State-of-the-Art Age Determination Methods for Amphibians and Reptiles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
3. Methods of Age Estimation
3.1. Capture–Mark–Recapture Method
3.2. Size Frequency Method
3.3. Sclerocronology
3.4. Other Morphological Methods
3.5. Molecular Methods
3.6. Biochemical Methods
4. Future Research Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Magalhães, J.P.; Costa, J.A. database of vertebrate longevity records and their relation to other life-history traits. J. Evol. Biol. 2009, 22, 1770–1774. [Google Scholar]
- Mezzasalma, M.; Andreone, F.; Glaw, F.; Guarino, F.M.; Odierna, G.; Petraccioli, A.; Picariello, O. Changes in heterochromatin content and ancient chromosome fusion in the endemic Malagasy boid snakes Sanzinia and Acrantophis (Squamata: Serpentes). Salamandra 2019, 55, 140–144. [Google Scholar]
- Mezzasalma, M.; Guarino, F.M.; Odierna, G. Lizards as model organisms of sex chromosome evolution: What we really know from a systematic distribution of available data? Genes 2021, 12, 1341. [Google Scholar] [CrossRef] [PubMed]
- Petraccioli, A.; Guarino, F.M.; Kupriyanova, L.; Mezzasalma, M.; Odierna, G.; Picariello, O.; Capriglione, T. Isolation and characterization of interspersed repeated sequences in the common lizard, Zootoca vivipara, and their conservation in Squamata. Cytog. Genome Res. 2019, 157, 65–76. [Google Scholar]
- Reinke, B.A.; Cayuela, H.; Janzen, F.J.; Lemaître, J.F.; Gaillard, J.M.; Lawing, A.M.; Iverson, J.B.; Christiansen, D.J.; Martínez-Solano, I.; Sánchez-Montes, G.; et al. Diverse aging rates in ectothermic tetrapods provide insights for the evolution of aging and longevity. Science 2022, 376, 1459–1466. [Google Scholar] [CrossRef]
- Stott, I.; Salguero-Gómez, R.; Jones, O.R.; Ezard, T.H.G.; Gamelon, M.; Lachish, S.; Lebreton, J.D.; Simmonds, E.G.; Gaillard, J.M. Life histories are not just fast or slow. Trends Ecol. Evol. 2024, 39, 830–840. [Google Scholar] [CrossRef]
- Voituron, J.; de Fraipont, M.; Issartel, J.; Guillaume, O.; Clobert, J. Extreme lifespan of the human fish (Proteus anguinus): A challenge for ageing mechanisms. Biol. Lett. 2010, 7, 105–107. [Google Scholar]
- Eckart, F.; Kappeler, P.M.; Krauss, C. Highly variable lifespan in an annual reptile, Labord’s chameleon (Furcifer labordi). Sci. Rep. 2017, 7, 11397. [Google Scholar] [CrossRef]
- Hemelaar, A. Age, growth and other population characteristics of Bufo bufo from different latitudes and altitudes. J. Herpetol. 1988, 22, 369–388. [Google Scholar] [CrossRef]
- Wapstra, E.; Swan, R.; O’Reilly, J.M. Geographic variation in age and size at maturity in a small Australian viviparous skink. Copeia 2001, 3, 646–655. [Google Scholar] [CrossRef]
- Morrison, C.; Hero, J.M. Geographic variation in life history characteristics of amphibians: A review. J. Anim. Ecol. 2003, 72, 270–279. [Google Scholar] [CrossRef]
- Guarino, F.M.; Di Già, I.; Sindaco, R. Age structure by skeletochronology in a declining population of Rana temporaria from northern Italy. Acta Zool. Acad. Sci. Hung. 2008, 54, 99–112. [Google Scholar]
- Zhang, L.; Lu, X. Amphibians live longer at higher altitudes but not at higher latitudes. Biol. J. Linn. Soc. 2012, 106, 623–632. [Google Scholar] [CrossRef]
- Sinsch, U. Review: Skeletochronological assessment of demographic life-history traits in amphibians. Herpetol. J. 2015, 25, 5–13. [Google Scholar]
- Stark, G.; Meiri, S. Cold and dark captivity: Drivers of amphibian longevity. Glob. Ecol. Biogeogr. 2018, 27, 1384–1397. [Google Scholar] [CrossRef]
- Stark, G.; Tamar, K.; Itescu, Y.; Feldman, A.; Meiri, S. Cold and isolated ectotherms: Drivers of reptilian longevity. Biol. J. Linn. Soc. 2018, 125, 730–740. [Google Scholar] [CrossRef]
- Castanet, J. Age estimation and Longevity in reptiles. Gerontology 1994, 40, 174–192. [Google Scholar] [CrossRef]
- Zarse, K.; Terao, T.; Tian, J.; Iwata, N.; Ishii, N.; Ristow, M. Low-dose lithium uptake promotes longevity in humans and metazoans. Eur. J. Nutr. 2011, 50, 387–389. [Google Scholar] [CrossRef]
