DHEA and Cortisol in Rainbow Trout (Oncorhynchus mykiss): Effect of Sex, Sexual Maturity, and Acute Stress Exposure
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Matrices
2.3. Hormones Extraction and Radioimmunoassays (RIAs)
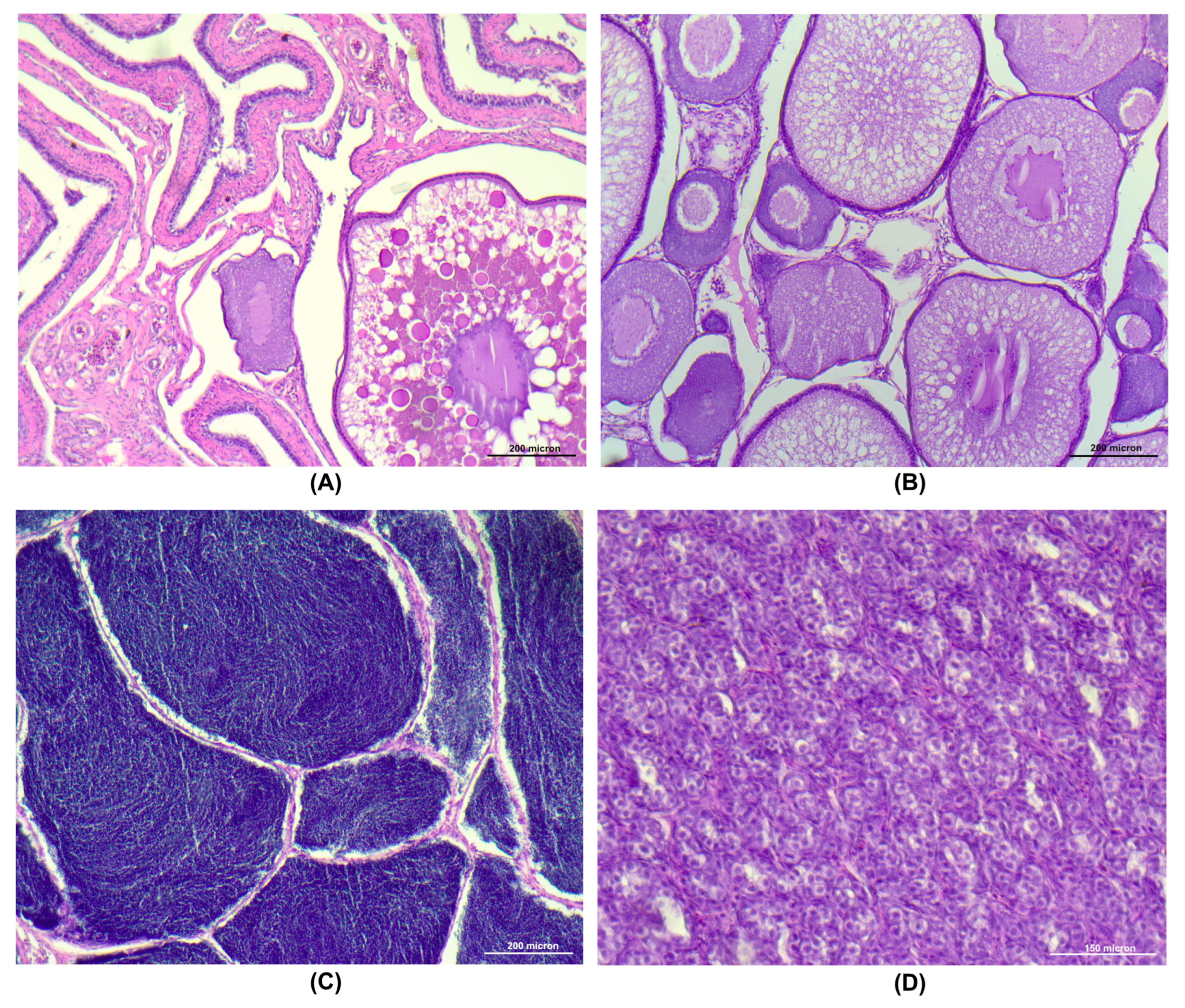
2.4. Gonad Histology
2.5. Statistical Analysis
3. Results
3.1. Fish
3.2. RIAs Validation
3.3. Hormone Concentration in the Serum
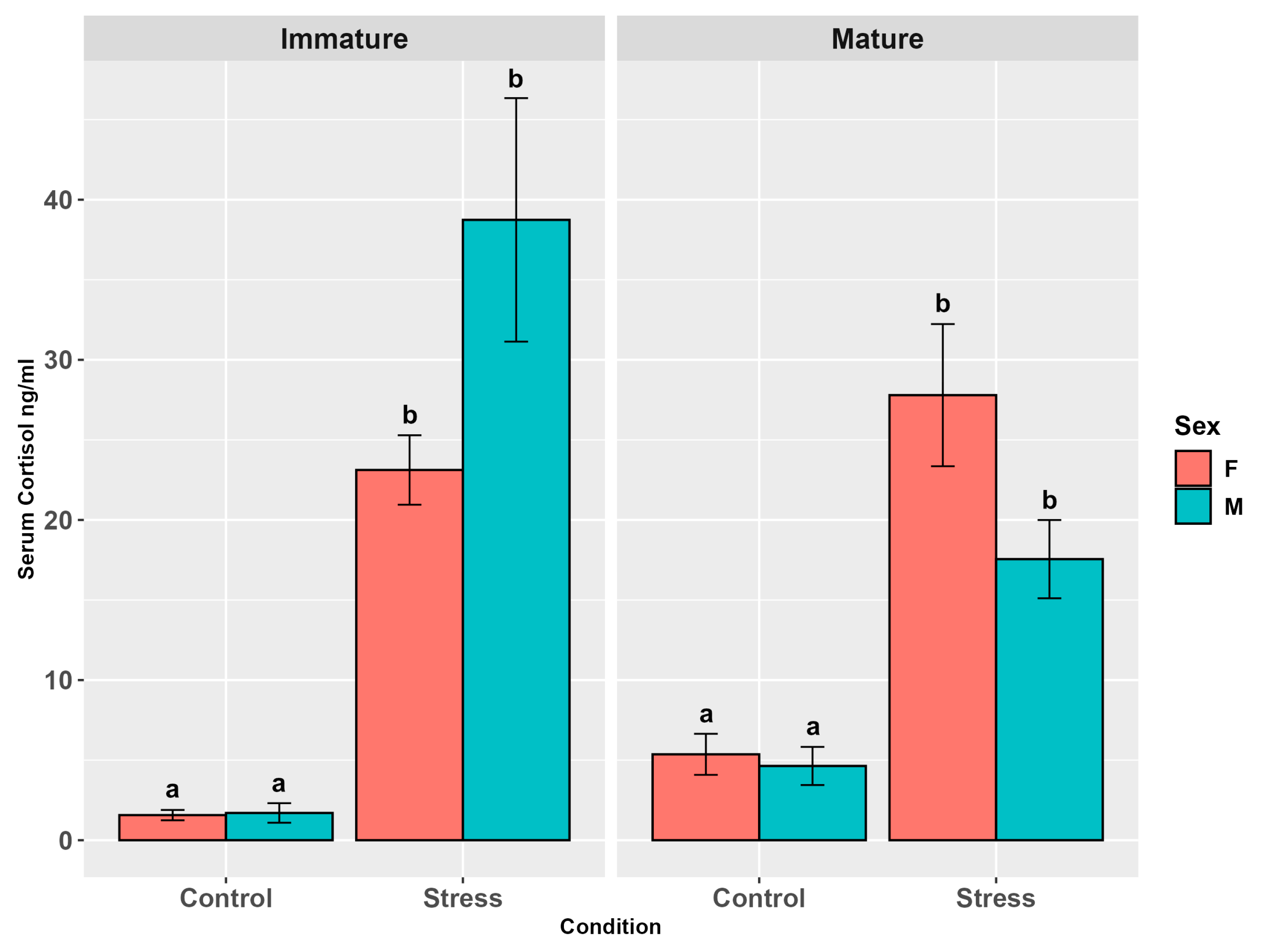
3.3.1. Cortisol
3.3.2. DHEA
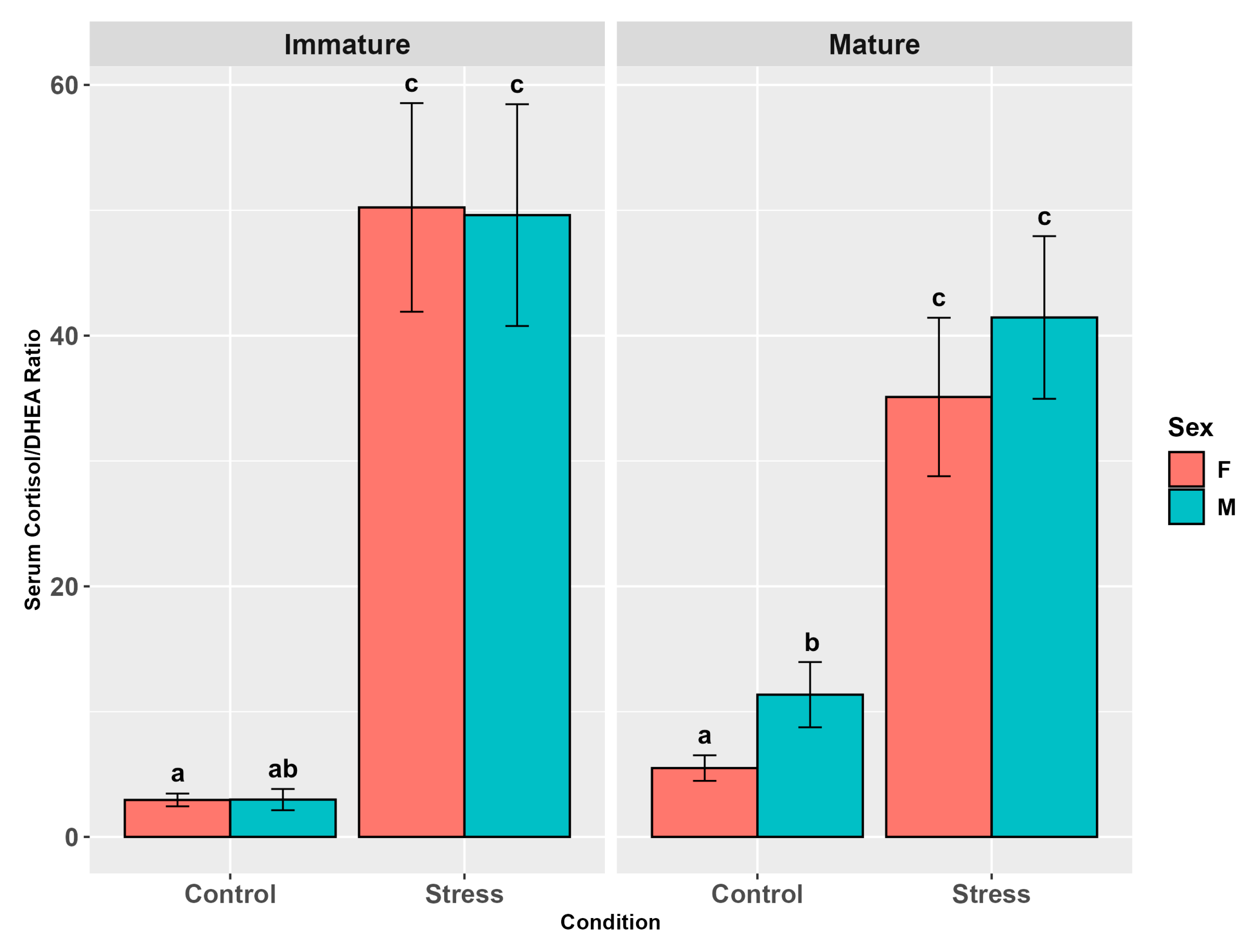
3.3.3. Cortisol/DHEA Ratio
3.4. Hormone Concentration in Alternative Matrices
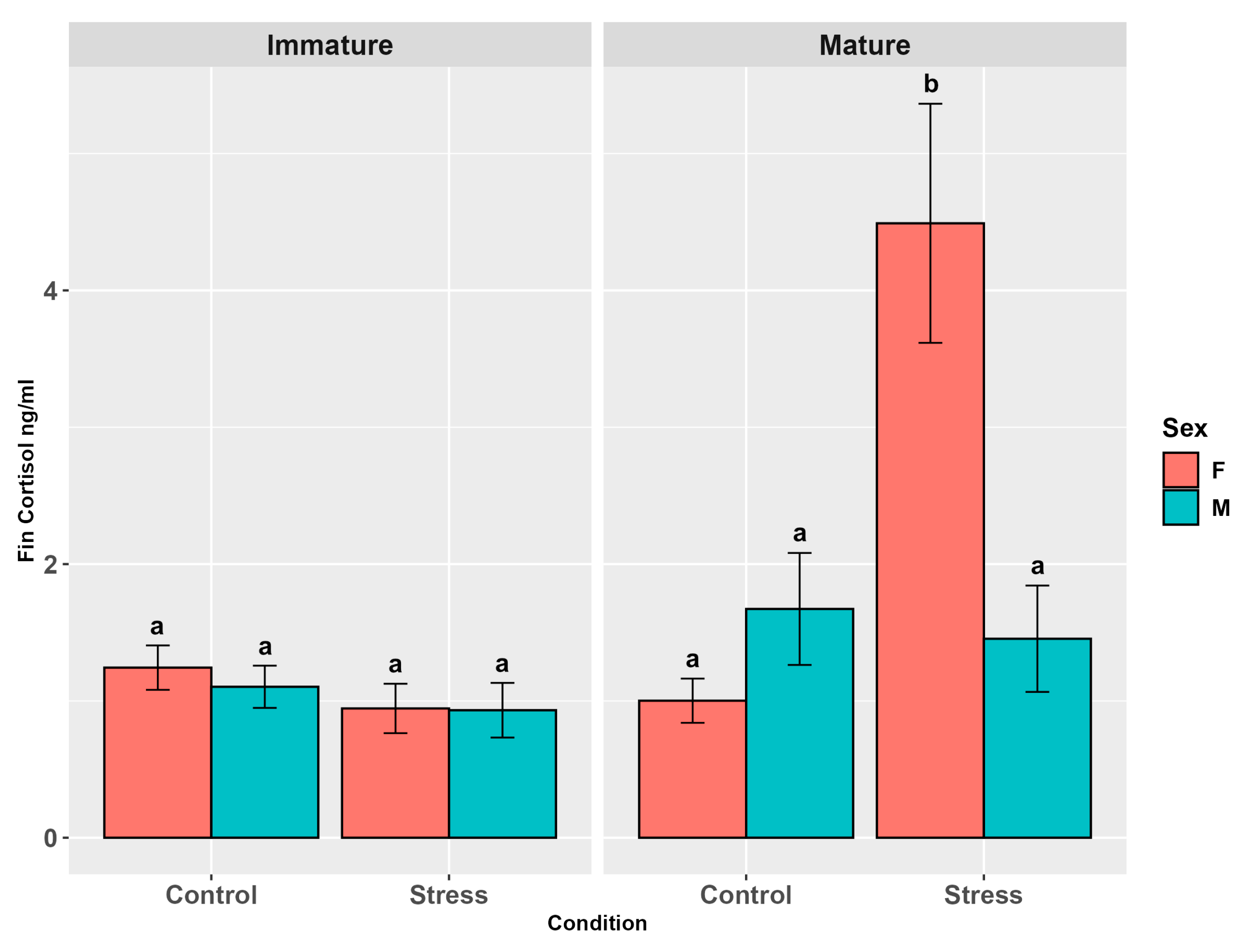
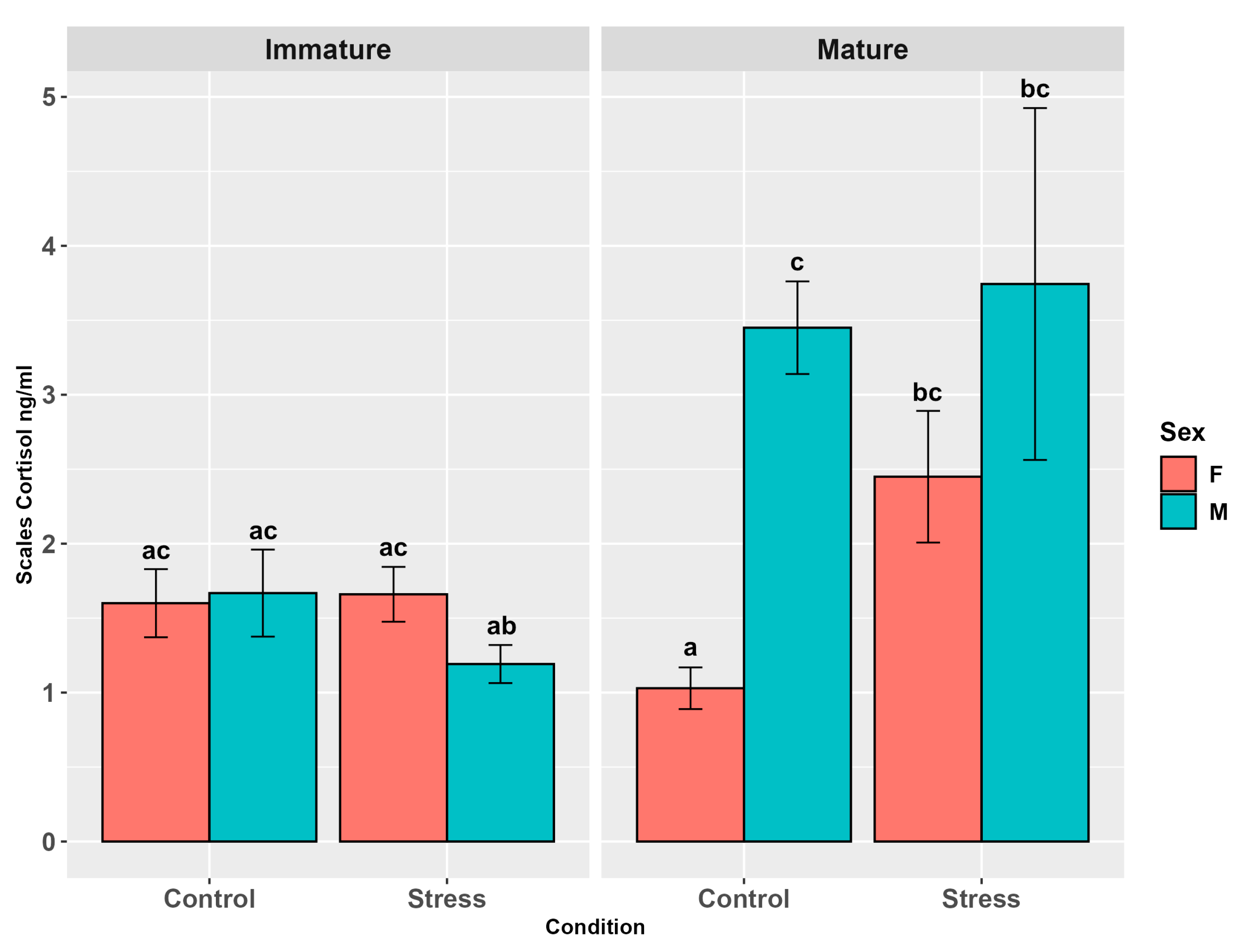
3.4.1. Cortisol
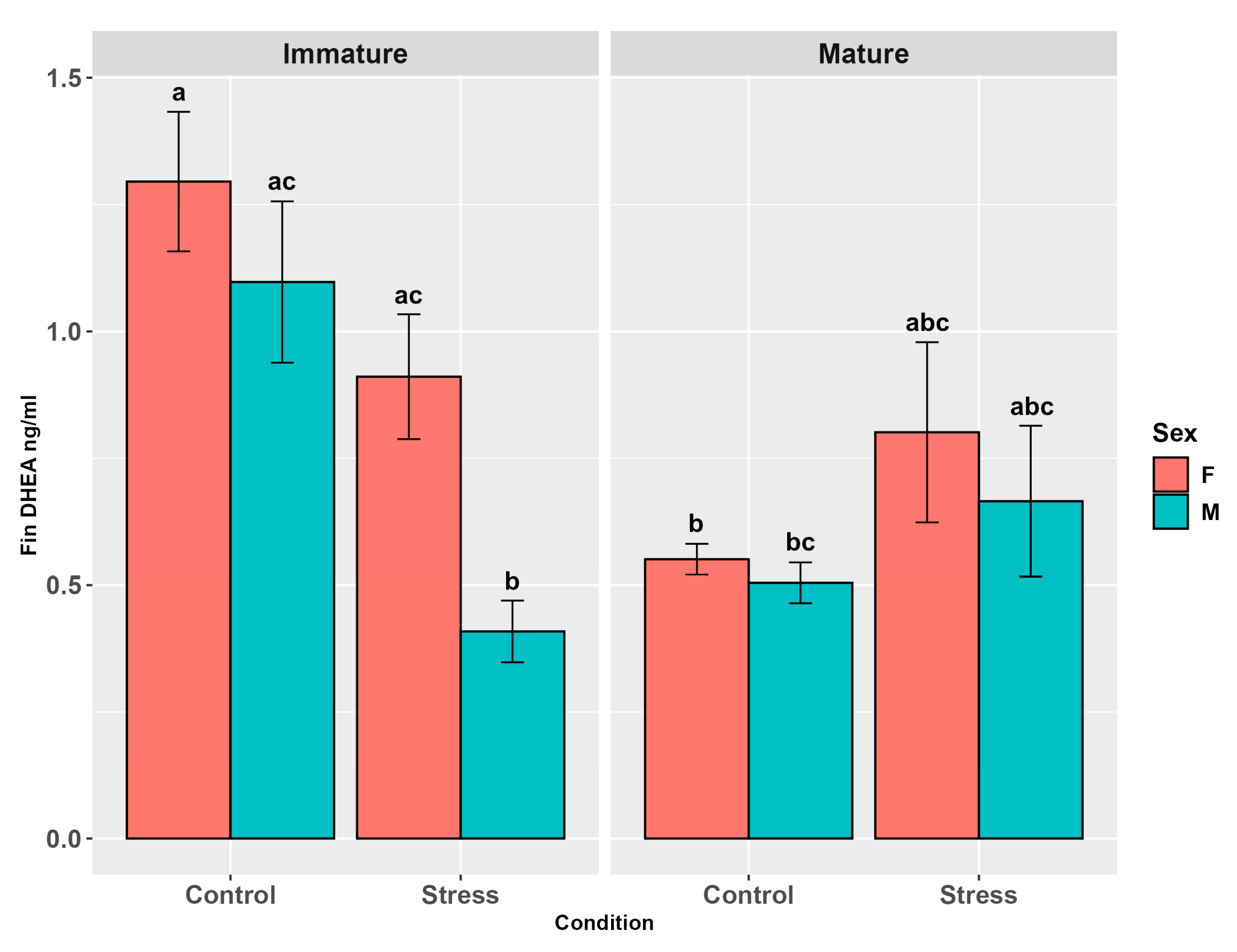
3.4.2. DHEA
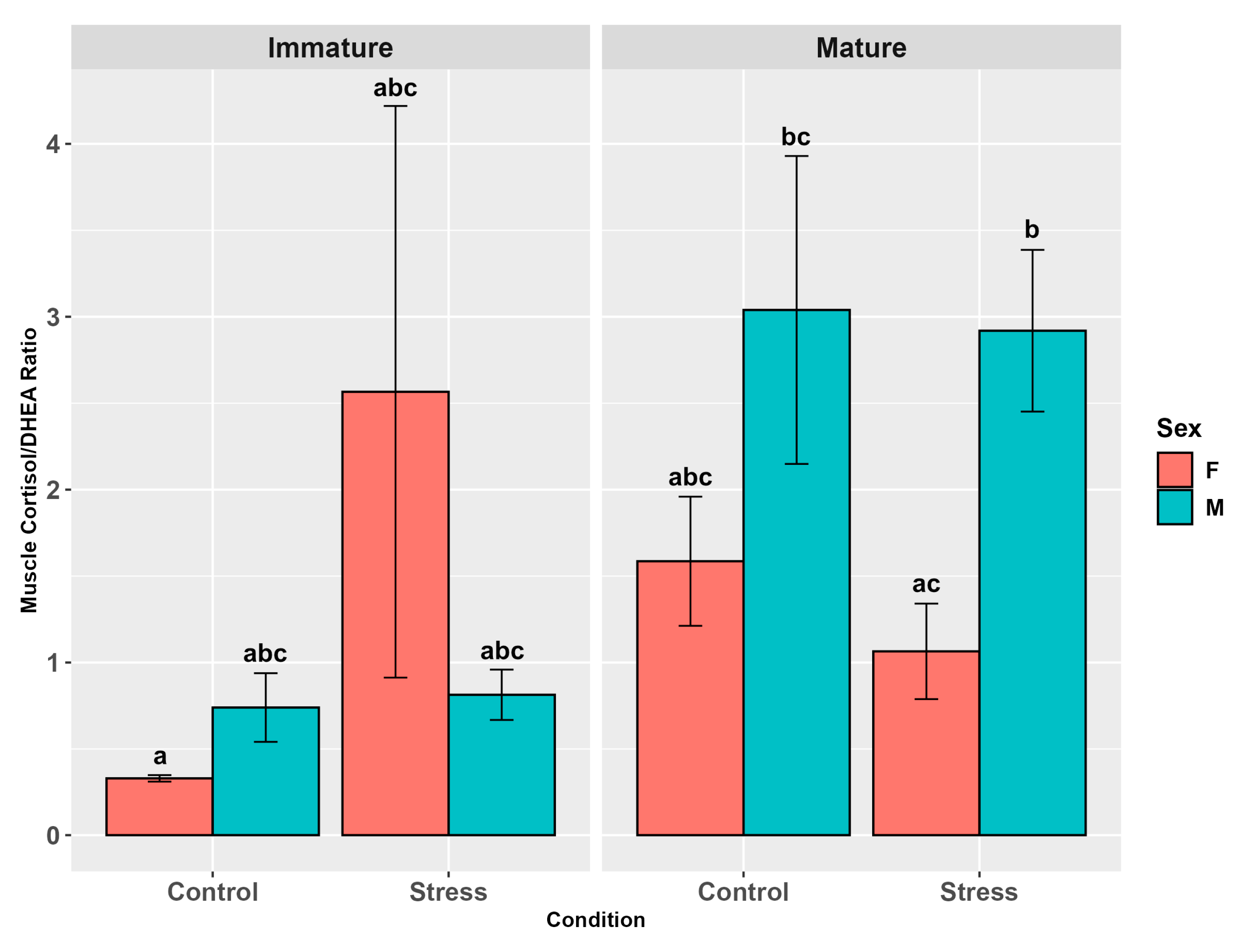
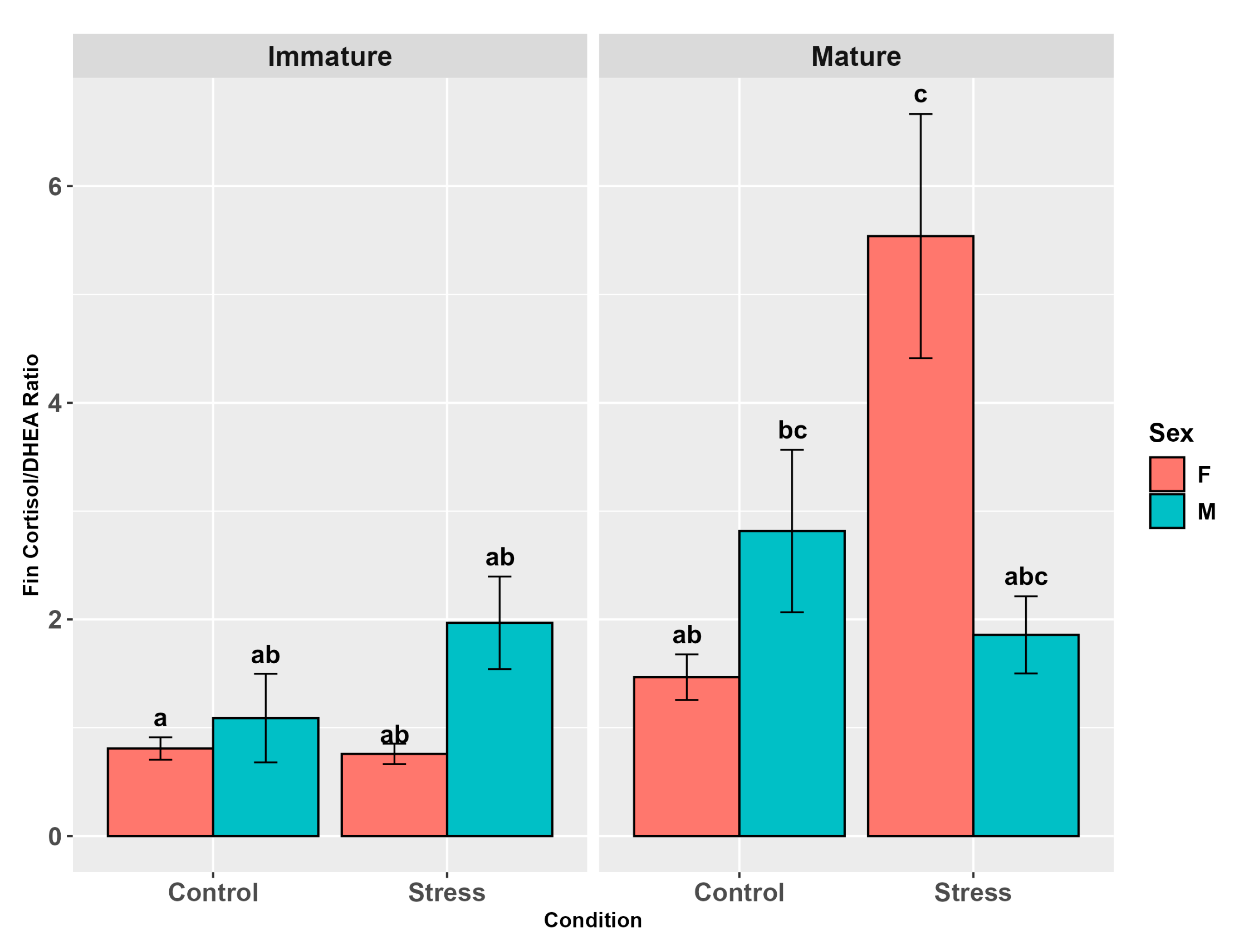
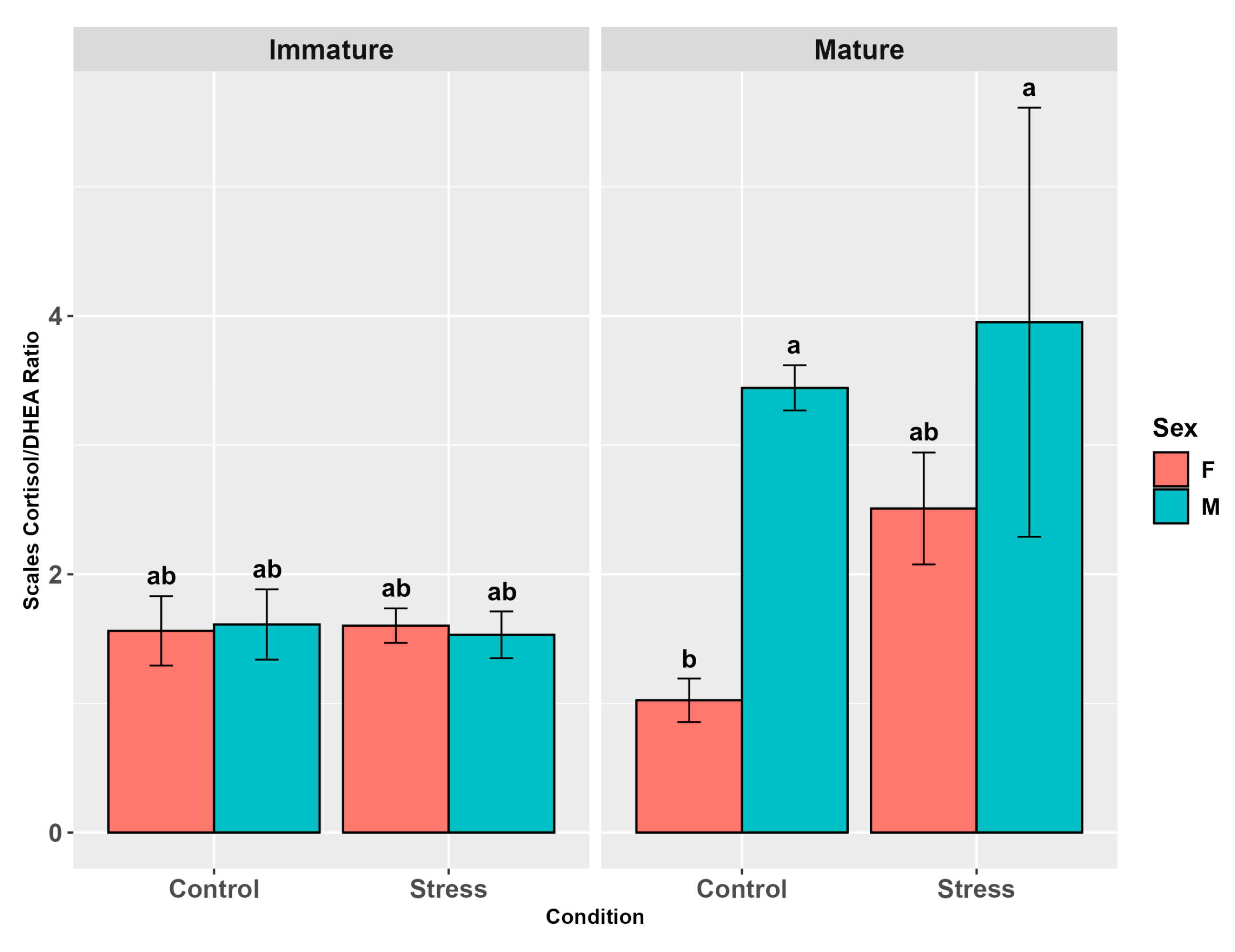
3.4.3. Cortisol/DHEA Ratio
3.5. Correlation Among Matrices
3.6. Morphological Evaluation of the Gonads
4. Discussion
4.1. Analytic Protocols
4.2. Cortisol in Serum
4.3. DHEA in Serum
4.4. Cortisol in Alternative Matrices
4.5. DHEA in Alternative Matrices
4.6. Cortisol/DHEA Ratio
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DHEA | Dehydroepiandrosterone |
| DHEAS | Dehydroepiandrosterone Sulfate |
| P450c17 | 17 alphahydroxylase/17,20 lyase |
| 11βHSD2 | 11betaHydroxysteroid Dehydrogenase type 2 |
| HPA | Hypothalamic–Pituitary–Adrenal axis |
| HPI | Hypothalamic–Pituitary–Inter-renal axis |
| ACTH | Adrenocorticotropic Hormone |
| RIA | RadioImmunoAssay |
| PBS | Phosphate-Buffered Saline |
| BSA | Bovine Serum Albumin |
| CA | Cortical Alveoli |
| POFs | Post-Ovulatory Follicles |
References
- Rutkowski, K.; Sowa, P.; Rutkowska-Talipska, J.; Kuryliszyn-Moskal, A.; Rutkowski, R. Dehydroepiandrosterone (DHEA): Hypes and hopes. Drugs 2014, 74, 1195–1207. [Google Scholar] [CrossRef]
- Gabai, G.; Mongillo, P.; Giaretta, E.; Marinelli, L. Do dehydroepiandrosterone (DHEA) and its sulfate (DHEAS) play a role in the stress response in domestic animals? Front. Vet. Sci. 2020, 7, 588835. [Google Scholar] [CrossRef] [PubMed]
