The Spatiotemporal Distribution Patterns and Coexistence Mechanisms of Two Musk Deer Species in Mt. Gongga, Sichuan Province, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
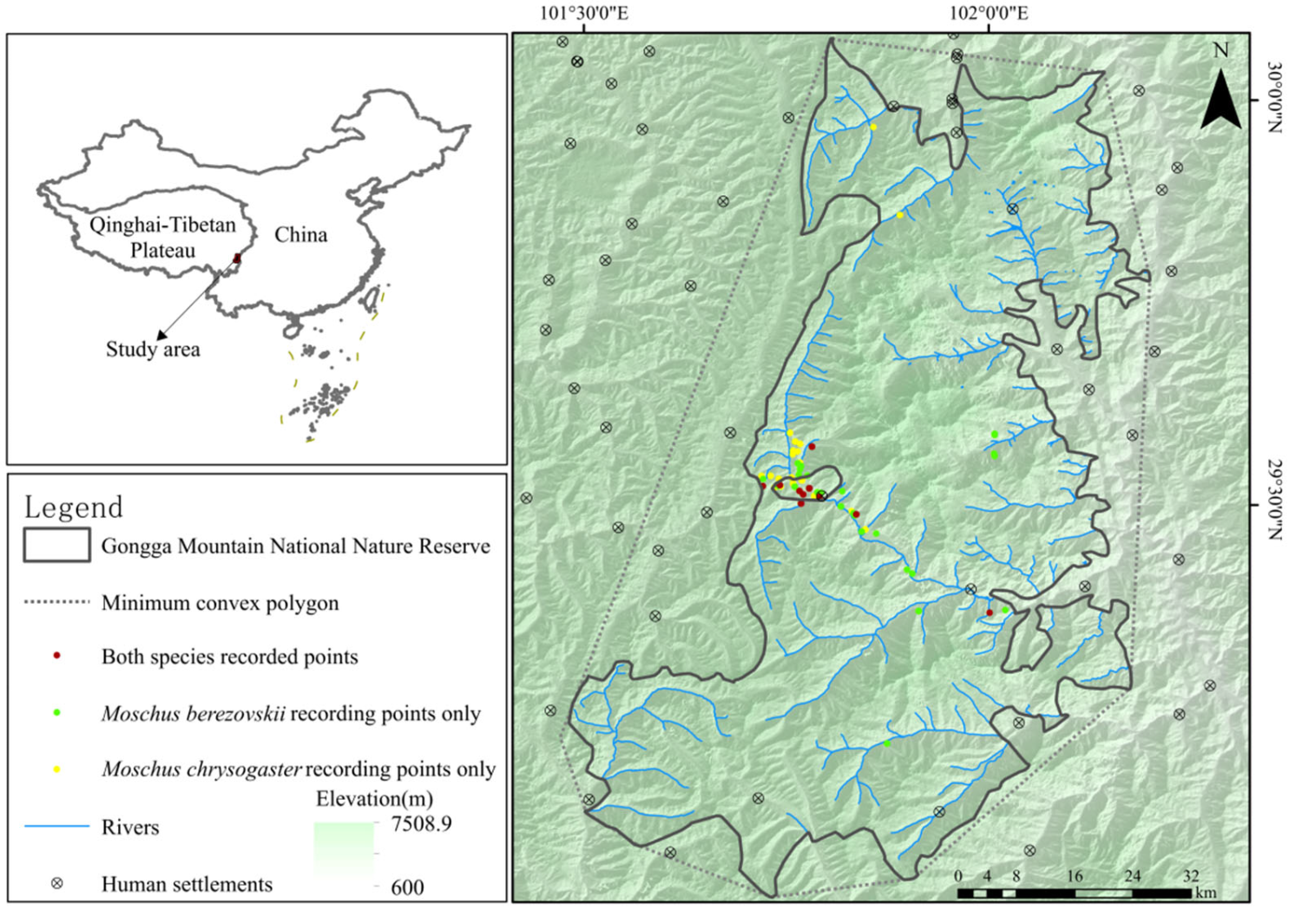
2.1. Study Area
2.2. Species Data Source and Processing
2.3. Environmental Data Source and Processing
2.4. MaxEnt Modeling
2.5. Kernel Density Estimation
3. Results
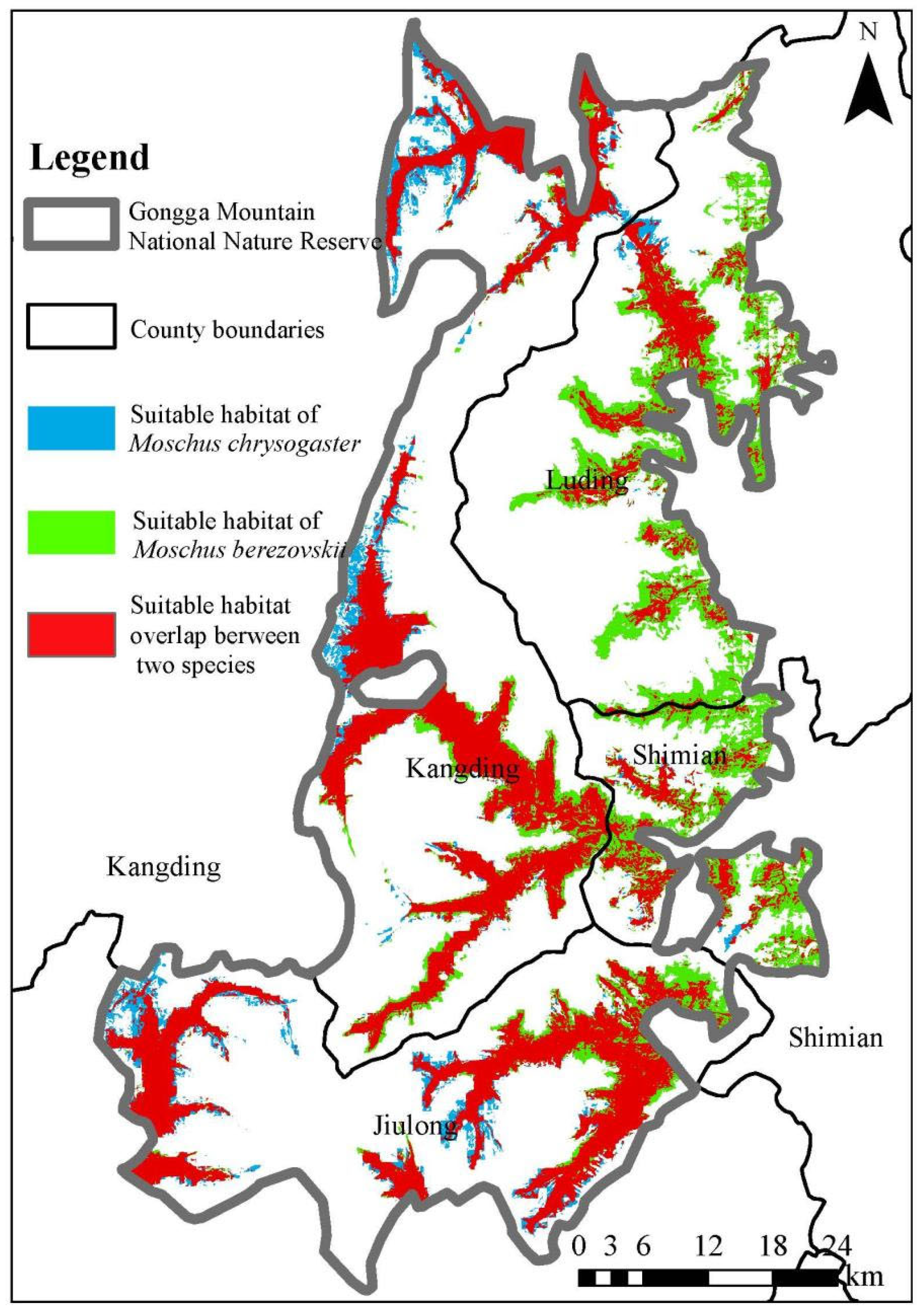
3.1. Spatial Distribution Patterns
3.2. Correlation Between Habitat Suitability and Environmental Variables
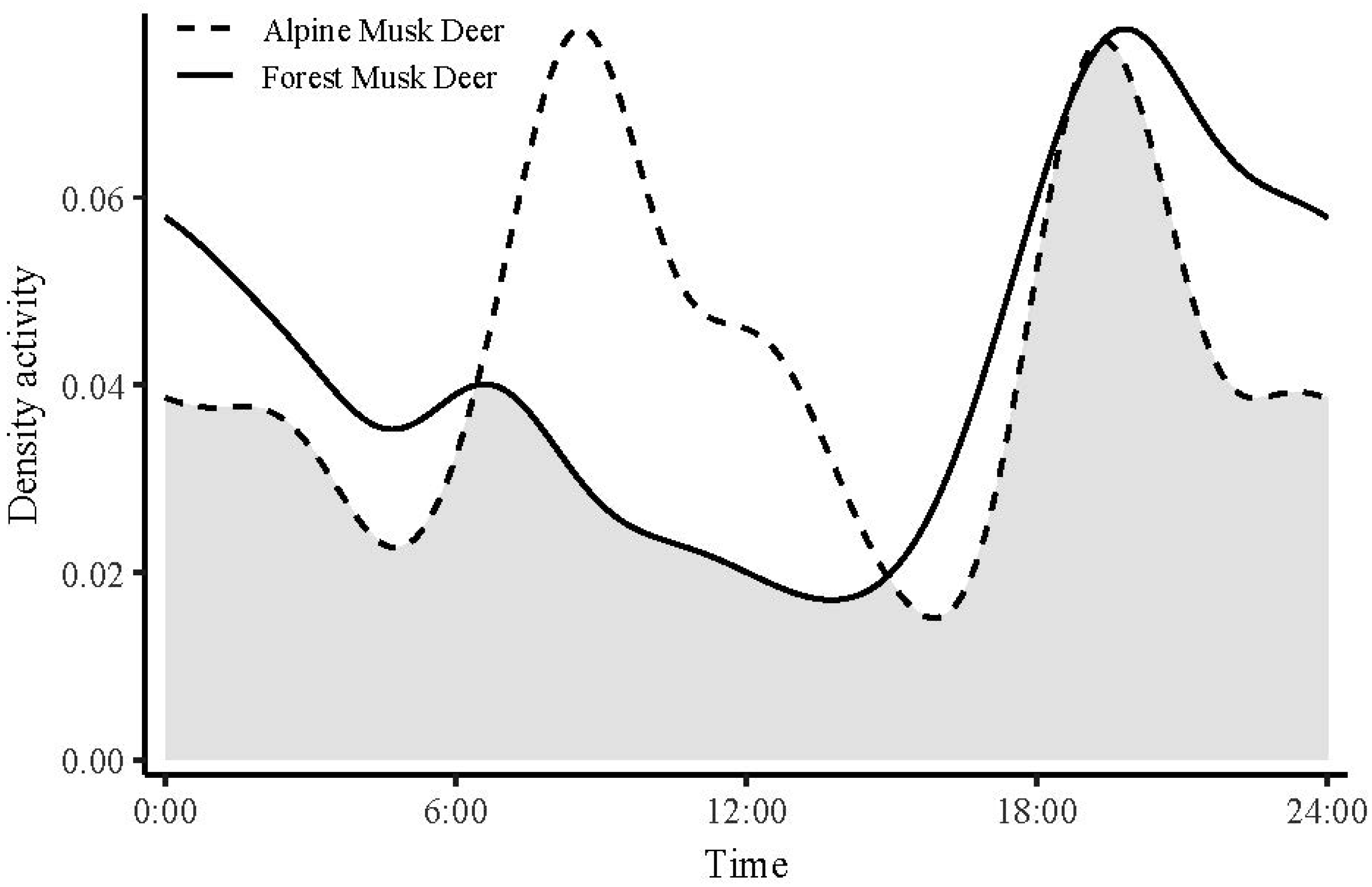
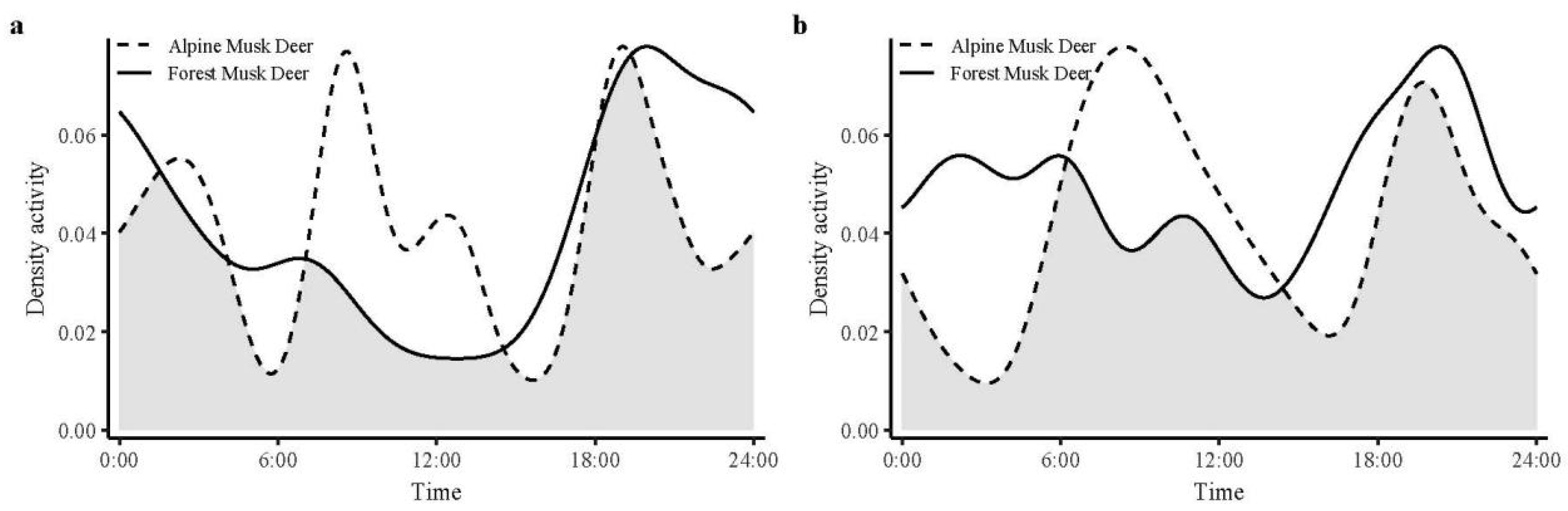
3.3. Temporal Distribution Patterns
4. Discussion
4.1. Spatial Ecological Niche
4.2. Temporal Ecological Niche
4.3. Coexistence Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hutchinson, G.E. Homage to Santa-Rosalia of why are there so many kinds of animals. Am. Nat. 1959, 93, 145–159. [Google Scholar] [CrossRef]
- Schoener, T.W. Resource partitioning in ecological communities. Science 1974, 185, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Namgail, T. Winter habitat partitioning between asiatic Ibex and blue sheep in Ladakh, Northern India. J. Mt. Ecol. 2006, 8, 7–13. [Google Scholar]
- Felton, A.M.; Hedwall, P.O.; Felton, A.; Wam, H.K. Forage availability, supplementary feed and ungulate density: Associations with ungulate damage in pine production forests. For. Ecol. Manag. 2022, 513, 120187. [Google Scholar] [CrossRef]
- Sheng, H.L.; Liu, Z.X. Chinese Musk Deer; Shanghai Science and Technology Press: Shanghai, China, 2007. (In Chinese) [Google Scholar]
- Liu, Z.X.; Sheng, H.L. A review of ecological research and protection sssues of musk deer in China. Chin. J. Zool. 2000, 3, 54–57. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Q.S.; Meng, X.X.; Xia, L.; Feng, Z.J. Conservation status and causes of decline of musk deer (Moschus spp.) in China. Biol. Conserv. 2003, 109, 333–342. [Google Scholar] [CrossRef]