- Coutts, F.; Palmos, A.B.; Duarte, R.R.R.; De Jong, S.; Lewis, C.M.; Dima, D.; Powell, T.R. The polygenic nature of telomere length and the anti-ageing properties of lithium. Neuropsychopharmacology 2019, 44, 757–765. [Google Scholar] [PubMed]
- Smirina, E. Age determination and longevity in amphibians. Gerontology 1994, 40, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, D.; Tomašević, N.; Ficetola, G.F.; Crnobrnja-Isailović, J.; Miaud, C. Bergmann’s rule in amphibians: Combining demographic and ecological parameters to explain body size variation among populations in the common toad Bufo bufo. J. Zool. Syst. Evol. Res. 2009, 47, 171–180. [Google Scholar] [CrossRef]
- Werner, Y.; Frankenberg, E.; Volokita, M.; Harari, R. Longevity of geckos (Reptilia: Lacertilia: Gekkonoidea) in captivity: An analytical review incorporating new data. Isr. J. Zool. 1993, 39, 105–124. [Google Scholar]
- Read, F.L.; Hohn, A.; Lockyer, C.H. A review of age estimation methods in marine mammals with special reference to monodontid. NAMMCO Sci. Publ. 2018, 10. [Google Scholar] [CrossRef]
- Denton, J.S.; Beebee, T.J.C. Density related features of natterjack toad (Bufo calamita) populations in Britain. J. Zool. 1993, 229, 105–119. [Google Scholar] [CrossRef]
- Driscoll, D.A. Skeletochronological assessment of age structure and population stability for two threatened frog species. Austr. J. Ecol. 1999, 24, 182–189. [Google Scholar] [CrossRef]
- Guarino, F.M.; De Pous, P.; Crottini, A.; Mezzasalma, M.; Andreone., F. Age structure and growth in a population of Pelobates varaldii (Anura, Pelobatidae) from northwestern Morocco. Amphib. Reptil. 2011, 32, 550–556. [Google Scholar]
- Sinsch, U.; Dehling, J.M. Tropical anurans mature early and die young: Evidence from eight Afromontane Hyperolius species and a meta-analysis. PLoS ONE 2017, 12, e0171666. [Google Scholar] [CrossRef] [PubMed]
- Atkins, Z.S.; Clemann, N.; Chapple, D.G.; Edwards, A.M.; Sinsch, U.; Hantzschmann, A.M.; Schroder, M.; Scroggie, M.P.; Robert, K.A. Demographic and life history variation in two sky-island populations of an endangered alpine lizard. J. Zool. 2020, 310, 34–44. [Google Scholar] [CrossRef]
- Ngo, B.V.; Ngo, C.D. Reproductive activity and advertisement calls of the Asian common toad Duttaphrynus melanostictus (Amphibia, Anura, Bufonidae) from Bach Ma National Park, Vietnam. Zool. Stud. 2012, 52, 12. [Google Scholar] [CrossRef]
- Jansen, F.T.; Paukstis, G.L.; Brodie, E.D. Observations on basking behavior of hatchling turtles in the wild. J. Herpetol. 1992, 26, 217–219. [Google Scholar] [CrossRef]
- Halliday, T.; Verrell, P. Body Size and Age in Amphibians and Reptiles. J. Herpetol. 1988, 22, 253–265. [Google Scholar] [CrossRef]
- Avens, L.; Snover, M.L. Age and age estimation in sea turtles. In The Biology of Sea Turtles; Wyneken, J., Lohmann, K.J., Musick, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2011; Volume III, pp. 97–134. [Google Scholar]
- Castanet, J.; Francillon-Vieillot, H.; Meunier, F.J.; de Ricqles, A. Bone and individual aging. In Bone Growth; Hall, B.K., Ed.; CRC Press: Boca Raton, FL, USA, 1993; pp. 245–283. [Google Scholar]
- El Mouden, E.H.; Znari, M.; Brown, R.P. Skeletochronology and mark ± recapture assessments of growth in the North African agamid lizard (Agama impalearis). J. Zool. 1999, 249, 455–461. [Google Scholar] [CrossRef]
- Kumbar, S.M.; Pancharatna, K. Annual Growth Layers in the Phalanges of the Indian Skipper Frog Rana cyanophlyctis (Schn.). Copeia 2002, 3, 870–872. [Google Scholar] [CrossRef]
- Barbault, R.; Mou, Y.P. A population analysis of the common wall lizard Podarcis muralis in Southwestern France. In Studies in Herpetology; Roček, Z., Ed.; Charles University Press: Prague, Czech Republic, 1986; pp. 513–515. [Google Scholar]
- James, C.G. Growth rates and age at maturity of simpatric scincid lizards (Ctenotus) in Central Australia. J. Herpetol. 1991, 15, 284–295. [Google Scholar] [CrossRef]
- Brown, W.S. Lifetime Reproduction in a Northern Metapopulation of Timber Rattlesnakes (Crotalus horridus). Herpetologica 2016, 72, 331–342. [Google Scholar] [CrossRef]
