Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns
Simple Summary
Abstract
1. Introduction
2. Examples of Emerging Applications of GM Animals for Agriculture and Aquaculture
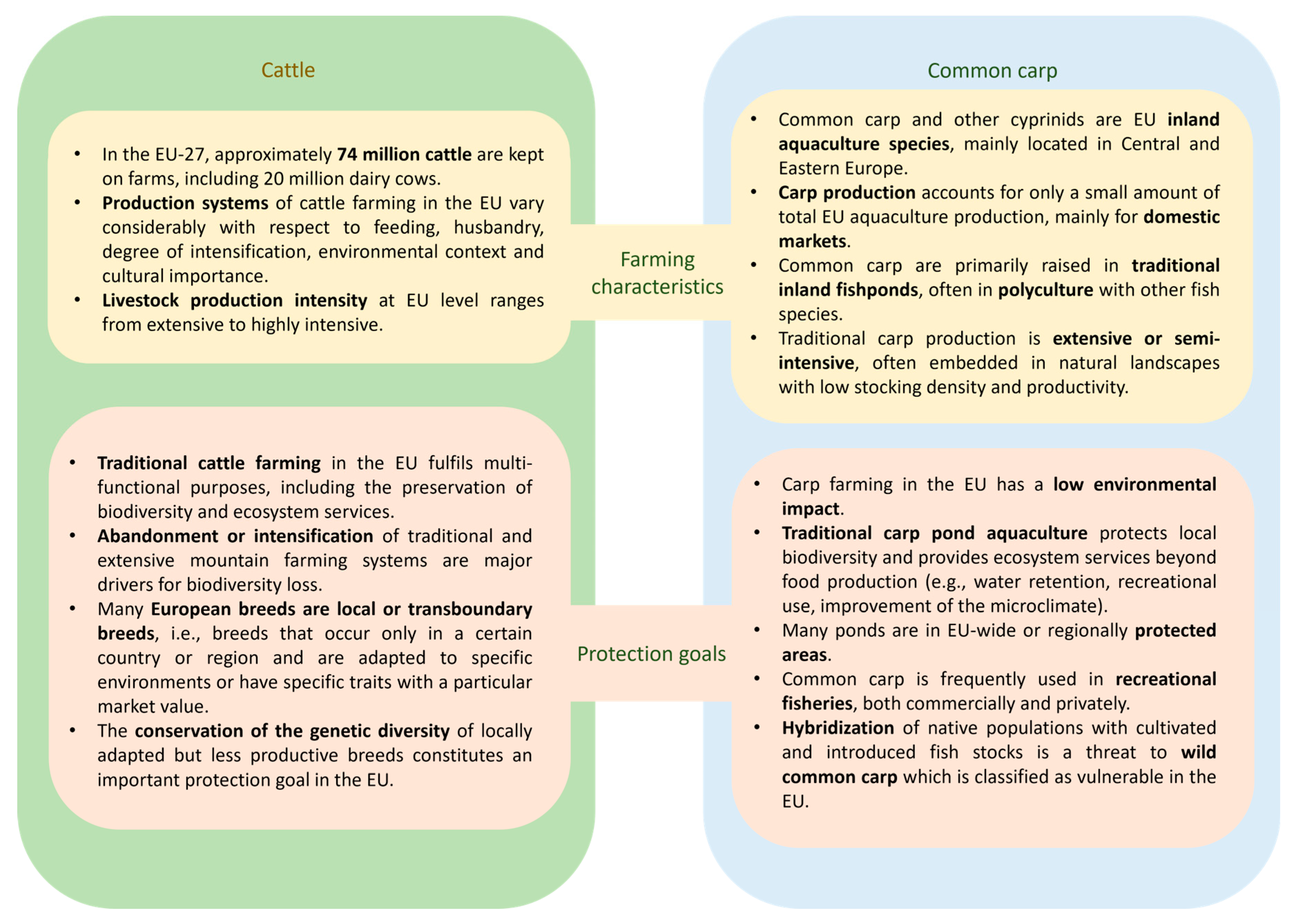
2.1. Slick-Haired Cattle
2.2. Growth-Enhanced Fish Including Common Carp
3. Knowledge Gaps Regarding GM Animals for Agriculture and Aquaculture
3.1. Slick-Haired Cattle
3.2. Growth-Enhanced Common Carp
4. Challenges for ERA of GM Animals for Agriculture and Aquaculture in the EU
4.1. Challenges with Regard to the Comparative Assessment of GM Farmed Animals
4.2. Challenges with Regard to the Assessment of Environmental Risks of GM Farmed Animals
4.2.1. Environmental Risks of GM Farmed Animals Are Linked to Their Farming Systems
4.2.2. Risks to Biodiversity and Nature Conservation Are Likely to Occur Through Stocking or Escape of GM Farmed Animals into Natural Habitats
4.3. Challenges with Regard to the Assessment of Animal Health and Welfare of GM Farmed Animals
5. Considerations for a Broader Assessment of GM Animals for Agriculture and Aquaculture Beyond ERA
5.1. Sustainability Analysis (SA) of GM Animals for Agriculture and Aquaculture
- The development of the GM slick-haired cattle is supposed to make milk and beef production more resilient to the effects of rising air temperatures and to mitigate thermal stress on cattle caused by the current and expected increase in global temperature levels [92,141]. Thermal stress is predicted to affect the health and welfare of cattle as well as their productivity, e.g., concerning milk yield in summer but also the overall environmental footprint of cattle production [30,142].
- Growth-enhanced GM fish, including carp, salmon, and other finfish species, have been developed to increase their production efficiency in aquaculture based on increased feed efficiencies, growth rates, and body weights, with potential benefits for competitiveness [109,137]. It has been suggested that such GM fish could also increase the global availability of foods produced from farmed fish and thus be beneficial for food security and the availability of an alternative protein source to meat produced from land-based farmed animals.
5.1.1. Framing a SA of GM Farmed Animals
5.1.2. Reference Systems and System Boundaries for the SA of GM Farmed Animals
Slick-Haired Cattle
Growth-Enhanced Carp
5.1.3. Issues to Be Addressed in the SA of GM Farmed Animals
Slick-Haired Cattle
Growth-Enhanced Carp
5.2. Broader Consideration of Governance Issues Raised by GMA Applications by Technology Assessment (TA)
- GM animals for biomedical purposes, addressing pigs as organ donors or model organisms, but also “biopharming” (production of biopharmaceuticals in animals) with goats, rabbits, and poultry;
- GM animals for sanitary, human and ecological purposes, applying new genomic techniques, e.g., gene drive approaches, mostly to insect populations and, more specifically, to malaria-transmitting mosquitos;
- GM livestock for food production purposes, for upscaling production rates, disease resistance, and/or for adaptation to (changing or diverse) agricultural environments.
