı-Herreros, M.; Aparicio, I.M.; Baro, F.J.; Garcı-Marı, L.J.; Gil, M.C. Standardization of sample preparation, staining and sampling methods for automated sperm head morphometry analysis of boar spermatozoa. Int. J. Androl. 2006, 29, 553–563. [Google Scholar] [CrossRef]
- Barrientos-Morales, M.; Hernández-González, R.; Domínguez-Mancera, B.; Hernández-González, J.A.; Rodríguez-Martínez, M.d.C. Is there a relationship between the PT-substructure status and acrosome loss of boar spermatozoa following freezing-thawing or acrosomal reaction? J. Anim. Vet. Adv. 2009, 8, 155–164. [Google Scholar]
- Wernicke, J.; Schirren, C.A. Morphologische Differenzierung menschlicher Spermatozoen mittels vorgefertigter Methodik Vergleichende Untersuchungen. Andrologia 1982, 14, 471–480. [Google Scholar] [CrossRef]
- Oettlé, E.E. Changes in acrosome morphology during cooling and freezing of dog semen. Anim. Reprod. Sci. 1986, 12, 145–150. [Google Scholar] [CrossRef]
- Oettlé, E.E.; Soley, J.T. Severe sperm abnormalities with subsequent recovery following on scrotal oedema and posthitis in a bulldog. J. Small Anim. Pract. 1986, 27, 477–484. [Google Scholar] [CrossRef]
- Riesenbeck, A.; Völger, D.; Hoffmann, B. Praxisnahe Bestimmung von Vitalitätsparametern zur Beurteilung von Rüdensperma. Tierärztl. Prax. 2001, 29, 116–120. [Google Scholar]
- Meissner, G. Morphologische Untersuchung von Eberspermien unter Verwendung von Testsimplets. Reprod. Domest. Anim. 1986, 22, 284–286. [Google Scholar]
- Schirren, C. Einführung in die Andrologie. Andrologia 1977, 9, 348. [Google Scholar]
- Kvác, M.; Sak, B.; Hanzlíková, D.; Kotilová, J.; Kvetonová, D. Molecular characterization of Cryptosporidium isolates from pigs at slaughterhouses in South Bohemia, Czech Republic. Parasitol. Res. 2009, 104, 425–428. [Google Scholar] [CrossRef] [PubMed]
- Nemejc, K.; Sak, B.; Kventonova, D.; Hanzal, V.; Jenikova, M.; Kvac, M. The first report of Cryptosporidium suis and Cryptosporidium pig genotype II in Eurasian wild boars (Sus scrofa) (Czech Republic). Vet. Parasitol. 2012, 184, 122–125. [Google Scholar] [CrossRef]
- Li, J.; Wang, S.; Zhu, X. Pathological changes following retrograde infusion of methyl violet in the parotid gland of the miniature pig. Chung Hua Kou Chiang Hsueh Tsa Chih 1999, 94, 91–93. [Google Scholar]
- Wekerle, L.; Sarlós, P. Vergleich verschiedener Färbemethoden von Eberspermien. Andrologia 1989, 21, 449–482. [Google Scholar]
- Didion, B.A.; Dobrinsky, J.R.; Giles, J.R.; Graves, C.N. Staining procedure to detect viability and the true acrosome reaction in spermatozoa of various species. Gamete Res. 1989, 22, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Kovács, A.; Foote, R.H. Viability and acrosome staining of bull, boar and rabbit spermatozoa. Biotech. Histochem. 1992, 67, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.A.; Vickers, S.E. Use of fluorescent probes to assess membrane integrity in mammalian spermatozoa. J. Reprod. Fertil. 1990, 88, 343–352. [Google Scholar] [CrossRef]