- Brown, W.S.; Kery, M.; Hines, J.E. Survival of timber rattlesnakes (Crotalus horridus) estimated by capture-recapture models in relation to age, sex, color morph, time, and birthplace. Copeia 2007, 3, 656–671. [Google Scholar] [CrossRef]
- Bell, G. The life of the smooth newt (Triturus vulgaris) after metamorphosis. Ecol. Monogr. 1977, 47, 279–299. [Google Scholar] [CrossRef]
- Daugherty, C.H.; Sheldon, A.C. Age-determination, growth and life history of a Montana population of the tailed frog (Ascaphus truei). Herpetologica 1982, 38, 461–468. [Google Scholar] [CrossRef]
- Tinkle, D.W. The life and demography of the side-blotched lizard, Uta stansburiana. Misc. Publ. Mus. Zool. Univ. Mich. 1967, 132, 1–182. [Google Scholar]
- Galan, P. Demography and population dynamics of the lacertid lizard Podarcis bocagei in north-west Spain. J. Zool. Lond. 1999, 249, 203–218. [Google Scholar]
- Bjorndal, K.A.; Bolten, A.B.; Koike, B.; Schroeder, B.A.; Shaver, D.J.; Teas, W.G.; Witzel, W.N. Somatic growth function for immature loggerhead sea turtles, Caretta caretta, in southeastern U.S. waters. Fish. Bull. 2001, 99, 240–246. [Google Scholar]
- Platz, J.E.; Lahtrop, A.; Hofbauer, L.; Vradenburg, M. Age distribution and longevity in the Ramsey Canyon leopard frog, Rana subaquavocalis. J. Herpetol. 1997, 31, 552–557. [Google Scholar] [CrossRef]
- Erismis, U.C.; Arikan, H.; Konuk, M.; Guarino, F.M. Age structure and growth in Caucasian parsley frogs Pelodytes caucasicus (Boulenger, 1896) from Turkey. Russ. J. Herpetol. 2009, 16, 19–26. [Google Scholar]
- Woodward, H.N.; Horner, J.R.; Farlow, J.O. Osteohistological evidence for determinate growth in the American alligator. J. Herpetol. 2011, 45, 339–342. [Google Scholar] [CrossRef]
- Wilkinson, P.M.; Rainwater, T.R.; Woodward, A.R.; Leone, E.H.; Carter, C. Determinate growth and reproductive lifespan in the American alligator (Alligator mississippiensis): Evidence from long-term recaptures. Copeia 2016, 104, 843–852. [Google Scholar] [CrossRef]
- Rainwater, T.R.; Woodward, H.N.; Woodward, A.R. Evidence of determinate growth in an American alligator (Alligator mississippiensis) based on long-term recapture and osteohistological confirmation. Anat. Rec. 2022, 305, 3101–3108. [Google Scholar]
- Hoffman, D.K.; Goldsmith, E.R.; Houssaye, A.; Maidment, S.C.R.; Felice, R.N.; Mannion, P.D. Evolution of growth strategy in alligators and caimans informed by osteohistology of the late Eocene early-diverging alligatoroid crocodylian Diplocynodon hantoniensis. J. Anat. 2025, 247, 165–178. [Google Scholar]
- Congdon, J.D.; Nagle, R.D.; Kinney, O.M.; van Loben Sels, R.C. Hypotheses of aging in a long-lived vertebrate, Blanding’s turtle (Emydoidea blandingii). Exp. Gerontol. 2001, 36, 813–827. [Google Scholar] [CrossRef]
- Tracy, C.R.; Tracy, R.C. Estimating age of desert tortoises (Gopherus agassizi) from scute rings. Copeia 1995, 4, 974–979. [Google Scholar]
- Thomas, R.B.; Beckman, D.W.; Thompson, K.; Buhlmann, K.A.; Gibbons, J.W.; Moll, D.L. Estimation of age for Trachemys scripta and Deirochelis reticularia by counting annual growth layers in claws. Copeia 1997, 4, 964–966. [Google Scholar]
- Galoyan, E.; Sopilko, N.; Kovalyeva, A.; Chamkina, A. Double-check in lizard age estimation: Use of phalanx bone and keratin claw sheath lamellas. Asian Herp. Res. 2024, 15, 59–61. [Google Scholar]
- Senning, W.C. A study of age determination and growth of Necturus maculosus based on parasphenoid bone. Am. J. Anat. 1940, 66, 483–495. [Google Scholar] [CrossRef]
- Peabody, F.E. Annual growth zones in living and fossil Vertebrates. J. Morphol. 1961, 108, 11–62. [Google Scholar] [CrossRef]
- Schroeder, E.E.; Baskett, T.E. Age estimation, growth rates and population structure in Missouri bullfrogs. Copeia 1968, 1968, 538–592. [Google Scholar] [CrossRef]
- Castanet, J.; Naulleau, J. La squelettochronologie chez les Reptiles. II. Résultats expérimentaux sur la signification des marques de croissance squelettiques chez les Serpents. Remarques sur la croissance et la longévités de la vipère aspic. Ann. Sci. Nat. Zool. 1985, 7, 41–62. [Google Scholar]