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perisse, I.V.; Fan, Z.; Singina, G.N.; White, K.L.; Polejaeva, I.A. Improvements in Gene Editing Technology Boost Its Applications in Livestock. Front. Genet. 2020, 11, 614688. [Google Scholar] [CrossRef]
- Tait-Burkard, C.; Doeschl-Wilson, A.; McGrew, M.J.; Archibald, A.L.; Sang, H.M.; Houston, R.D.; Whitelaw, C.B.; Watson, M. Livestock 2.0—Genome editing for fitter, healthier, and more productive farmed animals. Genome Biol. 2018, 19, 204. [Google Scholar] [CrossRef]
- Zhao, J.; Lai, L.; Ji, W.; Zhou, Q. Genome editing in large animals: Current status and future prospects. Natl. Sci. Rev. 2019, 6, 402–420. [Google Scholar] [CrossRef]
- Menchaca, A. Sustainable Food Production: The Contribution of Genome Editing in Livestock. Sustainability 2021, 13, 6788. [Google Scholar] [CrossRef]
- van Eenennaam, A.L. Application of genome editing in farm animals: Cattle. Transgenic Res. 2019, 28, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Parisi, C.; Rodriguez Cerezo, E. Current and Future Market Applications of New Genomic Techniques; Publications Office of the European Union: Reims, Luxembourg, 2021. [Google Scholar]
- Gratacap, R.L.; Wargelius, A.; Edvardsen, R.B.; Houston, R.D. Potential of Genome Editing to Improve Aquaculture Breeding and Production. Trends Genet. 2019, 35, 672–684. [Google Scholar] [CrossRef]
- Sovová, T.; Kerins, G.; Demnerová, K.; Ovesná, J. Genome Editing with Engineered Nucleases in Economically Important Animals and Plants: State of the Art in the Research Pipeline. Curr. Issues Mol. Biol. 2017, 21, 41–62. [Google Scholar] [CrossRef] [PubMed]
- Miklau, M.; Burn, S.-J.; Eckerstorfer, M.; Dolezel, M.; Greiter, A.; Heissenberger, A.; Hörtenhuber, S.; Zollitsch, W.; Hagen, K. Horizon scanning of potential environmental applications of terrestrial animals, fish, algae and microorganisms produced by genetic modification, including the use of new genomic techniques. Front. Genome Ed. 2024, 6, 1376927. [Google Scholar] [CrossRef]
- EFSA Panels on GMO and AHAW. Guidance on the risk assessment of food and feed from genetically modified animals and on animal health and welfare aspects. EFSA J. 2012, 10, 2501. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms. Guidance on the environmental risk assessment of genetically modified animals. EFSA J. 2013, 11, 190. [Google Scholar] [CrossRef]
- van Eenennaam, A.L. New Genomic Techniques (NGT) in animals and their agri/food/feed products. EFSA Support. Publ. 2023, 20, 8311E. [Google Scholar] [CrossRef]
- Kawall, K.; Cotter, J.; Then, C. Broadening the GMO risk assessment in the EU for genome editing technologies in agriculture. Environ. Sci. Eur. 2020, 32, 1–24. [Google Scholar] [CrossRef]
- Hallerman, E.M.; Bredlau, J.P.; Camargo, L.S.A.; Dagli, M.L.Z.; Karembu, M.; Ngure, G.; Romero-Aldemita, R.; Rocha-Salavarrieta, P.J.; Tizard, M.; Walton, M.; et al. Towards progressive regulatory approaches for agricultural applications of animal biotechnology. Transgenic Res. 2022, 31, 167–199. [Google Scholar] [CrossRef]
- Okoli, A.S.; Blix, T.; Myhr, A.I.; Xu, W.; Xu, X. Sustainable use of CRISPR/Cas in fish aquaculture: The biosafety perspective. Transgenic Res. 2022, 31, 1–21. [Google Scholar] [CrossRef]
- Blix, T.B.; Dalmo, R.A.; Wargelius, A.; Myhr, A.I. Genome editing on finfish: Current status and implications for sustainability. Rev. Aquacult. 2021, 13, 2344–2363. [Google Scholar] [CrossRef]
- Sonstegard, T.S.; Fahrenkrug, S.C.; Carlson, D. 307 Precision animal breeding to make genetically castrated animals for improved animal welfare and alternative breeding applications. J. Anim. Sci. 2017, 95, 149–150. [Google Scholar] [CrossRef]
- Wray-Cahen, D.; Bodnar, A.; Rexroad, C.; Siewerdt, F.; Kovich, D. Advancing genome editing to improve the sustainability and resiliency of animal agriculture. CABI Agric. Biosci. 2022, 3, 1–17. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Yang, H.; Liu, D.; Cai, G.; Li, G.; Mo, J.; Wang, D.; Zhong, C.; Wang, H.; et al. Novel transgenic pigs with enhanced growth and reduced environmental impact. Elife 2018, 7, e34286. [Google Scholar] [CrossRef] [PubMed]
- Güralp, H.; Skaftnesmo, K.O.; Kjærner-Semb, E.; Straume, A.H.; Kleppe, L.; Schulz, R.W.; Edvardsen, R.B.; Wargelius, A. Rescue of germ cells in dnd crispant embryos opens the possibility to produce inherited sterility in Atlantic salmon. Sci. Rep. 2020, 10, 18042. [Google Scholar] [CrossRef] [PubMed]
- de Graeff, N.; Jongsma, K.R.; Johnston, J.; Hartley, S.; Bredenoord, A.L. The ethics of genome editing in non-human animals: A systematic review of reasons reported in the academic literature. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180106. [Google Scholar] [CrossRef]
- van Reenen, C.G. Assessing the Welfare of Transgenic Farm Animals. In Genetic Engineering in Livestock; Gethmann, C.F., Engelhard, M., Hagen, K., Boysen, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 119–143. ISBN 978-3-540-85842-3. [Google Scholar]
- Rehbinder, E.; Rehbinder, E.; Engelhard, M.; Hagen, K.; Jørgensen, R.B.; Pardo-Avellaneda, R.; Schnieke, A.; Thiele, F. The welfare of pharming animals. In Pharming; Springer: Berlin/Heidelberg, Germany, 2009; pp. 101–120. ISBN 978-3-540-85792-1. [Google Scholar]
- Stirling, A. “Opening Up” and “Closing Down”. Sci. Technol. Hum. Values 2007, 33, 262–294. [Google Scholar] [CrossRef]
- PR Newswire. FDA Makes Low-Risk Determination for Marketing of Products from Genome-Edited Beef Cattle After Safety Review. Available online: https://www.prnewswire.com/news-releases/fda-makes-low-risk-determination-for-marketing-of-products-from-genome-edited-beef-cattle-after-safety-review-301496923.html (accessed on 13 September 2025).
- Porto-Neto, L.R.; Bickhart, D.M.; Landaeta-Hernandez, A.J.; Utsunomiya, Y.T.; Pagan, M.; Jimenez, E.; Hansen, P.J.; Dikmen, S.; Schroeder, S.G.; Kim, E.-S.; et al. Convergent Evolution of Slick Coat in Cattle through Truncation Mutations in the Prolactin Receptor. Front. Genet. 2018, 9, 57. [Google Scholar] [CrossRef]
- Hansen, P.J. Prospects for gene introgression or gene editing as a strategy for reduction of the impact of heat stress on production and reproduction in cattle. Theriogenology 2020, 154, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Olson, T.A.; Lucena, C.; Chase, C.C.; Hammond, A.C. Evidence of a major gene influencing hair length and heat tolerance in Bos taurus cattle. J. Anim. Sci. 2003, 81, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Flórez Murillo, J.M.; Landaeta-Hernández, A.J.; Kim, E.-S.; Bostrom, J.R.; Larson, S.A.; Pérez O’Brien, A.M.; Montero-Urdaneta, M.A.; Garcia, J.F.; Sonstegard, T.S. Three novel nonsense mutations of prolactin receptor found in heat-tolerant Bos taurus breeds of the Caribbean Basin. Anim. Genet. 2021, 52, 132–134. [Google Scholar] [CrossRef]
- Dikmen, S.; Khan, F.A.; Huson, H.J.; Sonstegard, T.S.; Moss, J.I.; Dahl, G.E.; Hansen, P.J. The SLICK hair locus derived from Senepol cattle confers thermotolerance to intensively managed lactating Holstein cows. J. Dairy Sci. 2014, 97, 5508–5520. [Google Scholar] [CrossRef] [PubMed]
- Littlejohn, M.D.; Henty, K.M.; Tiplady, K.; Johnson, T.; Harland, C.; Lopdell, T.; Sherlock, R.G.; Li, W.; Lukefahr, S.D.; Shanks, B.C.; et al. Functionally reciprocal mutations of the prolactin signalling pathway define hairy and slick cattle. Nat. Commun. 2014, 5, 5861. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, H.L.; Contreras-Correa, Z.E.; Lemley, C.O.; Domenech-Pérez, K.; Muñiz-Colón, G. 87 Milk Yield, Vaginal Temperature, and Solar Radiation Exposure in Slick and Wild Type-Haired Puerto Rican Holstein Cows. J. Anim. Sci. 2023, 101, 64–65. [Google Scholar] [CrossRef]
- Contreras-Correa, Z.E.; Sánchez-Rodríguez, H.L.; Muñiz-Colón, G.; Lemley, C.O. 84 Puerto Rican Slick-Haired Holstein Cattle Exhibit Enhanced Mammary Gland Hemodynamics Compared with Their Wild-Type Haired Counterparts. J. Anim. Sci. 2023, 101, 63–64. [Google Scholar] [CrossRef]
- FDA. Heritable Intentional Genomic Alterations in Animals: Risk-Based Approach: CVM GFI #187A. 2024. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-187a-heritable-intentional-genomic-alterations-animals-risk-based-approach (accessed on 28 October 2024).