- Yao, G.; Wang, B.B.; Zhu, Y.; Wan, Q.H.; Fang, S.G. Low Population Density of the Endangered Forest Musk Deer, Moschus berezovskii, in China. Pak. J. Zool. 2015, 47, 325–333. [Google Scholar]
- Feng, H.; Wang, L.; Cao, F.; Ma, J.; Tang, J.; Feng, C.; Su, Z. Forest musk deer (Moschus berezovskii) in China: Research and protection. J. Vertebr. Biol. 2023, 72, 22067. [Google Scholar] [CrossRef]
- Wang, W.F.; Fu, X.; Sun, Y.M.; Gao, T.; Wang, X.H. Impacts of land use changes on habitat corridors for the wild forest musk deer at the northern Qinling Mountains, China. Reg. Environ. Change 2025, 25, 83. [Google Scholar] [CrossRef]
- Mi, S.H.; Zhao, C.; Sun, J.; Li, Z.Z.; Teng, L.W.; Liu, Z.S. Study on the habitat selection of alpine musk Deer in autumn. Sichuan J. Zool. 2021, 40, 169–175. (In Chinese) [Google Scholar]
- Shen, L.; Gao, H.; Wu, J.; Wang, G.; Liu, R.; Qi, J.; Zhang, X.; Zhang, A.; Chen, L.; Zhang, Y.; et al. Birth-site habitat selection of wild alpine musk deer (Moschus chrysogaster) in the northeastern qing-tibetan plateau of China. Biologia 2023, 78, 141–147. [Google Scholar] [CrossRef]
- Liu, Z.G.; Qiu, F.Y. The main vegetation types and their distribution in the Gongga Mountain region. Chin. J. Plant Ecol. 1986, 1, 26–34. (In Chinese) [Google Scholar]
- Li, P. Study of Physiological Characteristics of the Alpine Treeline Trees in Mt.Gongga. Master’s Thesis, Southwest University, Chongqing, China, 2008. (In Chinese). [Google Scholar]
- Qiao, J.; Jia, G.Q.; Zhou, H.M.; Gong, L.; Jiang, Y.; Xiao, N.W.; Gao, X.Q.; Wen, A.X.; Wang, J. Mammal and bird diversity recorded with camera traps in Gongga Mountain National Nature Reserve, Sichuan, China. Biodivers. Sci. 2022, 30, 116–123. (In Chinese) [Google Scholar] [CrossRef]