- Ryser, J. Determination of growth and maturation in the common frog, Rana temporaria, by skeletochronology. J. Zool. 1988, 216, 673–685. [Google Scholar] [CrossRef]
- Francillon-Vieillot, H.; Arntzen, W.J.; Geraudie, J. Age, growth and longevity of sympatric Triturus cristatus, T. marmoratus and their hybrids (Amphibia, Urodela): A skeletochronological comparison. J. Herpetol. 1990, 24, 13–22. [Google Scholar] [CrossRef]
- Goshe, L.R.; Snover, M.L.; Hohn, A.A.; Balazs, G.H. Validation of back-calculated body lengths and timing of growth mark deposition in Hawaiian green sea turtles. Ecol. Evol. 2016, 10, 3208–3215. [Google Scholar] [CrossRef]
- Pereira, M.E.; Bona, P.; Siroski, P.; Chinsamy, A. Analyzing the life history of Caimans: The growth dynamics of Caiman latirostris from an osteohistological approach. J. Morphol. 2025, 286, e70010. [Google Scholar]
- Comas, M.; Reguera, S.; Zamora-Camacho, F.J.; Salvadó, H.; Moreno-Rueda, G. Comparison of the effectiveness of phalanges vs. humeri and femurs to estimate age with skeletochronology. Anim. Biodiv. Conserv. 2016, 39, 237–240. [Google Scholar] [CrossRef]
- Castanet, J.; Newman, D.J.; Saint Girons, H. Skeletochronological data on the growth, age, and population structure of the tuatara, Sphenodon punctatus, on Stephens and Lady Alice islands, New Zealand. Herpetologica 1988, 44, 25–37. [Google Scholar]
- Avens, L.; Taylor, J.C.; Goshe, L.R.; Jones, T.T.; Hastings, M. Use of skeletochronological analysis to estimate the age of leatherback sea turtles Dermochelys coriacea in the western North Atlantic. Endanger. Species Res. 2009, 8, 165–177. [Google Scholar] [CrossRef]
- Scholtz, S.; Orliz, M.; Gonwuouo, L.N.; Kupfer, A. Demography and life history of a viviparous Central African caecilian amphibian. J. Zool. 2010, 280, 17–24. [Google Scholar] [CrossRef]
- Guarino, F.M. Structure of the femora and autotomous (postpygal) caudal vertebrae in the three-toed skink Chalcides chalcides (Reptilia: Squamata:Scincidae) and its applicability for age and growth rate determination. Zool. Anz. 2010, 248, 273–283. [Google Scholar] [CrossRef]
- Guarino, F.M.; Mezzasalma, M.; Odierna, G. Usefulness of postpygal caudal vertebrae and osteoderms for skeletochronology in the limbless lizard Anguis veronensis Pollini, 1818 (Squamata: Sauria: Anguidae). Herpetozoa 2016, 29, 69–75. [Google Scholar]
- Waye, H.L.; Gregory, P.T. Determining the age of garter snakes (Thamnophis spp.) by means of skeletochronology. Can. J. Zool. 1998, 76, 288–294. [Google Scholar] [CrossRef]
- Tucker, A. Validation of skeletochronology to determine age of freshwater crocodiles (Crocodylus johnstoni). Mar. Fresh Res. 1997, 48, 343–351. [Google Scholar] [CrossRef]
- Wake, M.H.; Nygren, K.M. Variation in scales in Dermophis mexicanus (Amphibia: Gymnophiona): Are scales of systematic utility? Fieldiana Zool. 1987, 36, 1–8. [Google Scholar]
- de Buffrénil, V.; Quilhac, A.; Castanet, J. Cyclical growth and skeletochronology. In Vertebrate Skeletal Histology and Palaeohistology; de Buffrénil, V., de Ricqlès, A., Zylberberg, L., Padian, K., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 626–644. [Google Scholar]
- Zhang, Y.; Bi, J.; Ning, Y.; Feng, J. Methodology Advances in Vertebrate Age Estimation. Animals 2024, 14, 343. [Google Scholar] [CrossRef]
- Rosauer, E.A.; Redmond, J.R. Comparative crystallography of vertebrate otoconia. J. Laryngol. Otol. 1985, 9, 21–28. [Google Scholar] [CrossRef]
- Castanet, J.; Cheylan, M. Les marques de croissance des os el des ecailles comme indicaleur de rage chez Testudo hermanni et Testudo graeca (Reptilia, Chelonia, Testudinidae). Can. J. Zool. 1979, 57, 1649–1665. [Google Scholar] [CrossRef]
- Galbraith, D.A.; Brooks, R.J. Age Estimates for Snapping Turtles. J. Wildl. Manag. 1989, 53, 502–508. [Google Scholar] [CrossRef]
- Zug, G. Age determination in turtles. Herp. Circ. 1991, 20, 1–28. [Google Scholar]