- FDA. Heritable Intentional Genomic Alterations in Animals: The Approval Process: CVM GFI #187B. 2024. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cvm-gfi-187b-heritable-intentional-genomic-alterations-animals-approval-process (accessed on 28 October 2024).
- Nepomuceno, A.L.; Fuganti-Pagliarini, R.; Felipe, M.S.S.; Molinari, H.B.C.; Velini, E.D.; de Campos Pinto, E.R.; Dagli, M.L.Z.; Andrade Filho, G.; Fernandes, P.M.B. Brazilian biosafety law and the new breeding technologies. Front. Agr. Sci. Eng. 2020, 7, 204. [Google Scholar] [CrossRef]
- Fisheries and Oceans Canada. Summary of the Enviornmental and Indirect Human Health Risk Assessment of AquAdvantage Salmon.: Canadian Science Advisory Secretariat. Science Response 2013/23. Available online: https://waves-vagues.dfo-mpo.gc.ca/library-bibliotheque/361091.pdf (accessed on 12 November 2024).
- Government of Canada. AquAdvantage Salmon. Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/genetically-modified-foods-other-novel-foods/approved-products/aquadvantage-salmon.html (accessed on 12 November 2024).
- AquaBounty Technologies. AquaBounty Announces Plans to Cease Fish Farming Operations. 2024. Available online: https://investors.aquabounty.com/news-releases/news-release-details/aquabounty-announces-plans-cease-fish-farming-operations (accessed on 1 September 2025).
- US Food and Drug Administration. AquAdvantage Salmon. Available online: https://www.fda.gov/animal-veterinary/intentional-genomic-alterations-igas-animals/aquadvantage-salmon (accessed on 12 November 2024).
- Horváth, L.; Orbán, L. Genome and gene manipulation in the common carp. Aquaculture 1995, 129, 157–181. [Google Scholar] [CrossRef]
- Wu, G. Growth hormone gene transfer in common carp. Aquat. Living Resour. 2003, 16, 416–420. [Google Scholar] [CrossRef]
- Zhong, Z.; Niu, P.; Wang, M.; Huang, G.; Xu, S.; Sun, Y.; Xu, X.; Hou, Y.; Sun, X.; Yan, Y.; et al. Targeted disruption of sp7 and myostatin with CRISPR-Cas9 results in severe bone defects and more muscular cells in common carp. Sci. Rep. 2016, 6, 22953. [Google Scholar] [CrossRef]
- Aoki, M.; Wartenberg, P.; Grünewald, R.; Phillipps, H.R.; Wyatt, A.; Grattan, D.R.; Boehm, U. Widespread Cell-Specific Prolactin Receptor Expression in Multiple Murine Organs. Endocrinology 2019, 160, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Pozzebon, M.; Guldbrandtsen, B.; Sandøe, P. Gene Editing Cattle for Enhancing Heat Tolerance: A Welfare Review of the “PRLR-SLICK Cattle” Case. NanoEthics 2024, 18, 1–15. [Google Scholar] [CrossRef]
- Sosa, F.; Santos, J.E.P.; Rae, D.O.; Larson, C.C.; Macchietto, M.; Abrahante, J.E.; Amaral, T.F.; Denicol, A.C.; Sonstegard, T.S.; Hansen, P.J. Effects of the SLICK1 mutation in PRLR on regulation of core body temperature and global gene expression in liver in cattle. Animal 2022, 16, 100523. [Google Scholar] [CrossRef]
- Polsky, L.; von Keyserlingk, M.A.G. Invited review: Effects of heat stress on dairy cattle welfare. J. Dairy Sci. 2017, 100, 8645–8657. [Google Scholar] [CrossRef]
- Nardone, A.; Ronchi, B.; Lacetera, N.; Ranieri, M.S.; Bernabucci, U. Effects of climate changes on animal production and sustainability of livestock systems. Livest. Sci. 2010, 130, 57–69. [Google Scholar] [CrossRef]
- Pryce, J.E.; Haile-Mariam, M. Symposium review: Genomic selection for reducing environmental impact and adapting to climate change. J. Dairy Sci. 2020, 103, 5366–5375. [Google Scholar] [CrossRef]
- Hoffmann, I. Climate change and the characterization, breeding and conservation of animal genetic resources. Anim. Genet. 2010, 41, 32–46. [Google Scholar] [CrossRef]
- Contreras-Correa, Z.E.; Sánchez-Rodríguez, H.L.; Arick, M.A.; Muñiz-Colón, G.; Lemley, C.O. Thermotolerance capabilities, blood metabolomics, and mammary gland hemodynamics and transcriptomic profiles of slick-haired Holstein cattle during mid lactation in Puerto Rico. J. Dairy Sci. 2024, 107, 4017–4032. [Google Scholar] [CrossRef]
- Kim, W.-S.; Ghassemi Nejad, J.; Lee, H.-G. Impact of Cold Stress on Physiological, Endocrinological, Immunological, Metabolic, and Behavioral Changes of Beef Cattle at Different Stages of Growth. Animals 2023, 13, 1073. [Google Scholar] [CrossRef]
- Livestock Improvement Corporation. Slick Solutions: Research Advances in LIC’s Heat Tolerance Programme. 2023. Available online: https://www.lic.co.nz/news/slick-solutions-research-advances-in-lics-heat-tolerance-programme/ (accessed on 29 October 2024).
