Traumatic Oral Lesions in Loggerhead Sea Turtles (Caretta caretta) Linked to Polychaete (Laetmonice cf. hystrix) Ingestion: A Case Report from the Northern Adriatic Sea
Simple Summary
Abstract
1. Introduction
2. Case Presentation
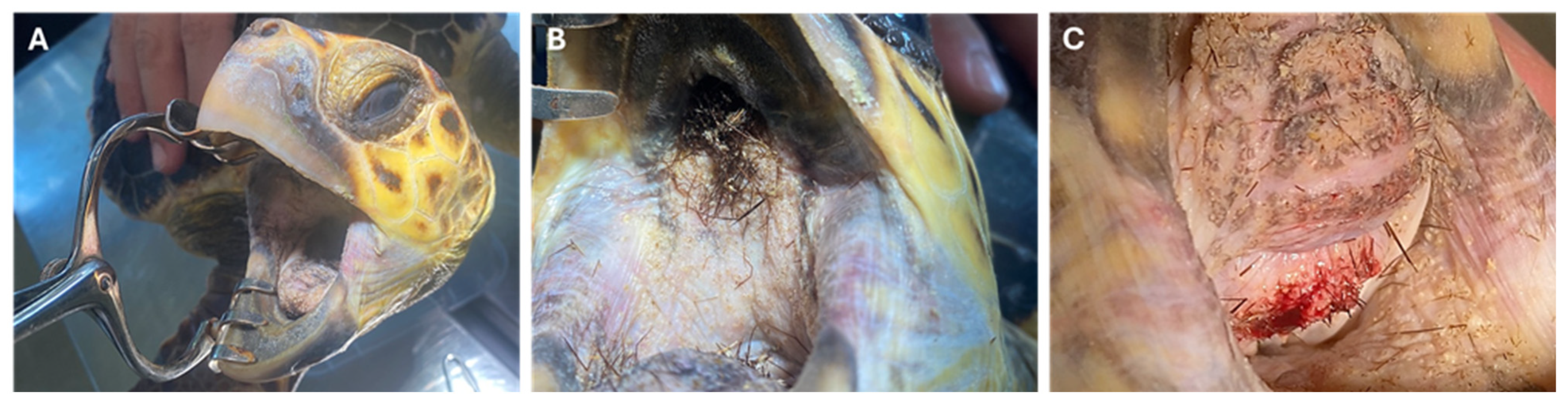
2.1. Rescue and Clinical Findings
2.2. Diagnostic Tests
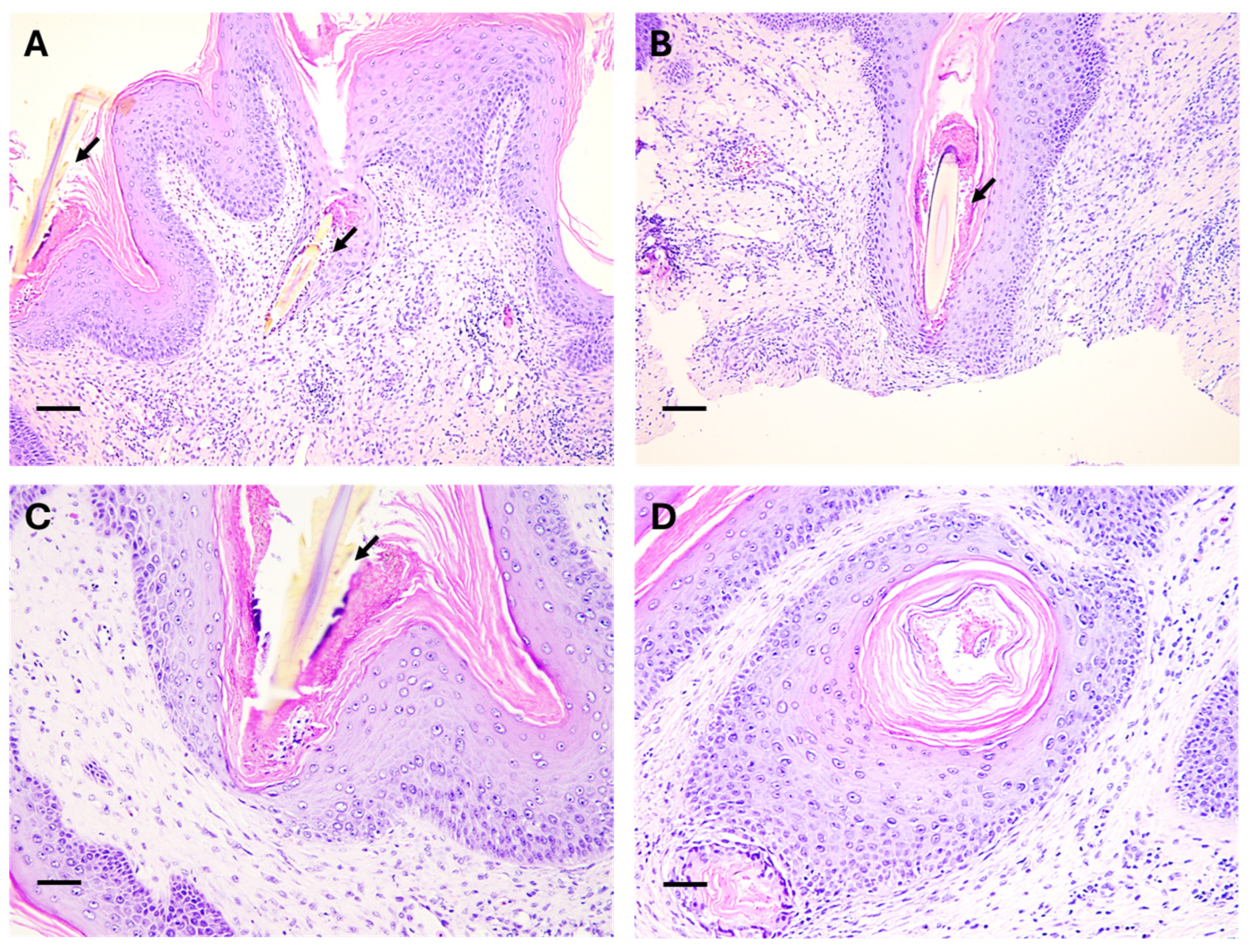
2.3. Identification of Foreign Bodies and Histological Analysis
2.4. Treatments and Outcome
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCL | Straight carapace length |
| SCW | Straight carapace width |
| HE | Haematoxylin Eosin |
| IM | Intra-Muscular |
References
- Casale, P. The IUCN Red List of Threatened Species: Caretta caretta Mediterranean Subpopulation. 2015. Available online: https://www.iucnredlist.org/species/83644804/83646294 (accessed on 4 July 2025).
- Zampollo, A.; Arcangeli, A.; Costantino, M.; Mancino, C.; Crosti, R.; Pietroluongo, G.; Giacoma, C.; Azzolin, M. Seasonal Niche and Spatial Distribution Modelling of the Loggerhead (Caretta caretta) in the Adriatic and Ionian Seas. Aquat. Conserv. Mar. Freshw. Ecosyst. 2022, 32, 1141–1155. [Google Scholar] [CrossRef]
- Burke, V.J.; Standora, E.A.; Morreale, S.J. Diet of Juvenile Kemp’s Ridley and Loggerhead Sea Turtles from Long Island, New York. Copeia 1993, 1993, 1176–1180. [Google Scholar] [CrossRef]
- Casale, P.; Broderick, A.; Camiñas, J.; Cardona, L.; Carreras, C.; Demetropoulos, A.; Fuller, W.; Godley, B.; Hochscheid, S.; Kaska, Y.; et al. Mediterranean Sea Turtles: Current Knowledge and Priorities for Conservation and Research. Endanger. Species Res. 2018, 36, 229–267. [Google Scholar] [CrossRef]
- Tomas, J.; Aznar, F.J.; Raga, J.A. Feeding Ecology of the Loggerhead Turtle Caretta caretta in the Western Mediterranean. J. Zool. 2001, 255, 525–532. [Google Scholar] [CrossRef]
- Revelles, M.; Cardona, L.; Aguilar, A.; Fernández, G. The Diet of Pelagic Loggerhead Sea Turtles (Caretta caretta) off the Balearic Archipelago (Western Mediterranean): Relevance of Long-Line Baits. J. Mar. Biol. Assoc. UK 2007, 87, 805–813. [Google Scholar] [CrossRef]