- Whitham, J.C.; Bryant, J.L.; Miller, L.J. Beyond glucocorticoids: Integrating dehydroepiandrosterone (DHEA) into animal welfare research. Animals 2020, 10, 1381. [Google Scholar] [CrossRef]
- Wiebe, R.H.; Williams, L.E.; Abee, C.R.; Yeoman, R.R.; Diamond, E.J. Seasonal changes in serum dehydroepiandrosterone, androstenedione, and testosterone levels in the squirrel monkey (Saimiri boliviensis boliviensis). Am. J. Primatol. 1988, 14, 285–291. [Google Scholar] [CrossRef]
- O'Brien, J.K.; Steinman, K.J.; Fetter, G.A.; Robeck, T.R. Androgen and glucocorticoid production in the male killer whale (Orcinus orca): Influence of age, maturity, and environmental factors. Andrology 2017, 5, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Wingfield, J.C.; Ramenofsky, M.; Hegner, R.E.; Ball, G.F. Whither the challenge hypothesis? Horm. Behav. 2020, 123, 104588. [Google Scholar] [CrossRef] [PubMed]
- Kusakabe, M.; Nakamura, I.; Young, G. 11β-Hydroxysteroid dehydrogenase complementary deoxyribonucleic acid in rainbow trout: Cloning, sites of expression, and seasonal changes in gonads. Endocrinology 2003, 144, 2534–2545. [Google Scholar] [CrossRef]
- Atanassova, N.; Koev, Y. Hydrohysteroid Dehydrogenases—Biological Role and Clinical Importance—Review. InTech 2012. [Google Scholar] [CrossRef]
- Phillips, D.M.; Lakshmi, V.; Monder, C. Corticosteroid 11β-dehydrogenase in rat testis. Endocrinology 1989, 125, 209–216. [Google Scholar] [CrossRef]
- Tang, J.; Chen, L.R.; Chen, K.H. The utilization of dehydroepiandrosterone as a sexual hormone precursor in premenopausal and postmenopausal women: An overview. Pharmaceuticals 2021, 15, 46. [Google Scholar] [CrossRef]
- Blauer, K.L.; Poth, M.; Rogers, W.M.; Bernton, E.W. Dehydroepiandrosterone antagonizes the suppressive effects of dexamethasone on lymphocyte proliferation. Endocrinology 1991, 129, 3174–3179. [Google Scholar] [CrossRef]