- Brooks, R.J.; Krawchuk, M.A.; Stevens, C.; Koper, N. Testing the precision and accuracy of age estimation using lines in scutes of Chelydra serpentina and Chrysemys picta. J. Herpetol. 1997, 31, 521–529. [Google Scholar] [CrossRef]
- Aresco, M.J.; Guyer, C. Efficacy of using scute annuli to determine growth histories and age of Gopherus polyphemus in Southern Alabama. Copeia 1998, 4, 1094–1100. [Google Scholar] [CrossRef]
- Litzgus, J.D.; Brooks, R.J. Testing the validity of counts of plastral scute rings in spotted turtles, Clemmys guttata. Copeia 1998, 1, 222–225. [Google Scholar] [CrossRef]
- Vlad, S.E.; Stănescu, F.; Cogălniceanu, D. Age estimation in tortoises: An evaluation of methods for the spur-thighed tortoise (Testudo graeca). Amphibia-Reptilia 2024, 45, 265–277. [Google Scholar] [CrossRef]
- Germano, D.J.; Bury, R.B. Age determination in turtles: Evidence of annual deposition of scute rings. Chelonian Conserv. Biol. 1998, 3, 123–132. [Google Scholar]
- Wilson, D.S.; Tracy, C.R.; Tracy, R.C. Estimating age of turtles from growth rings: A critical evaluation of the technique. Herpetologica 2003, 59, 178–194. [Google Scholar] [CrossRef]
- Peng, Z.; Zhang, L.; Lu, X. Global gaps in age data based on skeletochronology for amphibians. Integr. Zool. 2022, 17, 752–763. [Google Scholar] [CrossRef]
- Szekely, D.; Stănescu, F.; Szekely, P.; Telea, A.E.; Cogălniceanu, D. A review of age estimation methods in non-avian reptiles by growth marks in hard tissues. Integr. Zool. 2025, 20, 15–32. [Google Scholar] [CrossRef]
- Miaud, C.; Guyétant, R.; Elmberg, J. Variations in life-history traits in the common frog Rana temporaria (Amphibia: Anura): A literature review and new data from the French Alps. J. Zool. 1999, 249, 61–73. [Google Scholar] [CrossRef]
- Castanet, J.; Baez, M. Adaptation and evolution in Gallotia lizards from the Canary Islands: Age, growth maturity and longevity. Amphibia-Reptilia 1991, 12, 81–112. [Google Scholar] [CrossRef]
- Guarino, F.M.; Crottini, A.; Mezzasalma, M.; Randrianirina, J.E.; Andreone, F. A skeletochronological estimate of age and growth in a large riparian frog from Madagascar (Anura, Mantellidae, Mantidactylus). Herpetozoa 2019, 32, 39–44. [Google Scholar] [CrossRef]
- Ma, M.; Luo, S.; Tang, X.; Chen, Q. Age structure and growth pattern of a high-altitude lizard population based on age determination by skeletochronology. J. Exp. Zool. A Ecol. Integr. Physiol. 2022, 337, 491–500. [Google Scholar] [CrossRef] [PubMed]
- Seren, N.; Megía-Palma, R.; Simčič, T.; Krofel, M.; Guarino, F.M.; Pinho, C.; Žagar, A.; Carretero, M.A. Functional responses in a lizard along a 3.5-km altitudinal gradient. J. Biogeogr. 2023, 50, 2042–2056. [Google Scholar] [CrossRef]
- Hutton, J.M. Dermination of Living Nile Crocodiles from the age determination of living nile crocodiles from the cortical stratification of bone. Copeia 1986, 2, 332–341. [Google Scholar] [CrossRef]
- Bochaton, C.; De Buffrenil, V.; Lemoine, M.; Bailon, S.; Ineich, I. Body Location and Tail Regeneration Effectson Osteoderms Morphology—Are They Useful Tools for Systematic, Paleontology, and Skeletochronology in Diploglossine Lizards (Squamata, Anguidae)? J. Morphol. 2015, 276, 1333–1344. [Google Scholar] [CrossRef]
- David-Beal, T.; Chisaka, H.; Delgado, S.; Sire, J.Y. Amphibian teeth: Current knowledge, unanswered questions, and some directions for future research. Biol. Rev. 2007, 82, 49–81. [Google Scholar] [CrossRef] [PubMed]
- Razmadze, D.; Salomies, L.; Di Poi, N. Squamates as a model to understand key dental features of vertebrates. Dev. Biol. 2024, 516, 1–19. [Google Scholar] [CrossRef]
- Smirina, E.; Ananjeva, N. Growth layers in bones and acrodont teeth of the agamid lizard Laudakia stoliczkana (Blanford, 1875) (Agamidae, Sauria). Amphibia-Reptilia 2007, 28, 193–204. [Google Scholar] [CrossRef]
- Guarino, F.M.; Di Nocera, F.; Pollaro, F.; Galiero, G.; Iaccarino, D.; Iovino, D.; Mezzasalma, M.; Petraccioli, A.; Odierna, G.; Maio, N. Skeletochronology, age at maturity and cause of mortality of loggerhead sea turtles Caretta caretta stranded along the beaches of Campania (south-western Italy, western Mediterranean Sea). Herpetozoa 2020, 33, 39–51. [Google Scholar] [CrossRef]