- Mariani, G.; Bellucci, F.; Cocumelli, C.; Raso, C.; Hochscheid, S.; Roncari, C.; Nerone, E.; Recchi, S.; Di Giacinto, F.; Olivieri, V.; et al. Dietary Preferences of Loggerhead Sea Turtles (Caretta caretta) in Two Mediterranean Feeding Grounds: Does Prey Selection Change with Habitat Use throughout Their Life Cycle? Animals 2023, 13, 654. [Google Scholar] [CrossRef]
- Glazebrook, J.S.; Campbell, R.S.F. A Survey of the Diseases of Marine Turtles in Northern Australia. I. Farmed Turtles. Dis. Aquat. Org. 1990, 9, 83–95. [Google Scholar] [CrossRef]
- Glazebrook, J.S.; Campbell, R.S.F. A Survey of the Diseases of Marine Turtles in Northern Australia. II. Oceanarium-Reareo and Wild Turtles. Dis. Aquat. Org. 1990, 9, 97–104. [Google Scholar] [CrossRef]
- Orós, J.; Torrent, A.; Calabuig, P.; Déniz, S. Diseases and Causes of Mortality among Sea Turtles Stranded in the Canary Islands, Spain (1998–2001). Dis. Aquat. Org. 2005, 63, 13–24. [Google Scholar] [CrossRef]
- Orós, J.; Déniz, S.; Calabuig, P. Digestive Pathology of Sea Turtles Stranded in the Canary Islands between 1993 and 2001. Vet. Rec. 2004, 155, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Orós, J.; Montesdeoca, N.; Camacho, M.; Arencibia, A.; Calabuig, P. Causes of Stranding and Mortality, and Final Disposition of Loggerhead Sea Turtles (Caretta caretta) Admitted to a Wildlife Rehabilitation Center in Gran Canaria Island, Spain (1998–2014): A Long-Term Retrospective Study. PLoS ONE 2016, 11, e0149398. [Google Scholar] [CrossRef] [PubMed]
- George, R.H. Health Problems and Diseases of Sea Turtles. In The Biology of Sea Turtles, Volume I; CRC Press: Boca Raton, FL, USA, 1997; ISBN 978-0-203-73708-8. [Google Scholar]
- Torrent, A.; Déniz, S.; Ruiz, A.; Calabuig, P.; Sicilia, J.; Orós, J. Esophageal Diverticulum Associated with Aerococcus viridans Infection in a Loggerhead Sea Turtle (Caretta caretta). J. Wildl. Dis. 2002, 38, 221–223. [Google Scholar] [CrossRef] [PubMed]
- Stacy, B.A.; Wellehan, J.F.X.; Foley, A.M.; Coberley, S.S.; Herbst, L.H.; Manire, C.A.; Garner, M.M.; Brookins, M.D.; Childress, A.L.; Jacobson, E.R. Two Herpesviruses Associated with Disease in Wild Atlantic Loggerhead Sea Turtles (Caretta caretta). Vet. Microbiol. 2008, 126, 63–73. [Google Scholar] [CrossRef]