- Maninger, N.; Capitanio, J.P.; Mason, W.A.; Ruys, J.D.; Mendoza, S.P. Acute and chronic stress increase DHEAS concentrations in rhesus monkeys. Psychoneuroendocrinology 2010, 35, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Cardounel, A.; Gursoy, E.; Anderson, P.; Kalimi, M. Anti-stress effects of dehydroepiandrosterone: Protection of rats against repeated immobilization stress-induced weight loss, glucocorticoid receptor production, and lipid peroxidation. Biochem. Pharmacol. 2000, 59, 753–762. [Google Scholar] [CrossRef]
- MacDougall-Shackleton, S.A.; Bonier, F.; Romero, L.M.; Moore, I.T. Glucocorticoids and “stress” are not synonymous. Integr. Org. Biol. 2019, 1, obz017. [Google Scholar] [CrossRef]
- Otovic, P.; Hutchinson, E. Limits to using HPA axis activity as an indication of animal welfare. ALTEX-Altern. Anim. Exp. 2015, 32, 41–50. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Frongia, G.N.; Peric, T.; Leoni, G.; Satta, V.; Berlinguer, F.; Muzzeddu, M.; Prandi, A.; Naitana, S.; Comin, A. Assessment of cortisol and DHEA concentrations in Griffon vulture (Gyps fulvus) feathers to evaluate its allostatic load. Ann. Anim. Sci. 2020, 20, 85–96. [Google Scholar] [CrossRef]
- Laberge, F.; Yin-Liao, I.; Bernier, N.J. Temporal profiles of cortisol accumulation and clearance support scale cortisol content as an indicator of chronic stress in fish. Conserv. Physiol. 2019, 7, coz052. [Google Scholar] [CrossRef] [PubMed]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and sexual steroid concentrations in fattening pigs’ hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef] [PubMed]
- Lei, M.C.; Félix, L.; Cardoso, R.; Monteiro, S.M.; Silva, S.; Venâncio, C. Non-invasive biomarkers in saliva and eye infrared thermography to assess the stress response of calves during transport. Animals 2023, 13, 2311. [Google Scholar] [CrossRef] [PubMed]
- Fokidis, H.B.; Brock, T.; Newman, C.; Macdonald, D.W.; Buesching, C.D. Assessing chronic stress in wild mammals using claw-derived cortisol: A validation using European badgers (Meles meles). Conserv. Physiol. 2023, 11, coad024. [Google Scholar] [CrossRef] [PubMed]