- Guarino, F.M.; Andreone, F.; Mezzasalma, M.; Licata, F.; Puoti, S.; Santos, B.; Cocca, W.; Solofoniaina Fidy, J.; Ndriantsoa, S.N.; Noel, J.; et al. Life History Traits and Longevity of the Invasive Asian Common Toad Duttaphrynus melanostictus (Schneider, 1799) in Madagascar. Animals 2023, 13, 2099. [Google Scholar] [CrossRef]
- Guarino, F.M.; Cammarota, M.; Angelini, F. Skeletochronology on femurs and phalanxes from some species of Italian Amphibians. Riv. Idrobiol. 1999, 38, 55–59. [Google Scholar]
- Piantoni, C.; Ibargüengoitía, N.R.; Cussac, V.E. Growth and age of the southernmost distributed gecko of the world (Homonota darwini) studied by skeletochronology. Amphibia-Reptilia 2006, 27, 393–400. [Google Scholar] [CrossRef]
- Castanet, J.; Baez, M. Data on age and longevity in Gallotia galloti (Sauria, Lacertidae) assessed by skeletochronology. Herp. J. 1988, 1, 218–222. [Google Scholar]
- Woodward, H.N.; Padian, K.; Lee, A.H. Skeletochronology. In Bone Histology of Fossil Tetrapods: Advancing Methods, Analysis, and Interpretation; Padian, K., Lamm, E.-T., Eds.; University of California Press: Berkeley and Los Angeles, CA, USA, 2013; pp. 195–215. [Google Scholar]
- Hayashi, Y.; Tanaka, H. Age determination in the venomous snake, habu Trimesurus flavoviridis. Jpn. J. Exp. Med. 1981, 51, 209–213. [Google Scholar]
- Nishimura, M. Age estimation of habu, Trimesurus flavoviridis (Viperidae) using growth bands in angulares. Amphibia-Reptilia 1990, 11, 411–418. [Google Scholar]
- Minakami, K. An estimation of age and life-span of the genus Trimesurus (Reptilia, Serpentes, Viperidae) on Amani Oshima Island. Jpn. J. Herpetol. 1979, 13, 147–152. [Google Scholar] [CrossRef]
- Kidov, A.A.; Ivanov, A.A.; Ivolga, R.A.; Kondratova, T.E.; Kidova, E.A. Age Structure of the Population of Anguis colchica orientalis (Reptilia, Anguidae) in the Talysh Mountains. Biol. Bull. 2023, 40, 2857–2860. [Google Scholar] [CrossRef]
- Andreone, F.; Guarino, F.M. Giant and long-lived: Age structure in Macroscincus coctei, an extinct skink from Cape Verde. Amphibia Reptilia 2003, 4, 459–470. [Google Scholar]
- Chinsamy, A.; Valenzuela, N. Skeletochronology of the endangered side-neck turtle, Podocnemis expansa. S. Afr. J. Sci. 2008, 104, 311–314. [Google Scholar]
- Fornasiero, S.; Bonnet, X.; Dendi, F.; Zuffi, M.A.L. Growth, longevity and age at maturity in the European whip snakes, Hierophis viridiflavus and H. carbonarius. Acta Herpetol. 2016, 11, 135–149. [Google Scholar]
- Sinsch, U.; Leskovar, C.; Drobig, A.; König, A.; Rüdiger Gross, W. Life-history traits in green toad (Bufo viridis) populations: Indicators of habitat quality. Can. J. Zool. 2007, 65, 665–673. [Google Scholar] [CrossRef]
- Lyapkov, S.M. Skeletochronology of Amphibians and Reptiles: Fundamentals of Methodology, Variety of Problems, and Prospects. Biol. Bull. 2025, 52, 109. [Google Scholar] [CrossRef]
- Skutschas, P.P.; Kolchanov, V.V. Determination of individual age and ontogenetic stages of fossil tetrapods using paleohistological methods. Biol. Bull. 2025, 52, 112. [Google Scholar] [CrossRef]
- Schucht, P.J.; Klein, N.; Lambertz, M. What’s my age again? On the ambiguity of histology-based skeletochronology. Proc. R. Soc. B 2021, 288, 20211166. [Google Scholar] [CrossRef]
- Augusteyn, R.C. Growth of the eye lens: I. Weight accumulation in multiple species. Mol. Vis. 2014, 20, 410. [Google Scholar]
- Humphrey, R.R. The multiple testis in urodeles. Biol. Bull. 1922, 43, 45–67. [Google Scholar] [CrossRef]
- Tilley, S.G. Structure and dynamics of populations of the salamander Desmognathus ochrophaeus Cope in different habitats. Ecology 1974, 55, 808–817. [Google Scholar] [CrossRef]
- Dolmen, D. Skeletal growth marks and testis lobulation as criteria for age in Triturus species (Amphibia) in central Norway. Acta Zool. 1982, 63, 73–80. [Google Scholar] [CrossRef]