- Devlin, R.H.; Leggatt, R.A.; Benfey, T.J. Genetic modification of growth in fish species used in aquaculture: Phenotypic and physiological responses. Fish Physiol. 2020, 38, 237–272. [Google Scholar]
- Devlin, R.H.; Sundström, L.F.; Leggatt, R.A. Assessing Ecological and Evolutionary Consequences of Growth-Accelerated Genetically Engineered Fishes. BioScience 2015, 65, 685–700. [Google Scholar] [CrossRef]
- Sundt-Hansen, L.; Sundström, L.F.; Einum, S.; Hindar, K.; Fleming, I.A.; Devlin, R.H. Genetically enhanced growth causes increased mortality in hypoxic environments. Biol. Lett. 2007, 3, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tang, Z.; Liu, Z.; Shen, J.; Feng, H.; Xue, L. Transgenic red carp (Cyprinus carpio) with LcMSTN1 propeptide: Enhanced growth and unchanged muscle fat content. Aquaculture 2021, 541, 736795. [Google Scholar] [CrossRef]
- Fu, C.; Cui, Y.; Hung, S.S.O.; Zhu, Z. Growth and feed utilization by F 4 human growth hormone transgenic carp fed diets with different protein levels. J. Fish Biol. 1998, 53, 115–129. [Google Scholar] [CrossRef]
- Guo, W.; Fu, L.; Wu, Y.; Liu, H.; Yang, Y.; Hu, W.; Xie, S. Effects of dietary protein levels on growth and feed utilization in non-transgenic and growth-hormone-gene transgenic common carp (Cyprinus carpio L.). Aquac. Rep. 2021, 21, 100854. [Google Scholar] [CrossRef]
- Khalil, K.; Elayat, M.; Khalifa, E.; Daghash, S.; Elaswad, A.; Miller, M.; Abdelrahman, H.; Ye, Z.; Odin, R.; Drescher, D.; et al. Generation of Myostatin Gene-Edited Channel Catfish (Ictalurus punctatus) via Zygote Injection of CRISPR/Cas9 System. Sci. Rep. 2017, 7, 7301. [Google Scholar] [CrossRef]
- Duan, M.; Zhang, T.; Hu, W.; Sundström, L.F.; Wang, Y.; Li, Z.; Zhu, Z. Elevated ability to compete for limited food resources by ‘all-fish’ growth hormone transgenic common carp Cyprinus carpio. J. Fish Biol. 2009, 75, 1459–1472. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Wang, Y.-P.; Pei, D.-S.; Luo, D.-J.; Liao, L.-J.; Zhu, Z.-Y. A one-year investigation of the relationship between serum GH levels and the growth of F(4) transgenic and non-transgenic common carp Cyprinus carpio. J. Fish Biol. 2009, 75, 1092–1100. [Google Scholar] [CrossRef]
- Kishimoto, K.; Washio, Y.; Yoshiura, Y.; Toyoda, A.; Ueno, T.; Fukuyama, H.; Kato, K.; Kinoshita, M. Production of a breed of red sea bream Pagrus major with an increase of skeletal muscle mass and reduced body length by genome editing with CRISPR/Cas9. Aquaculture 2018, 495, 415–427. [Google Scholar] [CrossRef]
- Kim, J.; Cho, J.Y.; Kim, J.-W.; Kim, H.-C.; Noh, J.K.; Kim, Y.-O.; Hwang, H.-K.; Kim, W.-J.; Yeo, S.-Y.; An, C.M.; et al. CRISPR/Cas9-mediated myostatin disruption enhances muscle mass in the olive flounder Paralichthys olivaceus. Aquaculture 2019, 512, 734336. [Google Scholar] [CrossRef]
- Sun, Y.; Zheng, G.-D.; Nissa, M.; Chen, J.; Zou, S.-M. Disruption of mstna and mstnb gene through CRISPR/Cas9 leads to elevated muscle mass in blunt snout bream (Megalobrama amblycephala). Aquaculture 2020, 528, 735597. [Google Scholar] [CrossRef]
- Li, D.; Hu, W.; Wang, Y.; Zhu, Z.; Fu, C. Reduced swimming abilities in fast-growing transgenic common carp Cyprinus carpio associated with their morphological variations. J. Fish Biol. 2009, 74, 186–197. [Google Scholar] [CrossRef]
- Lee, S.J.; McPherron, A.C. Regulation of myostatin activity and muscle growth. Proc. Natl. Acad. Sci. USA 2001, 98, 9306–9311. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Davies, M.V.; Koniaris, L.G.; Haynes, P.; Esquela, A.F.; Tomkinson, K.N.; McPherron, A.C.; Wolfman, N.M.; Lee, S.-J. Induction of cachexia in mice by systemically administered myostatin. Science 2002, 296, 1486–1488. [Google Scholar] [CrossRef] [PubMed]
- Organisation for economic co-operation and development. Revised Consensus Document on Compositional Considerations for New Varieties of Soybean [Glycine max (L.) Merr]: And Allergens Environment Directorate: Key Food and Feed Nutrients, Anti-nutrients, Toxicants. ENV/JM/MONO(2012)24; OECD Environment, Health and Safety Publications No 25, 2012. Available online: https://one.oecd.org/document/env/jm/mono(2012)24/en/pdf (accessed on 18 November 2024).
- Organisation for economic co-operation and development. Consensus Document on the Biology of Atlantic Salmon (Salmo salar): ENV/JM/MONO(2017)64; Series on Harmonisation of Regulatory Oversight in Biotechnology No. 64, 2017. Available online: https://one.oecd.org/document/ENV/JM/MONO(2017)64/en/pdf (accessed on 14 November 2024).
- Cimadori, I.; Di Concetto, A.; Grieger, K. The Protection of Selectively Bred and Gene Edited Farm Animals under EU Law. Eur. J. Risk Regul. 2025, 1–17. [Google Scholar] [CrossRef]
- van Marle-Köster, E.; Visser, C. Unintended consequences of selection for increased production on the health and welfare of livestock. Arch. Anim. Breed. 2021, 64, 177–185. [Google Scholar] [CrossRef]
- EFSA Panel on Genetically Modified Organisms. Guidance on the environmental risk assessment of genetically modified plants. EFSA J. 2010, 8, 1879. [Google Scholar] [CrossRef]
- Bartz, R.; Heink, U.; Kowarik, I. Proposed definition of environmental damage illustrated by the cases of genetically modified crops and invasive species. Conserv. Biol. 2010, 24, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Campanati, C.; Willer, D.; Schubert, J.; Aldridge, D.C. Sustainable Intensification of Aquaculture through Nutrient Recycling and Circular Economies: More Fish, Less Waste, Blue Growth. Rev. Fish. Sci. Aquac. 2022, 30, 143–169. [Google Scholar] [CrossRef]
- Casimiro, A.C.R.; Garcia, D.A.Z.; Vidotto-Magnoni, A.P.; Britton, J.R.; Agostinho, A.A.; de Almeida, F.S.; Orsi, M.L. Escapes of non-native fish from flooded aquaculture facilities: The case of Paranapanema River, southern Brazil. Zoologia 2018, 35, 1–6. [Google Scholar] [CrossRef]
- Føre, H.M.; Thorvaldsen, T. Causal analysis of escape of Atlantic salmon and rainbow trout from Norwegian fish farms during 2010–2018. Aquaculture 2021, 532, 736002. [Google Scholar] [CrossRef]
- Bojarski, B.; Jakubiak, M.; Szczerbik, P.; Bień, M.; Klaczak, A.; Stański, T.; Witeska, M. The Influence of Fish Ponds on Fish Assemblages of Adjacent Watercourses. Pol. J. Environ. Stud. 2022, 31, 609–617. [Google Scholar] [CrossRef]
- Freyhof, J.; Kottelat, M. Cyprinus carpio: The IUCN Red List of Threatened Species. 2008. e.T6181A12559362. Available online: https://www.iucnredlist.org/species/6181/12559362 (accessed on 13 September 2025).
- Vitál, Z.; Józsa, V.; Specziár, A.; Mozsár, A.; Lehoczky, I.; Kovács, B.; Hliwa, P.; Boros, G. Source of bigheaded carp (Hypophthalmichthys spp.) in Lake Balaton, Hungary: Natural recruitment or continuous escapement from aquaculture? Inland Waters 2017, 7, 218–226. [Google Scholar] [CrossRef]
- Cowx, I.G. Characterisation of inland fisheries in Europe. Fish. Manag. Ecol. 2015, 22, 78–87. [Google Scholar] [CrossRef]
- Carpio, A.J.; de Miguel, R.J.; Oteros, J.; Hillström, L.; Tortosa, F.S. Angling as a source of non-native freshwater fish: A European review. Biol. Invasions 2019, 21, 3233–3248. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Mehner, T. Socio-economic characterisation of specialised common carp (Cyprinus carpio L.) anglers in Germany, and implications for inland fisheries management and eutrophication control. Fish. Res. 2003, 61, 19–33. [Google Scholar] [CrossRef]
- Specziár, A.; Turcsányi, B. Effect of stocking strategy on distribution and recapture rate of common carp Cyprinus carpio L., in a large and shallow temperate lake: Implications for recreational put-and-take fisheries management. J. Appl. Ichthyol. 2014, 30, 887–894. [Google Scholar] [CrossRef]
- Macklin, R.; Brazier, B.; Harrison, S.; Chapman, D.; Vilizzi, L. A review of the status and range expansion of common carp (Cyprinus carpio L.) in Ireland. AI 2016, 11, 75–82. [Google Scholar] [CrossRef]
- Kennedy, D.A.; Kurath, G.; Brito, I.L.; Purcell, M.K.; Read, A.F.; Winton, J.R.; Wargo, A.R. Potential drivers of virulence evolution in aquaculture. Evol. Appl. 2016, 9, 344–354. [Google Scholar] [CrossRef]
- Uchii, K.; Minamoto, T.; Honjo, M.N.; Kawabata, Z. Seasonal reactivation enables Cyprinid herpesvirus 3 to persist in a wild host population. FEMS Microbiol. Ecol. 2014, 87, 536–542. [Google Scholar] [CrossRef]
- Battaglini, L.; Bovolenta, S.; Gusmeroli, F.; Salvador, S.; Sturaro, E. Environmental Sustainability of Alpine Livestock Farms. Ital. J. Anim. Sci. 2014, 13, 3155. [Google Scholar] [CrossRef]
- Brade, W.; Flachowsky, G. (Eds.) Rinderzucht und Rindfleischerzeugung: Empfehlungen für Die Praxis; Bundesforschungsanst. für Landwirtschaft (FAL): Braunschweig, Germany, 2007; ISBN 978-3-86576-038-8. [Google Scholar]
- FAO. The State of the World’s Animal Genetic Resources for Food and Agriculture—In Brief; Food and Agriculture Organization of the United Nations: Rome, Italy, 2007; ISBN 978-92-5-105763-6. [Google Scholar]
- EFSA. Scientific Opinion on the overall effects of farming systems on dairy cow welfare and disease. EFSA J. 2009, 7, 1143. [Google Scholar] [CrossRef]
- Kuzma, J.; Grieger, K.; Cimadori, I.; Cummings, C.L.; Loschin, N.; Wei, W. Parameters, practices, and preferences for regulatory review of emerging biotechnology products in food and agriculture. Front. Bioeng. Biotechnol. 2023, 11, 1256388. [Google Scholar] [CrossRef]
- Squire, G.R.; Brooks, D.R.; Bohan, D.A.; Champion, G.T.; Daniels, R.E.; Haughton, A.J.; Hawes, C.; Heard, M.S.; Hill, M.O.; May, M.J.; et al. On the rationale and interpretation of the Farm Scale Evaluations of genetically modified herbicide-tolerant crops. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2003, 358, 1779–1799. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.N.; Rothery, P.; Clark, S.J.; Heard, M.S.; Hawes, C. Design, analysis and statistical power of the Farm-Scale Evaluations of genetically modified herbicide-tolerant crops. J. Appl. Ecol. 2003, 40, 17–31. [Google Scholar] [CrossRef]
- Copernicus Land Monitoring Service. Available online: https://land.copernicus.eu/en (accessed on 19 March 2025).