- Caracappa, S.; Persichetti, M.F.; Piazza, A.; Caracappa, G.; Gentile, A.; Marineo, S.; Crucitti, D.; Arculeo, M. Incidental Catch of Loggerhead Sea Turtles (Caretta caretta) along the Sicilian Coasts by Longline Fishery. PeerJ 2018, 6, e5392. [Google Scholar] [CrossRef] [PubMed]
- Parga, M.L. Hooks and Sea Turtles: A Veterinarian’s Perspective. Bull. Mar. Sci. 2012, 88, 731–741. [Google Scholar] [CrossRef]
- Glazebrook, J.S.; Campbell, R.S.F.; Thomas, A.T. Studies on an Ulcerative Stomatitis-Obstructive Rhinitis-Pneumonia Disease Complex in Hatching and Juvenile Sea Turtles Chelonia mydas and Caretta caretta. Dis. Aquat. Org. 1993, 16, 133–147. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Balazs, G.H.; Spraker, T.R.; Murakawa, S.K.K.; Zimmerman, B. Pathology of Oropharyngeal Fibropapillomatosis in Green Turtles Chelonia mydas. J. Aquat. Anim. Health 2002, 14, 298–304. [Google Scholar] [CrossRef]
- Bjorndal, K.; Bolten, A.; Martins, H. Somatic Growth Model of Juvenile Loggerhead Sea Turtles Caretta caretta: Duration of Pelagic Stage. Mar. Ecol. Prog. Ser. 2000, 202, 265–272. [Google Scholar] [CrossRef]
- Casal, A.B.; Camacho, M.; López-Jurado, L.F.; Juste, C.; Orós, J. Comparative Study of Hematologic and Plasma Biochemical Variables in Eastern Atlantic Juvenile and Adult Nesting Loggerhead Sea Turtles (Caretta caretta). Vet. Clin. Pathol. 2009, 38, 213–218. [Google Scholar] [CrossRef]
- Tomè, P.; Pesaro, S.; Orioles, M.; Pascotto, E.; Cadamuro, A.; Galeotti, M. InfoFaunaFVG: A Novel Progressive Web Application for Wildlife Surveillance. Eur. J. Wildl. Res. 2023, 69, 38. [Google Scholar] [CrossRef]
- Deem, S.L.; Norton, T.M.; Mitchell, M.; Segars, A.; Alleman, A.R.; Cray, C.; Poppenga, R.H.; Dodd, M.; Karesh, W.B. Comparison of Blood Values in Foraging, Nesting, and Stranded Loggerhead Turtles (Caretta caretta) Along the Coast of Georgia, USA. J. Wildl. Dis. 2009, 45, 41–56. [Google Scholar] [CrossRef]
- Teare, J.A. (Ed.) Species360 Physiological Reference Intervals for Captive Wildlife; Species360: Bloomington, MN, USA, 2013; Available online: https://Zims.Species360.Org/Main.Aspx (accessed on 5 November 2016).