- Bruce, R.C.; Castanet, J.; Francillon-Vieillot, H. Skeletochronological analysis of variation in age structure, body size, and life-history in three species of Demognathine salamanders. Herpetologica 2002, 58, 181–193. [Google Scholar] [CrossRef]
- Vaiserman, A.; Krasnienkov, D. Telomere Length as a Marker of Biological Age: State-of-the-Art, Open Issues, and Future Perspectives. Front. Gene 2021, 11, 630186. [Google Scholar] [CrossRef] [PubMed]
- Scott, N.M.; Haussmann, M.F.; Elsey, R.M.; Trosclai, P.L.; Vleck, C.M. Telomere Length Shortens with Body Length in Alligator mississippiensis. Southeast. Nat. 2006, 5, 685–692. [Google Scholar] [CrossRef]
- Bronikowski, A.M. The evolution of aging phenotypes in snakes: A review and synthesis with new data. Age 2008, 30, 169–176. [Google Scholar] [CrossRef]
- Xu, M.; Wu, B.; Yan, P.; Zhu, H. Telomere Length Shortens with Age in Chinese Alligators (Alligator sinensis). J. Appl. Anim. Res. 2009, 36, 109–112. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Montes, G.; Solano, I.M.; Díaz-Paniagua, C.; Vilches, A.; Ariño, A.H.; Gomez-Mestre, I. Telomere attrition with age in a wild amphibian population. Biol. Lett. 2020, 16, 20200168. [Google Scholar] [CrossRef]
- Ujvari, B.; Biro, P.A.; Charters, J.E.; Brown, G.; Heasman, K.; Beckmann, C.; Madsen, T. Curvilinear telomere length dynamics in a squamate reptile. Funct. Ecol. 2017, 31, 753–759. [Google Scholar] [CrossRef]
- Rollings, N.; Friesen, C.R.; Whittington, C.M.; Johansson, R.; Shine, R.; Olsson, M. Sex- and tissue-specific differences in telomere length in a reptile. Ecol. Evol. 2019, 9, 6211–6219. [Google Scholar] [CrossRef]
- Burraco, P.; Comas, M.; Reguera, S.; Zamora-Camacho, F.J.; Moreno-Rueda, G. Telomere length mirrors age structure along a 2200-m altitudinal gradient in a Mediterranean lizard. Comp. Biochem. Phys. Part A Mol. Integr. Physiol. 2020, 247, 110741. [Google Scholar] [CrossRef]
- Moore, L.; Le, T.; Fan, G. DNA Methylation and Its Basic Function. Neuropsychopharmacology 2013, 38, 23–38. [Google Scholar] [CrossRef]
- Mattei, A.L.; Bailly, N.; Meissner, A. DNA methylation: A historical perspective. Trends Genet. 2022, 38, 676–707. [Google Scholar] [CrossRef] [PubMed]
- De Paoli-Iseppi, R.; Deagle, B.E.; Polanowski, A.M.; McMahon, C.R.; Dickinson, J.L.; Hindell, M.A.; Jarman, S.N. Age estimation in a long-lived seabird (Ardenna tenuirostris) using DNA methylation-based biomarkers. Mol. Ecol. Resour. 2019, 19, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Morselli, M.; Bennett, R.; Shaidani, N.I.; Horb, M.; Peshkin, L.; Pellegrini, M. Age-associated DNA methylation changes in Xenopus frogs. Epigenetics 2023, 18, 2201517. [Google Scholar] [CrossRef] [PubMed]
- Zoller, J.A.; Parasyraki, E.; Lu, A.T.; Haghani, A.; Niehrs, C.; Horvath, S. DNA methylation clocks for clawed frogs reveal evolutionary conservation of epigenetic aging. Geroscience 2024, 46, 945–960. [Google Scholar] [CrossRef]
- Day, K.; Waite, L.L.; Thalacker-Mercer, A.; West, A.; Bamman, M.M.; Brooks, J.D.; Myers, R.M.; Absher, D. Differential DNA methylation with age displays both common and dynamic features across human tissues that are influenced by CpG landscape. Genome Biol. 2013, 14, R102. [Google Scholar] [CrossRef]
- Freire-Aradas, A.; Phillips, C.; Girón-Santamaría, L.; Mosquera-Miguel, A.; Gómez-Tato, A.; de Cal, M.Á.C.; Álvarez-Dios, J.; Lareu, M.V. Tracking age-correlated DNA methylation markers in the young. Forensic Sci. Int. Genet. 2018, 36, 50–59. [Google Scholar] [CrossRef]
- Csapó, J.; Csapó-Kiss, Z.; Némethy, S.; Folestad, S.; Tivesten, A.; Martin, T.G. Age determination based on amino acid racemization: A new possibility. Amino Acids 1994, 7, 317–325. [Google Scholar] [CrossRef][Green Version]
- Prastyo, E.; Auerkari, E.I.; Suhartono, A.W.; Pasaribu, R.S.; Putra, A.G.A.; Auerkari, P. Performance of Forensic Age Estimation by Aspartic Acid Racemization and DNA Methylation: A Systematic Review. F1000Research 2024, 13, 1368. [Google Scholar] [CrossRef]