- Yang, Q.S.; Feng, Z.J.; Wang, Z.W.; Liu, W.L.; Li, X.C.; Silang, O.Z. Research on the territory of the apline musk deer in the southeastern Xizang. Acta Theriol. Sin. 1998, 2, 8–10+12–15. (In Chinese) [Google Scholar] [CrossRef]
- Wisdom, M.J.; Nielson, R.M.; Rowland, M.M.; Proffitt, K.M. Modeling Landscape Use for Ungulates: Forgotten Tenets of Ecology, Management, and Inference. Front. Ecol. Evol. 2020, 8, 211. [Google Scholar] [CrossRef]
- Bowyer, R.T.; Kie, J.G. Effects of scale on interpreting life-history characteristics of ungulates and carnivores. Divers. Distrib. 2006, 12, 244–257. [Google Scholar] [CrossRef]
- Liu, Y.C.; Wu, H.; Liu, S.H. Research Advances in Habitat Selection of Terrestrial Wildlife from Spatial Scales Perspective. J. Green Sci. Technol. 2015, 12, 5–8+13. (In Chinese) [Google Scholar]
- Shi, J.B.; Dunbar, R.; Buckland, D.; Miller, D. Daytime activity budgets of feral goats (Capra hircus) on the Isle of Rum: Influence of season, age, and sex. Can. J. Zool.-Rev. Can. De Zool. 2003, 81, 803–815. [Google Scholar] [CrossRef]
- Phillips, S.J.; Dudík, M. Modeling of species distributions with Maxent: New extensions and a comprehensive evaluation. Ecography 2008, 31, 161–1750. [Google Scholar] [CrossRef]
- Swets, J.A. Measuring the accuracy of diagnostic systems. Science 1988, 240, 1285–1293. [Google Scholar] [CrossRef]
- Liu, C.R.; White, M.; Newell, G. Selecting thresholds for the prediction of species occurrence with presence-only data. J. Biogeogr. 2013, 40, 778–789. [Google Scholar] [CrossRef]
- Warren, D.L.; Glor, R.E.; Turelli, M. ENMTools: A toolbox for comparative studies of environmental niche models. Ecography 2010, 33, 607–611. [Google Scholar] [CrossRef]