- Cook, N.J. Minimally invasive sampling media and the measurement of corticosteroids as biomarkers of stress in animals. Can. J. Anim. Sci. 2012, 92, 227–259. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Qulu, L.A. Stress, hypothalamic-pituitary-adrenal axis, hypothalamic-pituitary-gonadal axis, and aggression. Metab. Brain Dis. 2024, 39, 1613–1636. [Google Scholar] [CrossRef]
- Fernandez-Novo, A.; Pérez-Garnelo, S.S.; Villagrá, A.; Pérez-Villalobos, N.; Astiz, S. The effect of stress on reproduction and reproductive technologies in beef cattle—A review. Animals 2020, 10, 2096. [Google Scholar] [CrossRef]
- Murugananthkumar, R.; Sudhakumari, C.C. Understanding the impact of stress on teleostean reproduction. Aquac. Fish. 2022, 7, 553–561. [Google Scholar] [CrossRef]
- Milla, S.; Wang, N.; Mandiki, S.N.M.; Kestemont, P. Corticosteroids: Friends or foes of teleost fish reproduction? Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2009, 153, 242–251. [Google Scholar] [CrossRef]
- Ahmed, T.; Qassem, M.; Kyriacou, P.A. Measuring stress: A review of the current cortisol and dehydroepiandrosterone (DHEA) measurement techniques and considerations for the future of mental health monitoring. Stress 2023, 26, 29–42. [Google Scholar] [CrossRef]
- Kamin, H.S.; Kertes, D.A. Cortisol and DHEA in development and psychopathology. Horm. Behav. 2017, 89, 69–85. [Google Scholar] [CrossRef]
- Kennedy, E.K.; Janz, D.M. The use of fish scale hormone concentrations in the assessment of long-term stress and associated adverse effects on reproductive endocrinology. Fishes 2022, 7, 393. [Google Scholar] [CrossRef]
- Kennedy, E.K.; Janz, D.M. Chronic stress causes cortisol, cortisone and DHEA elevations in scales but not serum in rainbow trout. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2023, 276, 111352. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, E.K.; Janz, D.M. First look into the use of fish scales as a medium for multi-hormone stress analyses. Fishes 2022, 7, 145. [Google Scholar] [CrossRef]