- Monum, T.; Jaikang, C.; Sinthubua, A.; Prasitwattanaseree, S.; Mahakkanukrauh, P. Age estimation using aspartic amino acid racemization from a femur. Aust. J. Forensic Sci. 2017, 51, 417–425. [Google Scholar] [CrossRef]
- Van Houtan, K.S.; Andrews, A.H.; Jones, T.T.; Murakawa, S.K.; Hagemann, M.E. Time in tortoiseshell: A bomb radiocarbon-validated chronology in sea turtle scutes. Proc. Biol. Sci. 2016, 283, 20152220. [Google Scholar] [CrossRef]
- Murray, I.W.; Wolf, B.O. Tissue carbon incorporation rates and diet-to-tissue discrimination in ectotherms: Tortoises are really slow. Physiol. Biochem. Zool. 2012, 85, 96–105. [Google Scholar]
- Grundler, M.R.; Pianka, E.R.; Pelegrin, N.; Cowan, M.A.; Rabosky, D.L. Stable isotope ecology of a hyper-diverse community of scincid lizards from arid Australia. PLoS ONE 2017, 12, e0172879. [Google Scholar] [CrossRef] [PubMed]
- Warne, R.W.; Wolf, B.O. Nitrogen stable isotope turnover and discrimination in lizards. Rapid Commun. Mass Spectr. 2021, 35, e9030. [Google Scholar] [CrossRef] [PubMed]
- Guarino, F.M.; Tessa, G.; Mercurio, V.; Andreone, F. Rapid sexual maturity and short life span in the blue-legged frog and the rainbow frog from the arid Isalo Massif, southern-central Madagascar. Zoology 2010, 113, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Cabezas-Cartes, F.; Boretto, J.M.; Ibarguengoytía, N.R. Effects of climate and latitude on age at maturity and longevity of lizards studied by skeletochronology. Integr. Comp. Biol. 2018, 58, 1086–1097. [Google Scholar] [CrossRef]
- Hantzschmann, A.M.; Gollmann, B.; Gollmann, G.; Sinsch, U. The fast slow continuum of longevity among yellow-bellied toad populations (Bombina variegata): Intrinsic and extrinsic drivers of variation. Peer J. 2019, 7, e8233. [Google Scholar] [CrossRef]
- Zamaletdinov, R.I.; Svinin, A.O.; Fayzulin, A.I.; Ermakov, O.A.; Mikhaylova, R.I.; Litvinchuk, S.N. Age structure of water frogs of the genus Pelophylax in the Middle Volga River region (European Russia). Animals 2025, 15, 1273. [Google Scholar] [CrossRef]
- Hemelaar, A. An improved method to estimate the number of year rings resorbed in phalanges of Bufo bufo (L.) and its application to populations from different latitudes and altitudes. Amphibia-Reptilia 1985, 6, 323–343. [Google Scholar] [CrossRef]
- Zug, G.; Wynn, A.H.; Ruckdeschel, C. Age determination of loggerhead sea turtles, Caretta caretta, by incremental growth marks in the skeleton. Smithson. Contrib. Zool. 1986, 427, 1–34. [Google Scholar] [CrossRef]
- Guarino, F.M.; Di Maio, A.; Caputo, V. Age estimation by phalangeal skeletochronology of Caretta caretta from Mediterranean Sea. Ital. J. Zool. 2004, 71 (Suppl. S2), 175–180. [Google Scholar] [CrossRef]
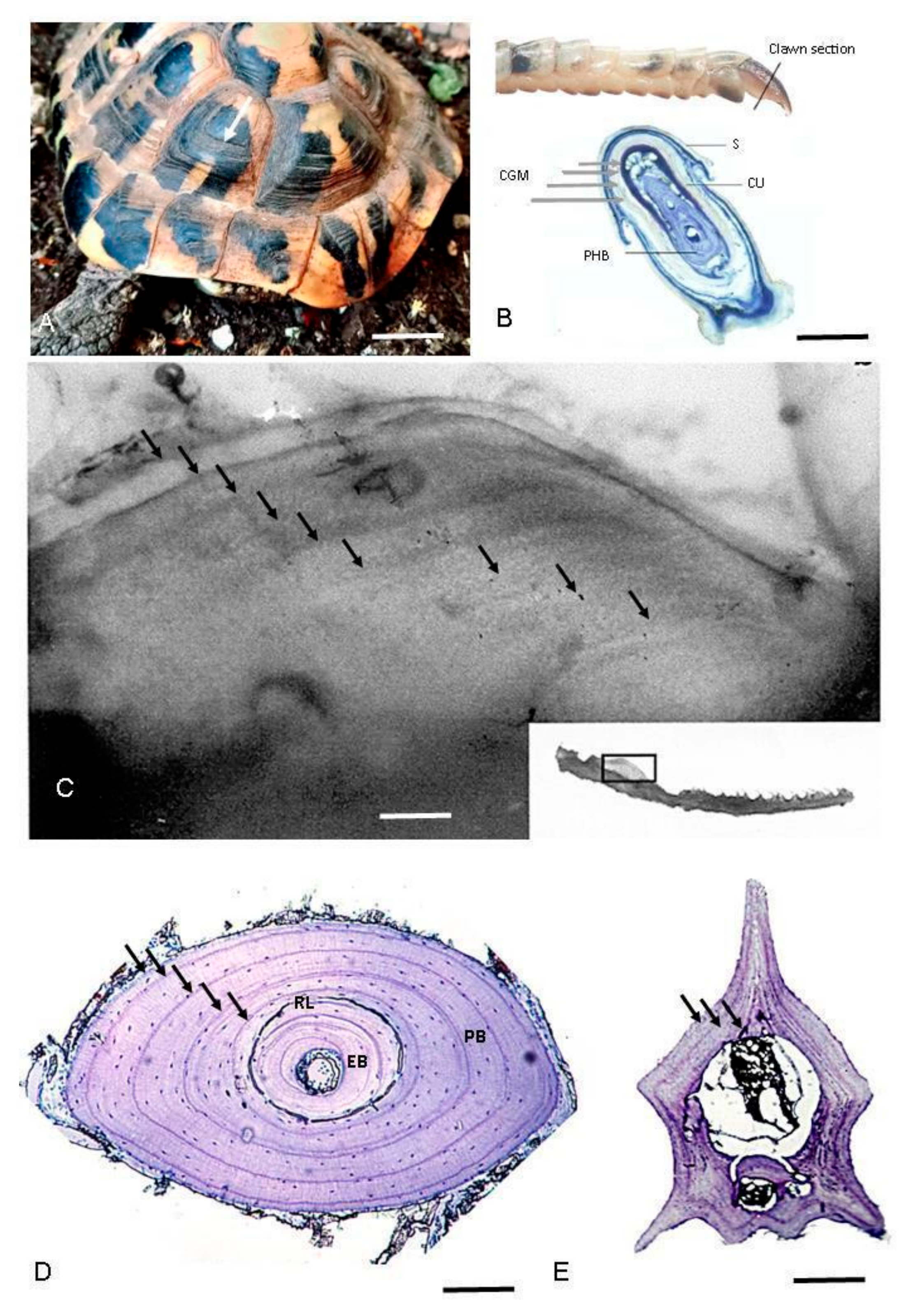
| Scutes Growth Marks  | Claws Growth Marks  | Skeletochronology | |||
|---|---|---|---|---|---|
Cranial Bones | Limb Long Bones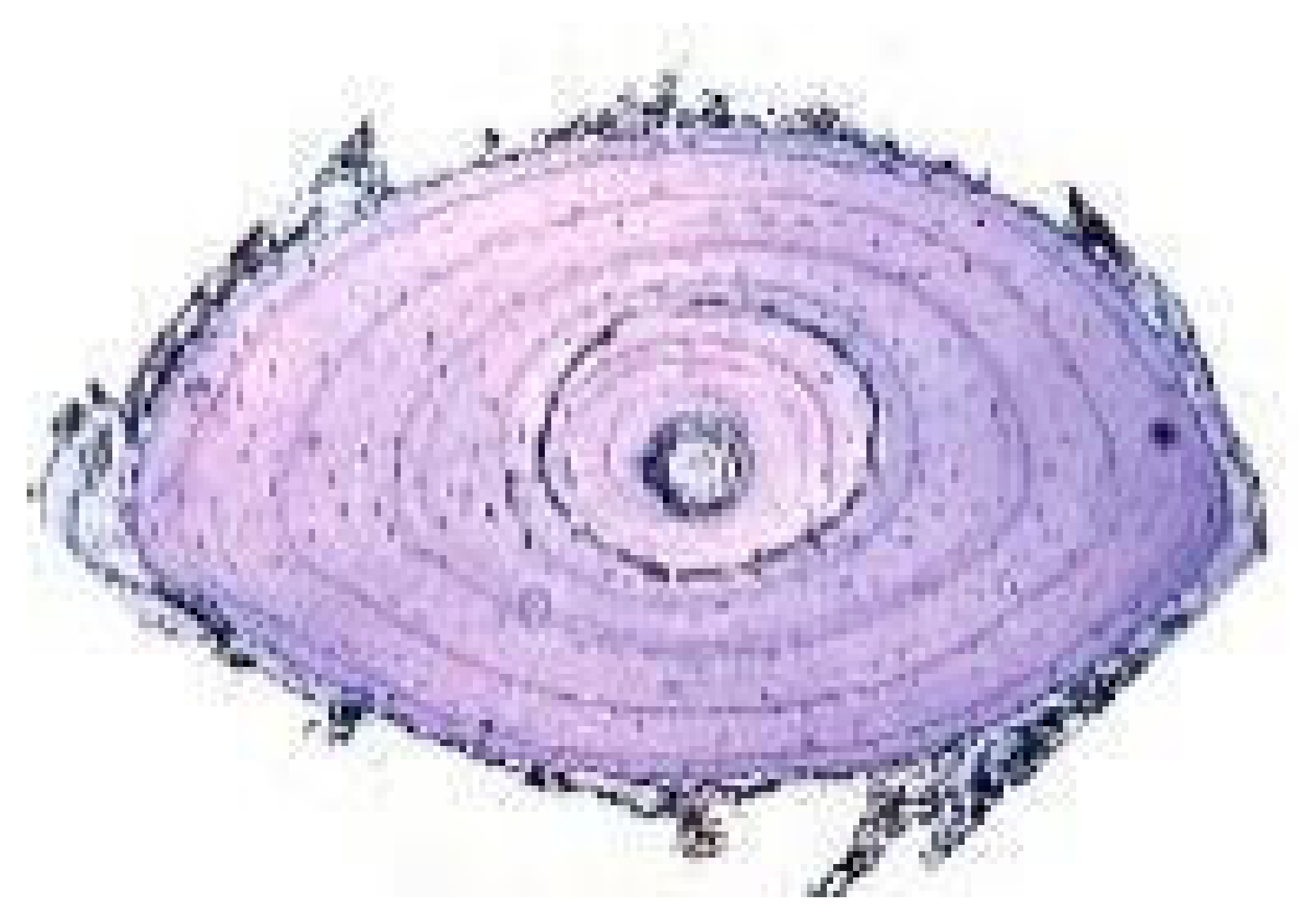 | Vertebrae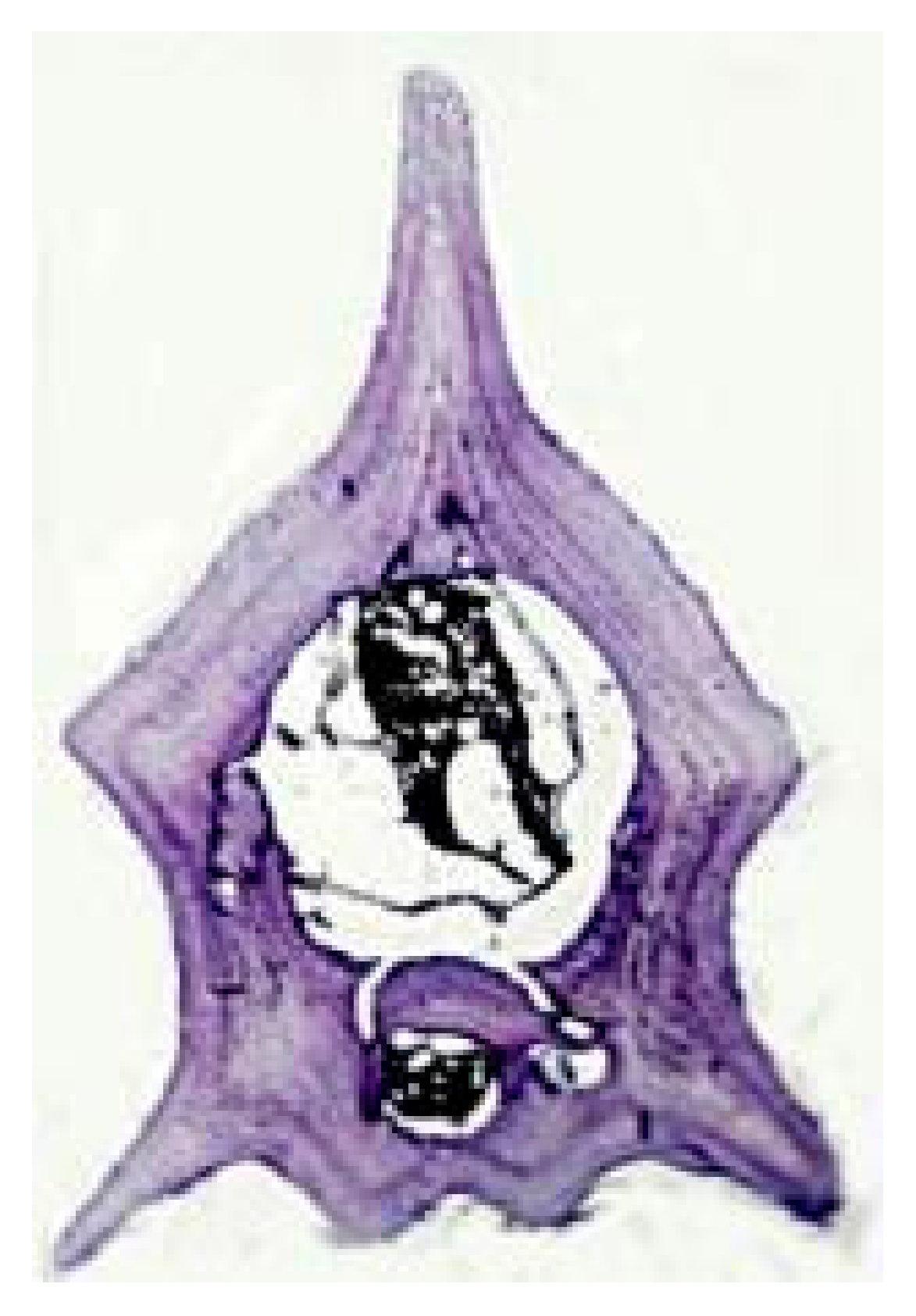 | |||
| Frogs | NA | NA | T 4 | T 9 | NT |
| Salamanders | NA | NA | T 5 | T 10 | NT |
| Caecilians | NA | NA | NT | NA | T 15 |
| Turtles | T 1 | T 2 | T 6 | T 11 | NT |
| Crocodiles | NT | NT | T 7 | T 12 | NT |
| Lizards | NT | T 3 | T 7 | T 13 | T 16 |
| Amphisbenians | NT | NA | NT | NA | NT |
| Snakes | NT | NA | T 8 | NA | T 17 |
| Tuataras | NT | NT | NT | T 14 | NT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guarino, F.M.; Mezzasalma, M. State-of-the-Art Age Determination Methods for Amphibians and Reptiles. Animals 2025, 15, 2722. https://doi.org/10.3390/ani15182722
Guarino FM, Mezzasalma M. State-of-the-Art Age Determination Methods for Amphibians and Reptiles. Animals. 2025; 15(18):2722. https://doi.org/10.3390/ani15182722
Chicago/Turabian StyleGuarino, Fabio Maria, and Marcello Mezzasalma. 2025. "State-of-the-Art Age Determination Methods for Amphibians and Reptiles" Animals 15, no. 18: 2722. https://doi.org/10.3390/ani15182722
APA StyleGuarino, F. M., & Mezzasalma, M. (2025). State-of-the-Art Age Determination Methods for Amphibians and Reptiles. Animals, 15(18), 2722. https://doi.org/10.3390/ani15182722