- Van Der Horst, G.; Maree, L. SpermBlue: A new universal stain for human and animal sperm which is also amenable to automated sperm morphology analysis. Biotech. Histochem. 2009, 84, 299–308. [Google Scholar] [CrossRef]
- Kondracki, S.; Wysokińska, A.; Kania, M.; Górski, K. Application of two staining methods for sperm morphometric evaluation in domestic pigs. J. Vet. Res. 2017, 61, 345–349. [Google Scholar] [CrossRef]
- Busch, W.; Waberski, D. Spermatologie. In Künstliche Besamung bei Haus- und Nutztieren, 1st ed.; Busch, W., Waberski, D., Eds.; Schattauer Verlag: Stuttgart, Germany, 2007; pp. 1–6. [Google Scholar]
- Szablicka, D.; Wysokińska, A.; Pawlak, A.; Roman, K. Morphometry of boar spermatozoa in semen stored at 17 °C—The influence of the staining technique. Animals 2022, 12, 1888. [Google Scholar] [CrossRef] [PubMed]
- Dott, H.M.; Foster, G.C. A technique for studying the morphology of mammalian spermatozoa which are eosinophilic in a differential “life-dead” stain. J. Reprod. Fertil. 1972, 29, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Wysokińska, A.; Chłopik, A. The occurrence of spermatozoa with acrosome reaction in semen of boars depending on staining method and storage duration. Acta Sci. Pol. Zootech. 2019, 18, 51–58. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 5th ed.; WHO: Geneva, Switzerland, 2010; Available online: https://iris.who.int/handle/10665/44261 (accessed on 28 April 2025).
- Paulenz, H.; Grevle, I.S.; Berg, K.A.; Thomassen, R. The use of a dichromatic stain method (Spermac®) for determining changes in the acrosomal integrity of boar semen during cryopreservation. Reprod. Domest. Anim. 1995, 30, 113–116. [Google Scholar] [CrossRef]
- Johnson, L.A.; Weitze, K.F.; Fiser, P.; Maxwell, W.M. Storage of boar semen. Anim. Reprod. Sci. 2000, 62, 143–172. [Google Scholar] [CrossRef]
- Busch, W.; Holzmann, A. Veterinärmedizinische Andrologie: Physiologie und Pathologie der Fortpflanzung bei männlichen Tieren, 1st ed.; Enke Verlag: Stuttgart, Germany, 2001; ISBN 3794519558. [Google Scholar]
- Gadea, J. Assessment of Boar Semen Morphology and Morphometry. In Spermatology. Methods in Molecular Biology; Álvarez-Rodríguez, M., Ed.; Humana: New York, NY, USA, 2025; Volume 2897, pp. 37–49. [Google Scholar]
- Blom, E. A one-minute live-dead sperm stain by means of eosin-nigrosin. Fertil. Steril. 1950, 1, 176–177. [Google Scholar] [CrossRef]
- Dott, H.M.; Foster, G.C. Preservation of differential staining of spermatozoa by formol citrate. J. Reprod. Fertil. 1975, 45, 57–60. [Google Scholar] [CrossRef][Green Version]
- Bamba, K. Evaluation of acrosomal integrity of boar spermatozoa by bright field microscopy using an eosin-nigrosin stain. Theriogenology 1988, 29, 1245–1251. [Google Scholar] [CrossRef]