- You, Z.Y.; Lu, B.G.; Du, B.B.; Yang, N. Spatio-temporal viche of sympatric tufted deer (Elaphodus cephalophus) and sambar (Rusa unicolor) based on camera traps in the Gongga Mountain National Nature Reserve, China. Animals 2022, 12, 2694. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.; Ridout, M.; Campbell, L.A. overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns, R Package version 0.3.9.; The R Foundation: Vienna, Austria, 2024. [Google Scholar] [CrossRef]
- Ridout, M.S.; Linkie, M. Estimating overlap of daily activity patterns from camera trap data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Wei, F.W.; Feng, Z.J.; Wang, Z.W. Research overview on wildlife habitat selection. Chin. J. Zool. 1998, 33, 49–53. (In Chinese) [Google Scholar]
- Wu, J.Y.; Wang, W. The Musk Deer of China; China Forestry Publishing House: Beijing, China, 2006. (In Chinese) [Google Scholar]
- Staudenmaier, A.R.; Shipley, L.A.; Bibelnieks, A.J.; Camp, M.J.; Thornton, D.H. Habitat use and spatio-temporal interactions of mule and white-tailed deer in an area of sympatry in NE Washington. Ecosphere 2021, 12, e03813.1-18. [Google Scholar] [CrossRef]
- Baasch, D.M. Resource Selection by White-Tailed Deer, Mule Deer, and Elk in Nebraska. Master’s Thesis, The University of Nebraska-Lincoln, Lincoln, NE, USA, 2008. [Google Scholar]
- Sun, J.X.; Li, J.Q.; Wan, Y.Q.; Xu, H.G. Study on the activity rhythms of nine ungulates in summer and autumn in Sichuan. J. Ecol. Rural Environ. 2018, 34, 1003–1009. (In Chinese) [Google Scholar] [CrossRef]
- Qiu, B.H.; Meng, Q.L.; Jin, Z.J.; Jiang, Q.; Wang, C. Analysis of daily activity rhythms of three ungulates in winter in Changbai Mountain Nature Reserve. Chin. J. Wildl. 2022, 43, 331–336. (In Chinese) [Google Scholar] [CrossRef]