- European Commission. Farming for Natura 2000: Guidance on How to Support Natura 2000 Farming Systems to Achieve Conservation objectives, Based on Member States Good Practice Experiences; Publications Office of the European Union: Reims, Luxembourg, 2018; ISBN 978-92-79-95905-9. [Google Scholar]
- Hoppichler, J.; Blab, A.; Götz, B.; Novak, H.; Oberleitner, I.; Paar, M.; Schwarzl, B.; Zehtner, G. Biodiversität im Alpengebiet: Evaluation und Bewertung. OECD Fallstudie. 2002. Available online: https://bab.gv.at/jdownloads/Publikationen/Archiv/BABF/Forschungsberichte/fb48.pdf (accessed on 30 August 2023).
- Ellmauer, T.; Kudrnovsky, H.; Schwarzl, B.; Weiss, M. GAP Post-2020 Wirkungsindikator I19A: Lebensräume von Gemeinschaftlichem Interesse, die von der Landwirtschaft Abhängig Sind, mit Stabilen Oder Zunehmenden Trends. 2020. Available online: www.umweltbundesamt.at (accessed on 30 August 2023).
- Hiemstra, S.J.; de Haas, Y.; Mäkit-Tanila, A.; Gandini, G. Local Cattle Breeds in Europe: Development of Policies and Strategies for Self-Sustaining Breeds; Hiemstra, S.J., Ed.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2010; ISBN 978-90-8686-697-7. [Google Scholar]
- European Regional Focal Point for Animal Genetic Resources. Animal Genetic Resources Strategy for Europe. 2022. Available online: https://www.animalgeneticresources.net/wp-content/uploads/2018/01/Final_AnGR-Strategy-_022022.pdf (accessed on 18 March 2025).
- Horváth, L.; Kovács, É.; Csorbai, B.; Hegyi, Á.; Lefler, K.; Müller, T.; Urbányi, B. Carp Breeding in the Carpathian Basin with a Sustainable Utilization of Renewable Natural Resources. Life 2022, 12, 1661. [Google Scholar] [CrossRef]
- Popp, J.; Békefi, E.; Duleba, S.; Oláh, J. Multifunctionality of pond fish farms in the opinion of the farm managers: The case of Hungary. Rev. Aquacult 2019, 11, 830–847. [Google Scholar] [CrossRef]
- Turkowski, K. Fish Farmers’ Perception of Ecosystem Services and Diversification of Carp Pond Aquaculture: A Case Study from Warmia and Mazury, Poland. Sustainability 2021, 13, 2797. [Google Scholar] [CrossRef]
- Kohlmann, K.; Kersten, P.; Flajšhans, M. Microsatellite-based genetic variability and differentiation of domesticated, wild and feral common carp (Cyprinus carpio L.) populations. Aquaculture 2005, 247, 253–266. [Google Scholar] [CrossRef]
- Klich, D.; Didkowska, A.; Pyziel-Serafin, A.M.; Perlińska-Teresiak, M.; Wołoszyn-Gałęza, A.; Żoch, K.; Balcerak, M.; Olech, W. Contact between European bison and cattle from the cattle breeders’ perspective, in the light of the risk of pathogen transmission. PLoS ONE 2023, 18, e0285245. [Google Scholar] [CrossRef]
- Moreau, D.T.R. Ecological risk analysis and genetically modified salmon: Management in the face of uncertainty. Annu. Rev. Anim. Biosci. 2014, 2, 515–533. [Google Scholar] [CrossRef]
- Cowx, I.G.; Bolland, J.D.; Nunn, A.D.; Kerins, G.; Stein, J.; Blackburn, J.; Hart, A.; Henry, C.; Britton, J.R.; Coop, G.; et al. Defining environmental risk assessment criteria for genetically modified fishes to be placed on the EU market. Extern. Sci. Rep. 2010, 7, 264. [Google Scholar] [CrossRef]
- Devos, Y.; Craig, W.; Devlin, R.H.; Ippolito, A.; Leggatt, R.A.; Romeis, J.; Shaw, R.; Svendsen, C.; Topping, C.J. Using problem formulation for fit-for-purpose pre-market environmental risk assessments of regulated stressors. EFS2 J. 2019, 17, e170708. [Google Scholar] [CrossRef]
- Norwegian Scientific Committee for Food and Environment. Environmental Risk Assessment of Genetically Modified Sterile VIRGIN Atlantic Salmon for Use in Research Trials in Aquaculture Sea-Cages: Scientific Opinion; VKM Report No. 20, 2023. Available online: https://vkm.no/english/riskassessments/allpublications/geneticallymodifiedsterilesalmonriskassessmentoffieldtrials.4.49914e7a18a5261030860bee.html (accessed on 12 November 2024).
- Stanković, D.; Crivelli, A.J.; Snoj, A. Rainbow Trout in Europe: Introduction, Naturalization, and Impacts. Rev. Fish. Sci. Aquac. 2015, 23, 39–71. [Google Scholar] [CrossRef]
- US Department of Agriculture. Invasive Carp: National Invasive Species Information Center. 2023. Available online: https://www.invasivespeciesinfo.gov/aquatic/fish-and-other-vertebrates/invasive-carp (accessed on 23 November 2023).