- Tamuli, M.K.; Watson, P.F. Use of a simple staining technique to distinguish acrosomal changes in the live sperm sub-population. Anim. Reprod. Sci. 1994, 35, 247–254. [Google Scholar] [CrossRef]
- Tsakmakidis, I.A.; Lymberopoulos, A.G.; Khalifa, T.A. Relationship between sperm quality traits and field-fertility of porcine semen. J. Vet. Sci. 2010, 11, 151–154. [Google Scholar] [CrossRef]
- Freneau, G.E.; Chenoweth, P.J.; Ellis, R.; Rupp, G. Sperm morphology of beef bulls evaluated by two different methods. Anim. Reprod. Sci. 2009, 118, 176–181. [Google Scholar] [CrossRef]
- Root Kustritz, M.V.; Olson, P.N.; Johnston, S.D.; Root, T.K. The effects of stains and investigators on assessment of morphology of canine spermatozoa. J. Am. Anim. Hosp. Assoc. 1998, 34, 348–352. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.W.; Maruyama, G.; Johnson, R.; Nelson, D.; Skon, L. Effects of cooperative, competitive, and individualistic goal structures on achievement: A meta-analysis. Psychol. Bull. 1981, 89, 47–62. [Google Scholar] [CrossRef]
- Hrudka, F. Cytochemical and ultrastructural abnormalities of the acrosome in spermatozoa of subfertile bulls. Theriogenology 1987, 27, 683–700. [Google Scholar]
- Kuster, C.E.; Hess, R.A.; Althouse, G.C. Immunohistochemical localization of aquaporins in the boar reproductive tract and their potential role in sperm membrane organization. Theriogenology 2004, 62, 789–804. [Google Scholar]
- Evans, G.; Maxwell, W.M. Salamon’s Artificial Insemination of Sheep and Goats; Butterworths: Sydney, Australia, 1987; ISBN 0409491672. [Google Scholar]
- Henkel, R.; Schill, W.B. Sperm preparation for ART. Hum. Reprod. Update 2003, 9, 275–284. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H. Role of the male in embryo development: Sperm quality and beyond. Reprod. Domest. Anim. 2007, 42 (Suppl. 2), 67–70. [Google Scholar]
- Oberlender, G.; Murgas, L.D.S.; Zangerônimo, M.G.; Silva, A.C.; Pereira, L.J.; Muzzi, R.A.L. Comparison of two different methods for evaluating boar semen morphology. Arch. Med. Vet. 2012, 44, 201–205. [Google Scholar] [CrossRef][Green Version]
- Czubaszek, M.; Andraszek, K.; Banaszewska, D.; Walczak-Jędrzejowska, R. Effect of the staining technique on morphological and morphometric parameters of boar sperm. PLoS ONE 2019, 14, e0214243. [Google Scholar] [CrossRef]
- Barth, A.D.; Oko, R.J. Abnormal Morphology of Bovine Spermatozoa; Iowa State University Press: Ames, IA, USA, 1989; ISBN 0813800727. [Google Scholar]

| Staining Method | Color Intensity (1–5) | Detail Recognition (1–5) | Contras (1–5) |
|---|---|---|---|
| Eosin | 3 (balanced color) | 5 (clear detail visibility) | 5 (marked difference) |
| Eosin–Nigrosin | 3 (balanced color) | 5 (clear detail visibility) | 5 (marked difference) |
| Diff-Quick® | 3 (balanced color) | 4 (acceptable detail level) | 5 (marked difference) |