- Ma, D.F.; Sun, Z.Y.; Hu, D.Z.; An, B.; Chen, L.Y.; Zhang, D.X.; Dong, K.; Zhang, L.X. Camera-trapping survey of the mammal diversity in the Qilian Mountains National Nature Reserve, Gansu Province. Acta Theriol. Sin. 2021, 41, 90–98. (In Chinese) [Google Scholar] [CrossRef]
- Li, P.; Zhang, Z.J.; Yang, H.; Wei, W.; Zhou, H.; Hong, M.S.; Fu, M.X.; Song, X.Q.; Yu, J. Study on activity rhythms of ungulates in Daxiangling Nature Reserve based on infrared camera trapping. J. Sichuan For. Sci. Technol. 2021, 42, 18–23. (In Chinese) [Google Scholar]
- Zha, M.H. Study on Habitat and Activity Rhythm of Forest Musk Deer (Moschus berezovskii) in Tangjiahe National Nature Reserve. Master’s Thesis, Beijing Forestry University, Beijing, China, 2019. (In Chinese). [Google Scholar]
- Yang, C.C. Activity Rhythm of Moschus berezovskii in Hunan Hupingshan National Nature Reserve by Camera Technology. Master’s Thesis, Central South University of Forestry & Technology, Changsha, China, 2021. (In Chinese). [Google Scholar]
- Chen, Y.; Xiao, Z.; Zhang, L.; Wang, X.; Li, M.; Xiang, Z. Activity rhythms of coexisting red serow and Chinese serow at Mt. Gaoligong as identified by camera traps. Animals 2019, 9, 1071. [Google Scholar] [CrossRef]
- Andreychev, A.; Kuznetsov, V.; Lapshin, A.; Alpeev, M. Activity of the Russian desman Desmana moschata (Talpidae, Insectivora) in its burrow. Therya 2020, 11, 161–167. [Google Scholar] [CrossRef]
- Hardin, G. Competitive exclusion principle. Science 1960, 131, 1292–12970. [Google Scholar] [CrossRef]
- Macarthur, R.; Levins, R. Limiting similarity, convergence, and divergence of coexisting species. Am. Nat. 1967, 101, 377–385. [Google Scholar] [CrossRef]