- Mazzi, G.; Feltracco, M.; Altavilla, L.; Alterio, A.; Barbaro, E.; Bortolini, M.; Malavasi, S.; Gambaro, A. Cortisol, cortisone and DHEAS in epidermis and scales of fish Aphanius fasciatus: HPLC-MS/MS measurement of stress indicators as proxies for natural and human-induced factors. Sci. Total Environ. 2023, 904, 166900. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations (FAO). Global Production by Production Source: Quantity (1950–2023). Available online: https://www.fao.org/fishery/statistics-query/en/global_production/global_production_quantity (accessed on 25 August 2025).
- Thorgaard, G.H.; Bailey, G.S.; Williams, D.; Buhler, D.R.; Kaattari, S.L.; Ristow, S.S.; Hansen, J.D.; Winton, J.R.; Bartholomew, J.L.; Nagler, J.J.; et al. Status and opportunities for genomics research with rainbow trout. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 133, 609–646. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G.; Sumpter, J.P.; Carragher, J.F.; Le Bail, P.Y. Effects of acute and chronic stress on the levels of circulating growth hormone in the rainbow trout, Oncorhynchus mykiss. Gen. Comp. Endocrinol. 1991, 83, 86–93. [Google Scholar] [CrossRef]
- Gesto, M.; Hernández, J.; López-Patiño, M.A.; Soengas, J.L.; Míguez, J.M. Is gill cortisol concentration a good acute stress indicator in fish? A study in rainbow trout and zebrafish. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2015, 188, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Gil, J.; Martín-Diana, A.B.; Bertotto, D.; Sanz-Calvo, M.Á.; Jover-Cerdá, M.; Tomás-Vidal, A. Effects of dietary barley on rainbow trout exposed to an acute stress challenge. Aquaculture 2019, 501, 32–38. [Google Scholar] [CrossRef]
- Bertotto, D.; Poltronieri, C.; Negrato, E.; Majolini, D.; Radaelli, G.; Simontacchi, C. Alternative matrices for cortisol measurement in fish. Aquac. Res. 2010, 41, 1261–1267. [Google Scholar] [CrossRef]
- Santos, D.; Rocha, E.; Malhão, F.; Lopes, C.; Gonçalves, J.F.; Madureira, T.V. Multi-parametric portfolio to assess the fitness and gonadal maturation in four key reproductive phases of brown trout. Animals 2021, 11, 1290. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2025. [Google Scholar]
- Schumann, S.; Negrato, E.; Piva, E.; Pietropoli, E.; Bonato, M.; Irato, P.; Marion, A.; Santovito, G.; Bertotto, D. Cortisol levels reveal species-specific stress condition in fish from PFAS polluted rivers. Chemosphere 2024, 363, 142925. [Google Scholar] [CrossRef]