| Spermac® | 5 (saturated color) | 5 (clear detail visibility) | 5 (marked difference) |
| Hemacolor® | 2 (faint color) | 4 (acceptable detail level) | 5 (marked difference) |
| Sangodiff-G® | 2 (faint color) | 4 (acceptable detail level) | 2 (minimal tonal difference) |
| Formol–Citrate–Rose Bengal stain | 5 (highly saturated) | 5 (clear detail visibility) | 5 (marked difference) |
| Testsimplets® | 4 (saturated color) | 5 (clear detail visibility) | 4 (noticeable but not sharp) |
| Methyl Violet | 2 (faint color) | 3 (unclear details) | 2 (minimal tonal difference) |
| Criteria | E | EN | DQ | H | SG | S | FB | TS | Ø | p-Value |
|---|---|---|---|---|---|---|---|---|---|---|
| Abnormal sperm | 26.53 a | 26.44 a | 22.56 b | 22.00 b | 18.31 c | 30.5 d | 26.58 d | 31.81 a | 25.59 | <0.0001 |
| Irregular head shape | 0.36 | 0.58 | 0.47 | 0.47 | 0.53 | 0.72 | 0.47 | 0.53 | 0.52 | 0.721 |
| Round head | 0.53 | 0.33 | 0.36 | 0.36 | 0.39 | 0.5 | 0.44 | 0.39 | 0.41 | 0.926 |
| Lanceolate head | 0.06 | 0.03 | 0.03 | 0 | 0 | 0 | 7.97 | 3.86 | 1.49 | <0.0001 |
| Pear-shaped head | 0 | 0.06 | 0 | 0.06 | 0 | 0 | 0.03 | 0.22 | 0.05 | 0.004 |
| Double head | 0 | 0.03 | 0 | 0.14 | 0.03 | 0.03 | 0.03 | 0 | 0.03 | 0.0353 |
| Acrosome size variations | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0.03 | 0.00 | 0 |
| Absence of acrosome | 0.11 | 0 | 0 | 0 | 1.19 | 1.14 | 0 | 0.17 | 0.33 | <0.0001 |
| Acrosome detachment | 4.53 | 2.22 | 1.22 | 1.25 | 0.53 | 6.31 | 1.5 | 0.44 | 2.25 | <0.0001 |
| Acrosome with vacuoles | 0 | 0.06 | 0.03 | 0 | 0 | 0.19 | 0.08 | 0 | 0.05 | 0.0007 |
| Total head/acrosome | 5.59 a | 3.31 b | 2.11 c | 2.28 c | 2.67 d | 8.89 d | 10.53 e | 5.64 a | 5.12 | <0.0001 |
| Asymmetrical midpiece | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | * |
| Broken midpiece | 1.69 | 0.5 | 1.53 | 0.78 | 1.06 | 1.42 | 0.28 | 0.31 | 0.95 | 0.0009 |
| Thickened/narrow midpiece | 0.08 | 0 | 0.14 | 0 | 0 | 0 | 0.14 | 0.03 | 0.05 | 0.468 |
| Total midpiece evaluation | 1.78 | 0.5 | 1.67 | 0.78 | 1.06 | 1.42 | 0.42 | 0.33 | 1 | 0.0009 |
| Coiled principal piece | 7.17 | 7.56 | 6.92 | 9.42 | 7.39 | 8.61 | 8.28 | 10.22 | 8.2 | 0.0014 |
| Rolled principal piece | 0.86 | 0.36 | 0.92 | 0.53 | 0.92 | 0.75 | 1.25 | 0.86 | 0.81 | 0.0888 |
| Bent principal piece | 1.92 | 1.33 | 3.94 | 3.06 | 2.11 | 1.25 | 1.03 | 1 | 1.96 | <0.0001 |
| Broken principal piece | 0.78 | 0.56 | 0.28 | 0.39 | 0.53 | 1.22 | 0.44 | 0.19 | 0.55 | 0.0002 |
| Total principal piece | 10.72 | 9.81 | 12.06 | 13.39 | 10.94 | 11.83 | 11.00 | 12.28 | 11.50 | 0.119 |
| Plasma droplets at neck | 1.47 | 6.72 | 1.11 | 0.81 | 0.97 | 3.22 | 1.31 | 7.5 | 2.89 | <0.0001 |
| Plasma droplets at midpiece | 3.08 | 3.47 | 1.83 | 1.56 | 1.08 | 2.06 | 1.75 | 4.97 | 2.48 | <0.0001 |
| Plasma droplets at principal piece | 0.06 | 0.14 | 0.03 | 0 | 0.03 | 0 | 0 | 0 | 0.03 | 0.002 |
| Total plasma droplet | 4.61 a | 10.33 b | 2.9 c | 2.36 c | 2.08 c | 5.28 a | 3.06 c | 12.47 b | 5.39 | <0.0001 |