- Desantis, S.; Galosi, L.; Santamaria, N.; Roncarati, A.; Biagini, L.; Rossi, G. Modulation of Morphology and Glycan Composition of Mucins in Farmed Guinea Fowl (Numida meleagris) Intestine by the Multi-Strain Probiotic Slab51®. Animals 2021, 11, 495. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hutchings, P.; Murray, A.; Xu, K. Laetmonice iocasica Sp. Nov., a New Polychaete Species (Annelida: Aphroditidae) from Seamounts in the Tropical Western Pacific, with Remarks on L. producta Grube, 1877. J. Oceanol. Limnol. 2021, 39, 1805–1816. [Google Scholar] [CrossRef]
- Parapar, J.; Moreira, J.; Gambi, M.C.; Caramelo, C. Morphology and Biology of Laetmonice producta producta Grube (Polychaeta: Aphroditidae) in the Bellingshausen Sea and Antarctic Peninsula (Southern Ocean, Antarctica). Ital. J. Zool. 2013, 80, 255–272. [Google Scholar] [CrossRef]
- Barnich, R. The Aphroditoidea (Annelida, Polychaeta) of the Mediterranean Sea. Abh. Senckenberg. Naturforschenden Ges. 2003, 559, 1–167. [Google Scholar]
- Pleijel, F. Polychaetes: British Phyllodocoideans, Typhloscolecoideans and Tomopteroideans. Synop. Br. Fauna N S 1991, 45, 202. [Google Scholar]
- Lai, O.R.; Marín, P.; Laricchiuta, P.; Marzano, G.; Crescenzo, G.; Escudero, E. Pharmacokinetics of Marbofloxacin in Loggerhead Sea Turtles (Caretta caretta) after Single Intravenous and Intramuscular Doses. J. Zoo Wildl. Med. 2009, 40, 501–507. [Google Scholar] [CrossRef]
- Casale, P.; Affronte, M.; Insacco, G.; Freggi, D.; Vallini, C.; Pino d’Astore, P.; Basso, R.; Paolillo, G.; Abbate, G.; Argano, R. Sea Turtle Strandings Reveal High Anthropogenic Mortality in Italian Waters. Aquat. Conserv. Mar. Freshw. Ecosyst. 2010, 20, 611–620. [Google Scholar] [CrossRef]
- Oliveira, R.E.M.; Pires, J.M.L.; Batista, J.S.; Attademo, F.L.N.; Farias, D.d.; Freire, A.C.B.; Bomfim, A.D.C.; Lima, L.R.P.; Oliveira, R.M.; Gavilan, S.A. Death of a Loggerhead Sea Turtle (Caretta caretta) from Ingestion of an Eel (Myrichthys ocellatus). Vet. Med. 2020, 65, 415–420. [Google Scholar] [CrossRef]
- Lazar, B.; Zavodnik, D.; Grbac, I.; Tvrtković, N. Diet Composition of the Loggerhead Sea Turtle, Caretta caretta, in the Northern Adriatic Sea: A Preliminary Study. In Proceedings of the 20th Annual Symposium on Sea Turtle Biology and Conservation (NMFS-SEFSC-477), Orlando, FL, USA, 29 February–4 March 2000; pp. 146–147. [Google Scholar]
- Patel, S.H.; Morreale, S.J.; Panagopoulou, A.; Bailey, H.; Robinson, N.J.; Paladino, F.V.; Margaritoulis, D.; Spotila, J.R. Changepoint Analysis: A New Approach for Revealing Animal Movements and Behaviors from Satellite Telemetry Data. Ecosphere 2015, 6, 1–13. [Google Scholar] [CrossRef]
- Sardá, R.; Pinedo, S.; Dueso, A. Estimating Secondary Production in Natural Populations of Polychaetes: Some General Constraints. Available online: https://www.ingentaconnect.com/contentone/umrsmas/bullmar/2000/00000067/00000001/art00037 (accessed on 5 August 2025).