- Ruane, N.M.; Bonga, S.W.; Balm, P.H.M. Differences between rainbow trout and brown trout in the regulation of the pituitary–interrenal axis and physiological performance during confinement. Gen. Comp. Endocrinol. 1999, 115, 210–219. [Google Scholar] [CrossRef]
- Pickering, A.D.; Pottinger, T.G. Stress responses and disease resistance in salmonid fish: Effects of chronic elevation of plasma cortisol. Fish Physiol. Biochem. 1989, 7, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Naderi, M.; Keyvanshokooh, S.; Ghaedi, A.; Salati, A.P. Effect of acute crowding stress on rainbow trout (Oncorhynchus mykiss): A proteomics study. Aquaculture 2018, 495, 106–114. [Google Scholar] [CrossRef]
- Bry, C. Plasma cortisol levels of female rainbow trout (Salmo gairdneri) at the end of the reproductive cycle: Relationship with oocyte stages. Gen. Comp. Endocrinol. 1985, 57, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Pottinger, T.G.; Balm, P.H.M.; Pickering, A.D. Sexual maturity modifies the responsiveness of the pituitary-interrenal axis to stress in male rainbow trout. Gen. Comp. Endocrinol. 1995, 98, 311–320. [Google Scholar] [CrossRef]
- Pottinger, T.G.; Carrick, T.R.; Hughes, S.E.; Balm, P.H.M. Testosterone, 11-ketotestosterone, and estradiol-17β modify baseline and stress-induced interrenal and corticotropic activity in trout. Gen. Comp. Endocrinol. 1996, 104, 284–295. [Google Scholar] [CrossRef]
- Sorwell, K.G.; Kohama, S.G.; Urbanski, H.F. Testosterone increases circulating dehydroepiandrosterone sulfate levels in the male rhesus macaque. Front. Endocrinol. 2014, 5, 101. [Google Scholar] [CrossRef]
- Mongillo, P.; Prana, E.; Gabai, G.; Bertotto, D.; Marinelli, L. Effect of age and sex on plasma cortisol and dehydroepiandrosterone concentrations in the dog (Canis familiaris). Res. Vet. Sci. 2014, 96, 33–38. [Google Scholar] [CrossRef]
- Chin, E.H.; Shah, A.H.; Schmidt, K.L.; Sheldon, L.D.; Love, O.P.; Soma, K.K. Sex differences in DHEA and estradiol during development in a wild songbird: Jugular versus brachial plasma. Horm. Behav. 2008, 54, 194–202. [Google Scholar] [CrossRef]