| Individual heads | 3.83 a | 2.5 b | 3.75 a | 3.19 ab | 1.56 c | 3.08 a | 1.58 c | 1.08 c | 2.57 | <0.0001 |
| Methods | Time 1 | Time 2 | Time 3 | Time 4 |
|---|---|---|---|---|
| Hemacolor | Pale color, fair detail visibility, good contrast across | Pale color, fair detail visibility, good contrast | Pale color, fair detail visibility, good contrast | 16.67% partially assessable, 33.33% with colored particles, 16.67% with yellowish areas. |
| Sangodiff-G® | Pale color, fair detail, poor contrast; 50% granular background, 25% stained head caps, 16.67% colored particles | Pale color, fair detail, poor contrast; 50% granular background, 25% stained head caps, 16.67% colored particles | Analyzability dropped to 91.67% | Analyzability dropped to 55.56% |
| Formol– Citrate–Rose Bengal stain | 100% Analyzable | 100% Analyzable | 100% Analyzable | 94.44% Analyzable |
| Testsimplets® | 100% Analyzable | 97.22% Analyzable | 72.22% Analyzable | 11.11% Analyzable |
| Methyl Violet | 100% Analyzable | 100% Analyzable | 0% Analyzable | 0% Analyzable |
| Criteria | Temperature | |||
|---|---|---|---|---|
| 18 °C | 38 °C | 6 °C | p-Value | |
| Morphological abnormalities | 30.59 | 31.34 | 31.26 | 0.908 |
| Irregular head shape | 0.48 | 0.33 | 0.56 | 0.047 |
| Round head | 0.38 | 0.41 | 0.32 | 0.626 |
| Lanceolate head | 9.91 | 8.9 | 8.95 | 0.526 |
| Pear-shaped head | 0.02 | 0.02 | 0.08 | <0.0001 |
| Double head | 0.05 | 0.01 | 0.03 | 0.0238 |
| Acrosome size variations (large/small) | 0 | 0 | 0.01 | 0 |
| Absence of acrosome | 0.34 | 0.25 | 0.28 | 0.29 |
| Acrosome detachment | 2.06 | 2.21 | 1.73 | 0.18 |
| Acrosome with vacuoles | 0 | 0.06 | 0.06 | 0.814 |
| Total head/acrosome | 13.23 | 12.19 | 12.03 | 0.548 |
| Asymmetrical midpiece | 0 | 0 | 0 | * |
| Broken midpiece | 1.05 | 0.76 | 0.93 | 0.467 |
| Thickened/narrow midpiece | 0.06 | 0.02 | 0.06 | 0.0519 |
| Total midpiece | 1.1 | 0.78 | 0.98 | 0.398 |
| Coiled principal piece | 6.78 | 7.56 | 8.11 | 0.0629 |
| Rolled principal piece | 0.48 | 0.83 | 0.83 | 0.0191 |
| Bent principal piece | 1.83 | 1.83 | 1.8 | 0.986 |
| Broken principal piece | 0.42 | 0.64 | 0.41 | 0.0595 |
| Total principal piece | 9.51 | 10.86 | 11.15 | 0.0721 |
| Plasma droplets at neck | 2.56 | 2.67 | 2.47 | 0.842 |
| Plasma droplets at midpiece | 2.25 | 2.33 | 2.04 | 0.637 |
| Plasma droplets at principal piece | 0.05 | 0.01 | 0.03 | 0.0007 |
| Total plasma droplet | 4.86 | 5.01 | 4.54 | 0.662 |
| Individual heads | 1.89 | 2.5 | 2.56 | 0.0975 |
| Staining Method | Preparation | Evaluation | Total Time | Vitality (%) | Vitality (Mean ± SD) | Total Exclu. Vitality (%) | Total Incl. Vitality (%) |
|---|---|---|---|---|---|---|---|
| Eosin | 3.17 ± 10.42 | 5.00 ± 12.78 | 8.17 ± 0.23.2 * | RT: 80.01 ± 12.23 | |||
| WB: 70.68 ± 16.10 | |||||||
| RF: 84.97 ± 8.96 | RT: 84 ± 5 | RT: 82.12 | RT: 84.67 | ||||