- Gordon, C.E. The coexistence of species. Rev. Chil. De Hist. Nat. 2000, 73, 175–198. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dayan, T. Partitioning of time as an ecological resource. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 153–181. [Google Scholar] [CrossRef]
- Xiang, D.; Meng, B.; Xie, B.; Huang, X.; Wang, C.; Ran, J.; Su, H.; Zhang, M. Daily activity rhythm of sympatric ungulate species in Fanjingshan Reserve, China. Glob. Ecol. Conserv. 2024, 56, e03271. [Google Scholar] [CrossRef]
- Chu, C.; Wang, Y.; Liu, Y.; Jiang, L.; He, F. Advances in species coexistence theory. Biodivers. Sci. 2017, 25, 345–354. [Google Scholar] [CrossRef]
- Ye, Z.-M.; He, Y.-D.; Huang, W.; Jin, X.-F.; Bergamo, P.J.; Yang, C.-F.; Ringler, E. Resource availability, competitor abundance and specialization affect competition among bumblebees. Behav. Ecol. 2025, 36, araf038. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, Z.X.; Teng, L.W.; Gao, H.; Sun, Y.J.; Wang, Z.Y.; Wang, J.F.; Ma, Z.Q.; Liu, Z.S. Summer foraging strategies of alpine musk deer (Moschus sifanicus) in Helan Mountains. Chin. J. Wildl. 2018, 39, 215–223. [Google Scholar] [CrossRef]


| Environmental Classification | Environmental Variable | Data Type | Source |
|---|---|---|---|
| Climate | Mean diurnal range (°C) | Continuous variable | https://worldclim.org |
| Temperature annual range (°C) | Continuous variable | ||
| Precipitation seasonality (%) | Continuous variable | ||
| Precipitation of driest quarter (mm) | Continuous variable | ||
| Topographic | Elevation (m) | Continuous variable | http://www.gscloud.cn |
| Slope (°) | Continuous variable | ||
| Vegetation | Net primary production(kg C/m2) | Continuous variable | http://loess.geodata.cn |
| Vegetation type | Categorical variable | https://data.ess.tsinghua.edu.cn | |
| Water source | Distance to river (m) | Continuous variable | http://loess.geodata.cn |