- Rege, J.; Garber, S.; Conley, A.J.; Elsey, R.M.; Turcu, A.F.; Auchus, R.J.; Rainey, W.E. Circulating 11-oxygenated androgens across species. J. Steroid Biochem. Mol. Biol. 2019, 190, 242–249. [Google Scholar] [CrossRef]
- Tan, M.R.; Aung, K.M.M.; Tan, J.Y.A.; Chua, K.X.; Doblado, G.J.; Chua, K.L.; Tham, V.Y.Y.; Lin, J.J.; Chaganty, V.; Yusoff, D.M.; et al. Dynamics of endogenous and water cortisol release in Asian Sea bass Lates calcarifer after acute stress in a farm scale recirculating aquaculture system. Aquac. Rep. 2024, 37, 102223. [Google Scholar] [CrossRef]
- Carbajal, A.; Reyes-López, F.E.; Tallo-Parra, O.; Lopez-Bejar, M.; Tort, L. Comparative assessment of cortisol in plasma, skin mucus and scales as a measure of the hypothalamic-pituitary-interrenal axis activity in fish. Aquaculture 2019, 506, 410–416. [Google Scholar] [CrossRef]
- Samaras, A.; Dimitroglou, A.; Kollias, S.; Skouradakis, G.; Papadakis, I.E.; Pavlidis, M. Cortisol concentration in scales is a valid indicator for the assessment of chronic stress in European sea bass, Dicentrarchus labrax L. Aquaculture 2021, 545, 737257. [Google Scholar] [CrossRef]
- Carbajal, A.; Monclús, L.; Tallo-Parra, O.; Sabes-Alsina, M.; Vinyoles, D.; López Béjar, M. Cortisol detection in fish scales by enzyme immunoassay: Biochemical and methodological validation. J. Appl. Ichthyol. 2018, 34, 967–970. [Google Scholar] [CrossRef]
- Roque d’Orbcastel, E.; Bettarel, Y.; Dellinger, M.; Sadoul, B.; Bouvier, T.; Amandé, J.M.; Dagorn, L.; Geffroy, B. Measuring cortisol in fish scales to study stress in wild tropical tuna. Environ. Biol. Fishes 2021, 104, 725–732. [Google Scholar] [CrossRef]
- Faught, E.; Vijayan, M.M. Maternal stress and fish reproduction: The role of cortisol revisited. Fish Fish. 2018, 19, 1016–1030. [Google Scholar] [CrossRef]
- Zhang, S.D.; Gant, T.W. Effect of pooling samples on the efficiency of comparative studies using microarrays. Bioinformatics 2005, 21, 4378–4383. [Google Scholar] [CrossRef] [PubMed]