- Wolfe, M.D.; Santucci, V.J.; Einfalt, L.M.; Wahl, D.H. Effects of Common Carp on Reproduction, Growth, and Survival of Largemouth Bass and Bluegills. Trans Am. Fish Soc. 2009, 138, 975–983. [Google Scholar] [CrossRef]
- Adámek, Z.; Anton Pardo, M.; Vilizzi, L.; Roberts, J. Successful reproduction of common carp Cyprinus carpio in irrigation waterways. Fish. Manag. Ecol. 2015, 22, 279–285. [Google Scholar] [CrossRef]
- Piczak, M.L.; Bzonek, P.A.; Pratt, T.C.; Sorensen, P.W.; Stuart, I.G.; Theÿsmeÿer, T.; Mandrak, N.E.; Midwood, J.D.; Cooke, S.J. Controlling common carp (Cyprinus carpio): Barriers, biological traits, and selective fragmentation. Biol. Invasions 2023, 25, 1317–1338. [Google Scholar] [CrossRef]
- Kohlmann, K.; Gross, R.; Muakaeva, A.; Kersten, P. Genetic variability and structure of common carp (Cyprinus carpio) populations throughout the distribution range inferred from allozyme, microsatellite and mitochondrial DNA markers. Aquat. Living Resour. 2003, 16, 421–431. [Google Scholar] [CrossRef]
- Naylor, R.; Hindar, K.; Fleming, I.; Goldburg, R.; Williams, S.; Volpe, J.; Whoriskey, F.; Eagle, J.; Kelso, D.; Mangel, M. Fugitive Salmon: Assessing the Risks of Escaped Fish from Net-Pen Aquaculture. BioScience 2005, 55, 427. [Google Scholar] [CrossRef]
- Hänfling, B.; Bolton, P.; Harley, M.; Carvalho, G.R. A molecular approach to detect hybridisation between crucian carp (Carassius carassius) and non-indigenous carp species (Carassius spp. and Cyprinus carpio). Freshw. Biol. 2005, 50, 403–417. [Google Scholar] [CrossRef]
- Šimková, A.; Vojtek, L.; Halačka, K.; Hyršl, P.; Vetešník, L. The effect of hybridization on fish physiology, immunity and blood biochemistry: A case study in hybridizing Cyprinus carpio and Carassius gibelio (Cyprinidae). Aquaculture 2015, 435, 381–389. [Google Scholar] [CrossRef]
- Tichopád, T.; Vetešník, L.; Šimková, A.; Rodina, M.; Franěk, R.; Pšenička, M. Spermatozoa morphology and reproductive potential in F1 hybrids of common carp (Cyprinus carpio) and gibel carp (Carassius gibelio). Aquaculture 2020, 521, 735092. [Google Scholar] [CrossRef]
- Tapkir, S.; Boukal, D.; Kalous, L.; Bartoň, D.; Souza, A.T.; Kolar, V.; Soukalová, K.; Duchet, C.; Gottwald, M.; Šmejkal, M. Invasive gibel carp (Carassius gibelio) outperforms threatened native crucian carp (Carassius carassius) in growth rate and effectiveness of resource use: Field and experimental evidence. Aquat. Conserv. 2022, 32, 1901–1912. [Google Scholar] [CrossRef]
- Ahti, P.A.; Kuparinen, A.; Uusi-Heikkilä, S. Size does matter—The eco-evolutionary effects of changing body size in fish. Environ. Rev. 2020, 28, 311–324. [Google Scholar] [CrossRef]
- Lian, H.; Hu, W.; Huang, R.; Du, F.; Liao, L.; Zhu, Z.; Wang, Y. Transgenic common carp do not have the ability to expand populations. PLoS ONE 2013, 8, e65506. [Google Scholar] [CrossRef]
- Cucherousset, J.; Sundt-Hansen, L.E.; Buoro, M.; Závorka, L.; Lassus, R.; Baekkelie, K.A.E.; Fleming, I.A.; Björnsson, B.T.; Johnsson, J.I.; Hindar, K. Growth-enhanced salmon modify stream ecosystem functioning. J. Fish Biol. 2021, 99, 1978–1989. [Google Scholar] [CrossRef]
- Maceda-Veiga, A.; López, R.; Green, A.J. Dramatic impact of alien carp Cyprinus carpio on globally threatened diving ducks and other waterbirds in Mediterranean shallow lakes. Biol. Conserv. 2017, 212, 74–85. [Google Scholar] [CrossRef]
- Huser, B.J.; Bajer, P.G.; Kittelson, S.; Christenson, S.; Menken, K. Changes to water quality and sediment phosphorus forms in a shallow, eutrophic lake after removal of common carp (Cyprinus carpio). Inland Waters 2022, 12, 33–46. [Google Scholar] [CrossRef]
- Chu, P.; Agapito-Tenfen, S.Z. Unintended Genomic Outcomes in Current and Next Generation GM Techniques: A Systematic Review. Plants 2022, 11, 2997. [Google Scholar] [CrossRef]
- Norris, A.L.; Lee, S.S.; Greenlees, K.J.; Tadesse, D.A.; Miller, M.F.; Lombardi, H.A. Template plasmid integration in germline genome-edited cattle. Nat. Biotechnol. 2020, 38, 163–164. [Google Scholar] [CrossRef] [PubMed]
- Schönhart, M. Heat stress impacts on cows in a case study landscape measured by an integrated modelling framework. Adv. Anim. Biosci. 2016, 7, 235–237. [Google Scholar] [CrossRef]
- Zentrich, E.; Iwersen, M.; Wiedrich, M.-C.; Drillich, M.; Klein-Jöbstl, D. Short communication: Effect of barn climate and management-related factors on bovine colostrum quality. J. Dairy Sci. 2019, 102, 7453–7458. [Google Scholar] [CrossRef]
- Nordlund, K.V.; Strassburg, P.; Bennett, T.B.; Oetzel, G.R.; Cook, N.B. Thermodynamics of standing and lying behavior in lactating dairy cows in freestall and parlor holding pens during conditions of heat stress. J. Dairy Sci. 2019, 102, 6495–6507. [Google Scholar] [CrossRef] [PubMed]
- Hempel, S.; Menz, C.; Pinto, S.; Galán, E.; Janke, D.; Estellés, F.; Müschner-Siemens, T.; Wang, X.; Heinicke, J.; Zhang, G.; et al. Heat stress risk in European dairy cattle husbandry under different climate change scenarios—Uncertainties and potential impacts. Earth Syst. Dynam. Discuss. 2019, 10, 859–884. [Google Scholar]
- Thornton, P.; Nelson, G.; Mayberry, D.; Herrero, M. Impacts of heat stress on global cattle production during the 21st century: A modelling study. Lancet Planet. Health 2022, 6, e192–e201. [Google Scholar] [CrossRef]
- Herzog, A.; Winckler, C.; Hörtenhuber, S.; Zollitsch, W. Environmental impacts of implementing basket fans for heat abatement in dairy farms. Animal 2021, 15, 100274. [Google Scholar] [CrossRef] [PubMed]
- Eckerstorfer, M.F.; Engelhard, M.; Heissenberger, A.; Simon, S.; Teichmann, H. Plants Developed by New Genetic Modification Techniques-Comparison of Existing Regulatory Frameworks in the EU and Non-EU Countries. Front. Bioeng. Biotechnol. 2019, 7, 26. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- Kjeldaas, S.; Dassler, T.; Antonsen, T.; Wikmark, O.-G.; Myhr, A.I. With great power comes great responsibility: Why ‘safe enough’ is not good enough in debates on new gene technologies. Agric. Hum. Values 2023, 40, 533–545. [Google Scholar] [CrossRef]
- Blix, T.B.; Myhr, A.I. A sustainability assessment framework for genome-edited salmon. Aquaculture 2023, 562, 738803. [Google Scholar] [CrossRef]
- Eckerstorfer, M.F.; Dolezel, M.; Miklau, M.; Greiter, A.; Heissenberger, A.; Kastenhofer, K.; Schulz, F.; Hagen, K.; Otto, M.; Engelhard, M. Environmental Applications of GM Microorganisms: Tiny Critters Posing Huge Challenges for Risk Assessment and Governance. Int. J. Mol. Sci. 2025, 26, 3174. [Google Scholar] [CrossRef] [PubMed]
- Beltrán, J.P.; Berbel, J.; Berdaji, I.; Bernabéu, R.; Boix Fayos, C.; Clotet Ballús, R.; Colomer Xena, Y.; Del Castillo Bilbao, M.D.; Flotats Ripoll, X.; Gil, J.C.; et al. The Impact of the European Green Deal from a Sustainable Global Food System Approach. Eur. Food Feed. Law Rev. 2022, 17, 2–38. [Google Scholar]
- Hallerman, E.; Bredlau, J.; Camargo, L.S.A.; Dagli, M.L.Z.; Karembu, M.; Kovich, D.; Muia, A.N.; Murrone, M.L.; Rocha-Salavarrieta, P.J.; Romero-Aldemita, R.; et al. Enabling regulatory policy globally will promote realization of the potential of animal biotechnology. CABI Agric. Biosci. 2024, 5, 1–28. [Google Scholar] [CrossRef]
- Dikmen, S.; Alava, E.; Pontes, E.; Fear, J.M.; Dikmen, B.Y.; Olson, T.A.; Hansen, P.J. Differences in Thermoregulatory Ability Between Slick-Haired and Wild-Type Lactating Holstein Cows in Response to Acute Heat Stress. J. Dairy Sci. 2008, 91, 3395–3402. [Google Scholar] [CrossRef]
- Rojas-Downing, M.M.; Nejadhashemi, A.P.; Harrigan, T.; Woznicki, S.A. Climate change and livestock: Impacts, adaptation, and mitigation. Clim. Risk Manag. 2017, 16, 145–163. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. SAFA (Sustainability Assessment of Food and Agricultural Systems) Guidelines; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2014; Available online: https://openknowledge.fao.org/items/84c84661-7172-415c-b66e-7c1eee5db675 (accessed on 13 September 2025).