| WB: 72 ± 6 | WB: 71.02 | WB: 72.14 | |||||
| RF: 88 ± 4 | RF: 85.34 | RF: 88.11 | |||||
| Eosin–Nigrosin | 3.67 ± 10.42 | 6.70 ± 11.65 | 10.37 ± 22.07 * | RT: 87.28± 7.87 | |||
| WB: 79.27 ± 9.72 | |||||||
| RF: 84.36 ± 8.49 | |||||||
| Diff-Quick® | 5.73 ± 11.97 | 5.03 ± 8.67 | 10.76 ± 20.94 | ||||
| Hemacolor® | 5.73 ± 15.28 | 5.03 ± 8.67 | 10.76 ± 23.95 | ||||
| Spermac® | 12.64 ± 10.09 | 6.90 ± 12.16 | 19.54 ± 22.25 | ||||
| Sangodiff-G® | 14.43 ± 8.02 | 5.20 ± 9.96 | 19.63 ± 17.98 | ||||
| Formol–Citrate–Rose Bengal | 45.49 ± 1.72 | 6.48 ± 10.96 | 51.97 ± 12.32 | ||||
| Methyl Violet | 60.46 ± 0.89 | 4.98 ± 5.82 | 65.44 ± 8.55 | ||||
| Testsimplets® | 60.22 ± 2.73 | 7.45 ± 18.95 | 67.67 ± 19.84 |
| Staining Method | Material Components | Materials (€) | Total Time (min) | Labor (€) | Total (€) | Cost Variation (€) |
|---|---|---|---|---|---|---|
| Formol–Citrate–Rose Bengal | 0.3 mL stain solution, 1 slide, 1 coverslip, 2 pipette tips | 0.15 | 51.97 | 21.65 | 21.80 | 19.47–24.95 |
| Methyl Violet | 0.05 mL Methyl Violet, 1 slide, 1 pipette tip | 0.17 | 65.44 | 27.27 | 27.44 | 24.54–30.71 |
| Eosin | mL Eosin solution, 1 slide, 2 pipette tips | 0.19 | 12.32 | 5.13 | 5.32 | 4.79–5.85 |
| Eosin–Nigrosin | 0.1 mL Eosin–Nigrosin solution, 2 slides, 2 pipette tips, 0.05 mL immersion oil | 0.20 | 15.35 | 6.39 | 6.59 | 5.93–7.25 |
| Diff-Quick® | 3 × 1.66 mL staining solution, 2 slides, 1 pipette tip | 1.22 | 10.76 | 4.49 | 5.71 | 5.14–6.28 |
| Spermac® | 4 × 0.25 mL staining solution, 2 slides, 1 pipette tip | 0.90 | 19.54 | 8.15 | 9.05 | 8.14–10.01 |
| Hemacolor® | 3 × 0.5–2 mL staining solution, 2 slides, 1 pipette tip | 0.46–1.36 | 10.76 | 4.49 | 4.95–5.85 | 4.46–6.43 |
| Sangodiff-G® | stain with fixative solution, 2 slides, 1 pipette tip | 0.83 | 19.63 | 8.18 | 9.01 | 8.11–9.91 |
| Testsimplets® | 1 pre-stained slide/coverslip, 1 pipette tip, 0.05 mL immersion oil | 1.70 | 67.67 | 28.19 | 29.89 | 27.08–32.78 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braune, A.; Wehrend, A.; Kauffold, J.; Farshad, A. Comparative Assessment of Sperm Morphology in Liquid-Preserved Boar Semen Using Cytological Stains. Animals 2025, 15, 2737. https://doi.org/10.3390/ani15182737
Braune A, Wehrend A, Kauffold J, Farshad A. Comparative Assessment of Sperm Morphology in Liquid-Preserved Boar Semen Using Cytological Stains. Animals. 2025; 15(18):2737. https://doi.org/10.3390/ani15182737
Chicago/Turabian StyleBraune, Annika, Axel Wehrend, Johannes Kauffold, and Abbas Farshad. 2025. "Comparative Assessment of Sperm Morphology in Liquid-Preserved Boar Semen Using Cytological Stains" Animals 15, no. 18: 2737. https://doi.org/10.3390/ani15182737
APA StyleBraune, A., Wehrend, A., Kauffold, J., & Farshad, A. (2025). Comparative Assessment of Sperm Morphology in Liquid-Preserved Boar Semen Using Cytological Stains. Animals, 15(18), 2737. https://doi.org/10.3390/ani15182737