- Bianchi, C.N.; Morri, C.; Chiantore, M.; Montefalcone, M.; Parravicini, V.; Rovere, A. Mediterranean Sea Biodiversity between the Legacy from the Past and a Future of Change. Life Mediterr. Sea Look Habitat Changes 2012, 1, 55. [Google Scholar]
- Giani, M.; Djakovac, T.; Degobbis, D.; Cozzi, S.; Solidoro, C.; Umani, S.F. Recent Changes in the Marine Ecosystems of the Northern Adriatic Sea. Estuar. Coast. Shelf Sci. 2012, 115, 1–13. [Google Scholar] [CrossRef]
- Çinar, M. Polychaetes from the Coast of Northern Cyprus (Eastern Mediterranean Sea), with Two New Records for the Mediterranean Sea. Cah. Biol. Mar. 2005, 46, 143–159. [Google Scholar]
- Matarrese, A.; Mastrototaro, F.; D’onghia, G.; Maiorano, P.; Tursi, A. Mapping of the Benthic Communities in the Taranto Seas Using Side-Scan Sonar and an Underwater Video Camera. Chem. Ecol. 2004, 20, 377–386. [Google Scholar] [CrossRef]
- Mencacci, R.; Aiudi, L.; Angelini, V.; Casale, P.; Cerritelli, G.; Lombardi Moraes, K.; Pari, S.; Luschi, P. Satellite Tracking Identifies Important Foraging Areas for Loggerhead Turtles Frequenting the Adriatic Sea, Central Mediterranean. Mediterr. Mar. Sci. 2023, 24, 377–383. [Google Scholar] [CrossRef]
- Desrina, J.V.; Verdegem, M.C.J.; Vlak, J.M. Polychaetes as Potential Risks for Shrimp Pathogen Transmission. Asian Fish. Sci. 2018, 31, 155–167. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pesaro, S.; Biagini, L.; De Bellis, D.; Dorigo, L.; Baggio, A.; Perlin, I.; Rossi, G. Traumatic Oral Lesions in Loggerhead Sea Turtles (Caretta caretta) Linked to Polychaete (Laetmonice cf. hystrix) Ingestion: A Case Report from the Northern Adriatic Sea. Animals 2025, 15, 2727. https://doi.org/10.3390/ani15182727
Pesaro S, Biagini L, De Bellis D, Dorigo L, Baggio A, Perlin I, Rossi G. Traumatic Oral Lesions in Loggerhead Sea Turtles (Caretta caretta) Linked to Polychaete (Laetmonice cf. hystrix) Ingestion: A Case Report from the Northern Adriatic Sea. Animals. 2025; 15(18):2727. https://doi.org/10.3390/ani15182727
Chicago/Turabian StylePesaro, Stefano, Lucia Biagini, Danilo De Bellis, Luca Dorigo, Alice Baggio, Isabella Perlin, and Giacomo Rossi. 2025. "Traumatic Oral Lesions in Loggerhead Sea Turtles (Caretta caretta) Linked to Polychaete (Laetmonice cf. hystrix) Ingestion: A Case Report from the Northern Adriatic Sea" Animals 15, no. 18: 2727. https://doi.org/10.3390/ani15182727
APA StylePesaro, S., Biagini, L., De Bellis, D., Dorigo, L., Baggio, A., Perlin, I., & Rossi, G. (2025). Traumatic Oral Lesions in Loggerhead Sea Turtles (Caretta caretta) Linked to Polychaete (Laetmonice cf. hystrix) Ingestion: A Case Report from the Northern Adriatic Sea. Animals, 15(18), 2727. https://doi.org/10.3390/ani15182727