- Purvis, B.; Mao, Y.; Robinson, D. Three pillars of sustainability: In search of conceptual origins. Sustain. Sci. 2019, 14, 681–695. [Google Scholar] [CrossRef]
- Bock, A.; Bontoux, L.; Rudkin, J. Concepts for a Sustainable EU Food System: Reflections from a Participary Process; Publications Office of the European Union: Luxembourg, 2022; Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC126575 (accessed on 13 September 2025).
- Alaoui, A.; Barão, L.; Ferreira, C.S.S.; Hessel, R. An Overview of Sustainability Assessment Frameworks in Agriculture. Land 2022, 11, 537. [Google Scholar] [CrossRef]
- El Hage, N. Guidelines for Sustainability Assessment in Food and Agriculture; Food and Agriculture Organization (FAO): Rome, Italy, 2012; Available online: https://orgprints.org/21169/ (accessed on 4 September 2025).
- Paçarada, R.; Hörtenhuber, S.; Hemme, T.; Wurzinger, M.; Zollitsch, W. Sustainability Assessment Tools for Dairy Supply Chains: A Typology. Sustainability 2024, 16, 4999. [Google Scholar] [CrossRef]
- Haque, M.M.; Alam, M.M.; Hoque, M.S.; Hasan, N.A.; Nielsen, M.; Hossain, M.I.; Frederiksen, M. Can Bangladeshi pangasius farmers comply with the requirements of aquaculture certification? Aquac. Rep. 2021, 21, 100811. [Google Scholar] [CrossRef]
- Kirchner, M.; Pölz, W.; Mayrhofer, H.; Hickersberger, M.; Sinabell, F. Carbon footprint of Austrian beef in an international context. Austrian J. Agric. Econ. Rural. Stud. 2023, 32, 20–26. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Abbott, J.K.; Fenichel, E.P.; Carpenter, S.R.; Hunt, L.M.; Alós, J.; Klefoth, T.; Cooke, S.J.; Hilborn, R.; Jensen, O.P.; et al. Opinion: Governing the recreational dimension of global fisheries. Proc. Natl. Acad. Sci. USA 2019, 116, 5209–5213. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Tillner, R.; Bork, M. Explaining participation rates in recreational fishing across industrialised countries. Fish. Manag. Ecol. 2015, 22, 45–55. [Google Scholar] [CrossRef]
- Arlinghaus, R. Catch uncertainty and recreational fishing attraction: Propositions and future research directions. Fish Fish. 2024, 25, 761–780. [Google Scholar] [CrossRef]
- Matern, S.; Radinger, J.; Klefoth, T.; Wolter, C.; Arlinghaus, R. Replicated whole-lake experiment reveals the ineffectiveness of stocking five example fish species in small lakes. Fish. Manag. Ecol. 2024, 32, 16. [Google Scholar] [CrossRef]
- Hörtenhuber, S.; Kasperczyk, N.; Ruckli, A.; Leeb, C.; Dippel, S. Deliverable 4.5 Report on SusPigSys toolbox for integrative system analysis. ResearchGate 2021, 29. [Google Scholar] [CrossRef]
- Ruckli, A.K.; Hörtenhuber, S.J.; Ferrari, P.; Guy, J.; Helmerichs, J.; Hoste, R.; Hubbard, C.; Kasperczyk, N.; Leeb, C.; Malak-Rawlikowska, A.; et al. Integrative Sustainability Analysis of European Pig Farms: Development of a Multi-Criteria Assessment Tool. Sustainability 2022, 14, 5988. [Google Scholar] [CrossRef]
- Guzmán-Luna, P.; Mauricio-Iglesias, M.; Flysjö, A.; Hospido, A. Analysing the interaction between the dairy sector and climate change from a life cycle perspective: A review. Trends Food Sci. Technol. 2022, 126, 168–179. [Google Scholar] [CrossRef]
- Noack, F.; Engist, D.; Gantois, J.; Gaur, V.; Hyjazie, B.F.; Larsen, A.; M’Gonigle, L.K.; Missirian, A.; Qaim, M.; Sargent, R.D.; et al. Environmental impacts of genetically modified crops. Science 2024, 385, eado9340. [Google Scholar] [CrossRef] [PubMed]
- Grunwald, A. Technology Assessment in Practice and Theory; Routledge: Oxfordshire, UK, 2019; ISBN 9781138337084. [Google Scholar]
- Kastenhofer, K. Debating the risks and ethics of emerging technosciences. Innov. Eur. J. Soc. Sci. Res. 2009, 22, 77–103. [Google Scholar] [CrossRef]
- Bosley, K.S.; Botchan, M.; Bredenoord, A.L.; Carroll, D.; Charo, R.A.; Charpentier, E.; Cohen, R.; Corn, J.; Doudna, J.; Feng, G.; et al. CRISPR germline engineering--the community speaks. Nat. Biotechnol. 2015, 33, 478–486. [Google Scholar] [CrossRef]
- Martani, A. Changing the regulation of human germline genome editing: What does a truly broad societal debate entail? Law Innov. Technol. 2024, 16, 687–714. [Google Scholar] [CrossRef]
- Lang, A.; Spök, A.; Gruber, M.-C.; Harrer, D.; Hammer, C.; Winkler, F.; Kaelin, L.; Hönigmayer, H.; Sommer, A.; Wuketich, M.; et al. (Eds.) Genome Editing: Interdisziplinäre Technikfolgenabschätzung; vdf Hochschulverlag AG an der ETH Zürich: Zollikon, Switzerland, 2019; ISBN 978-3-7281-3981-8. [Google Scholar]
- Rapley, D.; Wentworth, J. Genome Edited Animals (POSTbrief 50); UK Parliament’s Parliamentary Office of Science and Technology (POST): London, UK, 2022. [Google Scholar]
- Hammer, C.; Spök, A. Genome Editing in der Tierzucht. In Genome Editing—Interdisziplinäre Technikfolgenabschätzung; Lang, A., Spök, A., Gruber, M., Harrer, D., Hammer, C., Winkler, F., Kaelin, L., Hönigmayer, H., Eds.; vdf Hochschulverlag an der ETH Zürich: Zürich, Switzerland, 2019; pp. 219–237. [Google Scholar]
- Wright, W.; Tworek, H.J.S.; von Keyserlingk, M.A.G.; Koralesky, K.E.; Weary, D.M. Using animal history to inform current debates in gene editing farm animals: A systematic review. Front. Sustain. Food Syst. 2022, 6, 938085. [Google Scholar] [CrossRef]
- Harrison, R.; Dawkins, M.S. Animal Machines; CABI: Wallingford, UK, 2013; ISBN 978-1-78064-284-0. [Google Scholar]
- Kleter, G.; Sturme, M. Position Paper on Genome Editing: Potential, Expected and Socially Accepted Goals (Deliverable 5.1): RUMIGEN—Towards IMPROVEMENT of Ruminant Breeding Through Genomic and Epigenomic Approaches, H2020 Research and Innovation Action (Grant Agreement Number 101000226). 2022. Available online: https://ec.europa.eu/research/participants/documents/downloadPublic?documentIds=080166e5ecde2e64&appId=PPGMS (accessed on 4 June 2025).
- Mattalia, S.; Carabano, M.; Vinet, A.; Vandenplas, J. What Is the Expected Impact of Global Warming on the Genetic Abilities of Dairy Cows? Available online: https://rumigen.eu/wp-content/uploads/2023/11/PA_RUMIGEN_WP3_updated_compressed.pdf (accessed on 13 September 2025).