| Human disturbance | Distance to human settlement (m) | Continuous variable |
| Environmental Classification | Environmental Variable | Moschus berezovskii | Moschus chrysogaster |
|---|---|---|---|
| Climate | Mean diurnal range (°C) | 12.7 | 16.5 |
| Temperature annual range (°C) | 0.3 | 3 | |
| Precipitation seasonality (%) | 11.1 | 10.7 | |
| Precipitation of driest quarter (mm) | 3.9 | 4.3 | |
| Topographic | Elevation (m) | 20.3 | 6.2 |
| Slope (°) | 1.7 | 5.9 | |
| Vegetation | Net primary production(kg C/m2) | 17.7 | 21 |
| Vegetation type | 19.8 | 14.6 | |
| Water source | Distance to river (m) | 10.3 | 15.2 |
| Human disturbance | Distance to human settlement (m) | 2.1 | 2.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, N.; Du, Y.; Liu, M.; Jiang, Y.; He, X. The Spatiotemporal Distribution Patterns and Coexistence Mechanisms of Two Musk Deer Species in Mt. Gongga, Sichuan Province, China. Animals 2025, 15, 2701. https://doi.org/10.3390/ani15182701
Yang N, Du Y, Liu M, Jiang Y, He X. The Spatiotemporal Distribution Patterns and Coexistence Mechanisms of Two Musk Deer Species in Mt. Gongga, Sichuan Province, China. Animals. 2025; 15(18):2701. https://doi.org/10.3390/ani15182701
Chicago/Turabian StyleYang, Nan, Yuanhao Du, Mingyang Liu, Yong Jiang, and Xingcheng He. 2025. "The Spatiotemporal Distribution Patterns and Coexistence Mechanisms of Two Musk Deer Species in Mt. Gongga, Sichuan Province, China" Animals 15, no. 18: 2701. https://doi.org/10.3390/ani15182701
APA StyleYang, N., Du, Y., Liu, M., Jiang, Y., & He, X. (2025). The Spatiotemporal Distribution Patterns and Coexistence Mechanisms of Two Musk Deer Species in Mt. Gongga, Sichuan Province, China. Animals, 15(18), 2701. https://doi.org/10.3390/ani15182701