| Group | Sex | Maturity | Stress | Weight (g) | p < 0.05 |
|---|---|---|---|---|---|
| 1 | Female | Mature | Control | 975.77 ± 36.98 | a |
| 2 | Female | Mature | Stress | 1015.38 ± 40.33 | a |
| 3 | Female | Immature | Control | 1280.00 ± 70.67 | bc |
| 4 | Female | Immature | Stress | 1419.17 ± 63.89 | b |
| 5 | Male | Mature | Control | 1053.33 ± 57.23 | ac |
| 6 | Male | Mature | Stress | 955.83 ± 56.78 | a |
| 7 | Male | Immature | Control | 1461.67 ± 28.41 | b |
| 8 | Male | Immature | Stress | 1326.67 ± 54.74 | b |
| Cortisol | ||||
|---|---|---|---|---|
| Matrix | R2 | EY% | Intra | Inter |
| Serum | 0.9983 | 85 | 4.26 | 2.69 |
| Muscle | 0.9948 | 80 | 7.68 | 5.49 |
| Fin | 0.9943 | 84 | 5.81 | 4.85 |
| Scales | 0.9997 | 82 | 5.06 | 3.67 |
| DHEA | ||||
| Matrix | R2 | EY% | Intra | Inter |
| Serum | 0.9983 | 74 | 5.55 | 2.26 |
| Muscle | 0.9948 | 72 | 8.33 | 7.54 |
| Fin | 0.9943 | 71 | 4.94 | 3.77 |
| Scales | 0.9997 | 79 | 8.73 | 5.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meloni, A.; Bortoletti, M.; Negrato, E.; Fonsatti, E.; Radaelli, G.; Bertotto, D. DHEA and Cortisol in Rainbow Trout (Oncorhynchus mykiss): Effect of Sex, Sexual Maturity, and Acute Stress Exposure. Animals 2025, 15, 2710. https://doi.org/10.3390/ani15182710
Meloni A, Bortoletti M, Negrato E, Fonsatti E, Radaelli G, Bertotto D. DHEA and Cortisol in Rainbow Trout (Oncorhynchus mykiss): Effect of Sex, Sexual Maturity, and Acute Stress Exposure. Animals. 2025; 15(18):2710. https://doi.org/10.3390/ani15182710
Chicago/Turabian StyleMeloni, Andrea, Martina Bortoletti, Elena Negrato, Elisa Fonsatti, Giuseppe Radaelli, and Daniela Bertotto. 2025. "DHEA and Cortisol in Rainbow Trout (Oncorhynchus mykiss): Effect of Sex, Sexual Maturity, and Acute Stress Exposure" Animals 15, no. 18: 2710. https://doi.org/10.3390/ani15182710
APA StyleMeloni, A., Bortoletti, M., Negrato, E., Fonsatti, E., Radaelli, G., & Bertotto, D. (2025). DHEA and Cortisol in Rainbow Trout (Oncorhynchus mykiss): Effect of Sex, Sexual Maturity, and Acute Stress Exposure. Animals, 15(18), 2710. https://doi.org/10.3390/ani15182710