- Fischer, A.R.H.; Holstener, S.; Mikkelsen, R.B.; Klüver, L. Room of Acceptance Ex-Ante. Deliverable 2.1. 2022. Available online: https://rumigen.eu/wp-content/uploads/2023/02/RUMIGEN-D2.1-Room-of-acceptance-ex-ante.pdf (accessed on 13 September 2025).
- Dolezel, M.; Lang, A.; Greiter, A.; Miklau, M.; Eckerstorfer, M.; Heissenberger, A.; Willée, E.; Züghart, W. Challenges for the Post-Market Environmental Monitoring in the European Union Imposed by Novel Applications of Genetically Modified and Genome-Edited Organisms. BioTech 2024, 13, 14. [Google Scholar] [CrossRef] [PubMed]
- European Commission. Regulation of the European Parliament and of the Council on Plants Obtained by Certain New Genomic Techniques and Their Food and Feed, and Amending Regulation (EU) 2017/625 COM(2023) 411 Final, Brussels. 2023. Available online: https://food.ec.europa.eu/plants/genetically-modified-organisms/new-techniques-biotechnology_en (accessed on 11 September 2024).
- Dassler, T.; Myhr, A.I.; Lalyer, C.R.; Frieß, J.L.; Spök, A.; Liebert, W.; Hagen, K.; Engelhard, M.; Giese, B. Structured analysis of broader GMO impacts inspired by technology assessment to inform policy decisions. Agric. Hum. Values 2023, 41, 449–458. [Google Scholar] [CrossRef]
| No. | Specific Areas of Risks (According to the EFSA [11]) | Assessment for Slick-Haired Cattle | Assessment for Growth-Enhanced Carp |
|---|---|---|---|
| 1 | Persistence/invasiveness/gene transfer to wild and feral relatives. | Likelihood for hybridization of GM cattle with European bison is low as husbandry and breeding are largely under human control. | Hybridization of GM carp with wild carp populations as well as invasive cyprinids; Gene transfer of GM trait to offspring. |
| 2 | Horizontal gene transfer (due to introduction of recombinant DNA). | Not relevant as no recombinant DNA. | Not relevant as no recombinant DNA. |
| 3 | Potential changes in the susceptibility of GMA to pathogens, infections, or diseases. | Changes in the susceptibility of GM cattle to pathogens, infections or diseases. | Altered interactions between the GM carp and pathogens, infections and diseases. |
| 4 | Interaction with target organisms. | Not relevant as no target organism. | No risk area for GM carp. |
| 5 | Interaction with NTO (impacts on biotic components and processes). | Effects on different functional groups of NTOs of GM cattle (e.g., gut micro-organisms and endosymbionts). | Effects of GM carp on organisms, ecosystems, and biodiversity, e.g., through changes in consumption, predation, competition, due to hybridization, or through habitat alteration. |
| 6 | Interactions with the abiotic environment. | Altered emission of greenhouse gases or pollutants (e.g., ammonia and nitrate). | Altered tolerance to abiotic factors (e.g., oxygen) and effects on the abiotic environment (e.g., physical or chemical characteristics of the habitat). |
| 7 | Environmental impacts of the specific techniques used for management of the GMA. | Changes in husbandry and management or intended use of the GMA in novel environments. | Changes in the production system (incl. specific management practices) of GM carp, such as changes in fish diet and feeding due to specific requirements of GM carp. Changes in excretion due to changed protein metabolism. |
| 8 | Impacts of the GMA on non-GM animal health and welfare. | Changes in husbandry and management of GM cattle and their effects on non-GM cattle if raised together. | No risk area for GM carp. |
| 9 | Impacts on animal and human health. | Risks to farmers and workers; food safety of products from the GMA (e.g., due to differences in animal behavior and food quality) | |
| PS Aspects | A1 High Intensity Dairy PS | A2 Moderate Intensity Dairy PS | B1 High Intensity Beef PS | B2 Moderate Intensity Beef PS | B3 Suckler Cow PS |
|---|---|---|---|---|---|
| Typical housing systems | Confined all year round, mainly loose-house systems *; typically without pasture | Partially confined, mainly loose-house systems **, typically with pasture (summer half-year) | Confined all year round, mainly loose-house systems with slatted floor; without pasture | Partially confined, loose-house systems; typically with pasture (summer half-year) | Partially confined, mainly loose-house systems; typically with extensive pasturing (summer half-year) |
| Level of performance | >7000 kg milk per cow and year | <7000 kg milk per cow and year | >1000 g body mass gain per day | <1000 g body mass gain per day | <1000 g body mass gain per day |
| Feeding (type of diet) | TMR, maize and grass silage, concentrates, hay | maize and grass silages (partially TMR), pasture, hay, concentrates | TMR, maize and grass silage, concentrate, hay | maize and grass silages, pasture, concentrate, hay | maize and grass silages, pasture, hay, concentrate |
| Sustainability Dimension * | Sustainability Themes * | GM Slick-Haired Cattle | GM Growth-Enhanced Carp |
|---|---|---|---|
| Ecological integrity | Atmosphere | GHG emissions, air pollutants | GHG emissions |
| Water | Water withdrawal, water quality | Water quality | |
| Soil | Soil quality, land degradation | Soil quality, land degradation (feed- and pond-related) | |
| Biodiversity | Ecosystem, species and genetic diversity (e.g., more genetically uniform breeds) | Ecosystem, species and genetic diversity (e.g., preservation of wild stocks) | |
| Material and Energy | Feed and energy use, waste reduction | Feed and energy use, waste reduction | |
| Economic resilience and efficiency | Production efficiency | Economic viability, volume of production | Economic viability, volume of production |
| Economic resilience | (Stability of) net income Costs of production | Costs, increased independence from imported fish products, viability of non-GM carp production | |
| Social sustainability | Decent Livelihood | Job creation, farm income | Job creation, farm income |
| Fair trading practices | tbd | tbd | |
| Equality, non-discrimination, gender equality, vulnerable groups | Freedom of choice for non GM products | Freedom of choice for non GM products | |
| Human health and safety | Nutrition | Nutrition | |
| Good governance | Compliance with climate and agricultural policies | Compliance with policies for sustainable aquaculture | |
| Absence of hunger and thirst | Impact on food security | Impact on food security | |
| Animal Health and Welfare | Comfort | Thermal, physical, (when resting and during locomotion) | Potential occurrence of hunger in GM fish; stocking density |
| Good human–animal relationship | Human–animal relationship | tbd |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dolezel, M.; Eckerstorfer, M.F.; Miklau, M.; Greiter, A.; Heissenberger, A.; Hörtenhuber, S.; Burn, S.-J.; Zollitsch, W.; Kastenhofer, K.; Hagen, K.; et al. Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns. Animals 2025, 15, 2731. https://doi.org/10.3390/ani15182731
Dolezel M, Eckerstorfer MF, Miklau M, Greiter A, Heissenberger A, Hörtenhuber S, Burn S-J, Zollitsch W, Kastenhofer K, Hagen K, et al. Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns. Animals. 2025; 15(18):2731. https://doi.org/10.3390/ani15182731
Chicago/Turabian StyleDolezel, Marion, Michael F. Eckerstorfer, Marianne Miklau, Anita Greiter, Andreas Heissenberger, Stefan Hörtenhuber, Sarah-Joe Burn, Werner Zollitsch, Karen Kastenhofer, Kristin Hagen, and et al. 2025. "Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns" Animals 15, no. 18: 2731. https://doi.org/10.3390/ani15182731
APA StyleDolezel, M., Eckerstorfer, M. F., Miklau, M., Greiter, A., Heissenberger, A., Hörtenhuber, S., Burn, S.-J., Zollitsch, W., Kastenhofer, K., Hagen, K., & Engelhard, M. (2025). Governance Perspectives on Genetically Modified Animals for Agriculture and Aquaculture: Challenges for the Assessment of Environmental Risks and Broader Societal Concerns. Animals, 15(18), 2731. https://doi.org/10.3390/ani15182731