Analysis of Genetic Diversity and Race Genetic Structure of Major Horse Breeds in Xinjiang, China
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Sample Collection
2.3. DNA Extraction, PCR Amplification and Microsatellite Genotyping
2.4. Microsatellite Statistical Analysis
3. Results
3.1. Genotyping Results and Polymorphism of Microsatellite Loci
3.2. Genetic Diversity Among Five Horse Races in Xinjiang, China
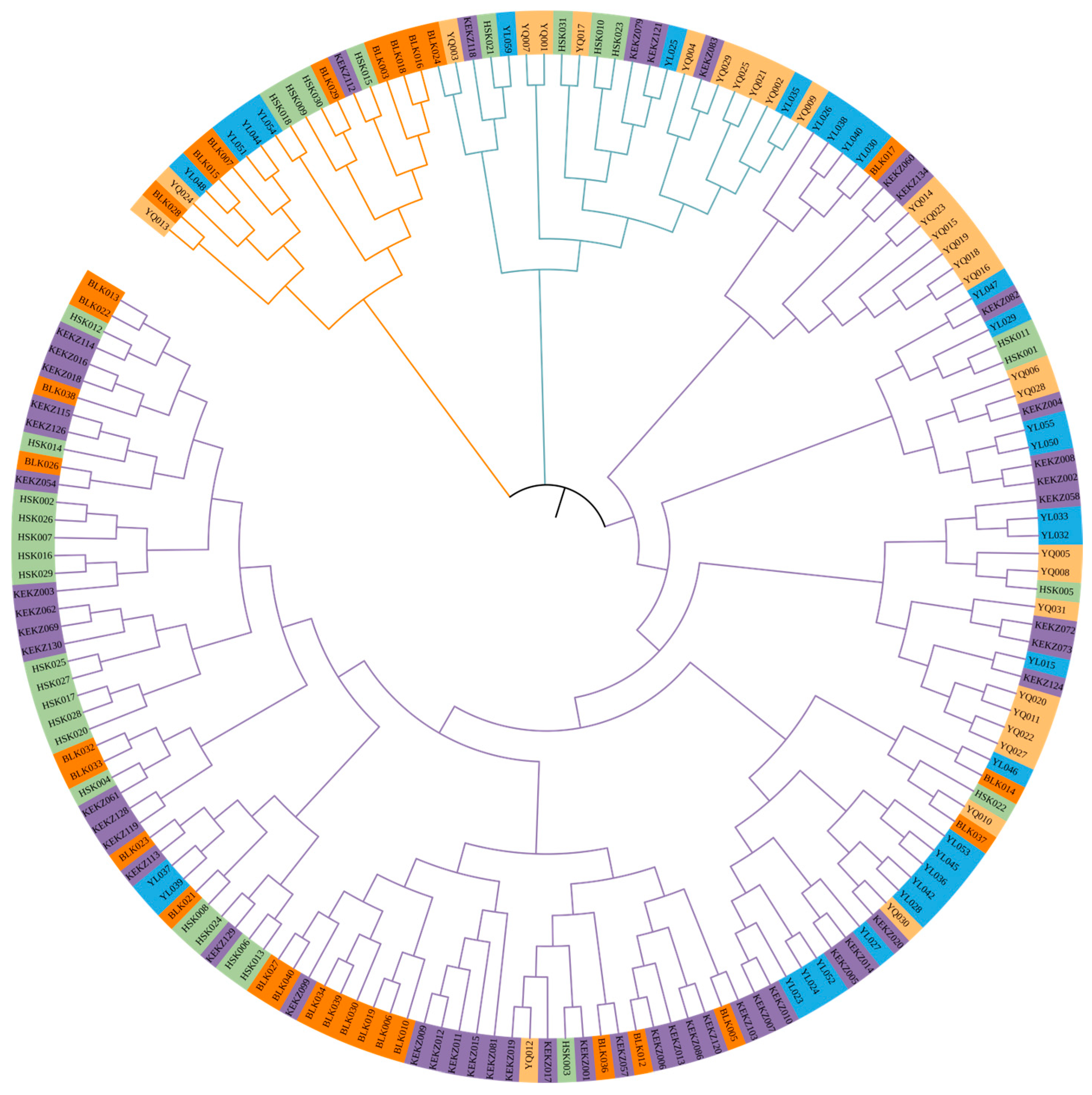
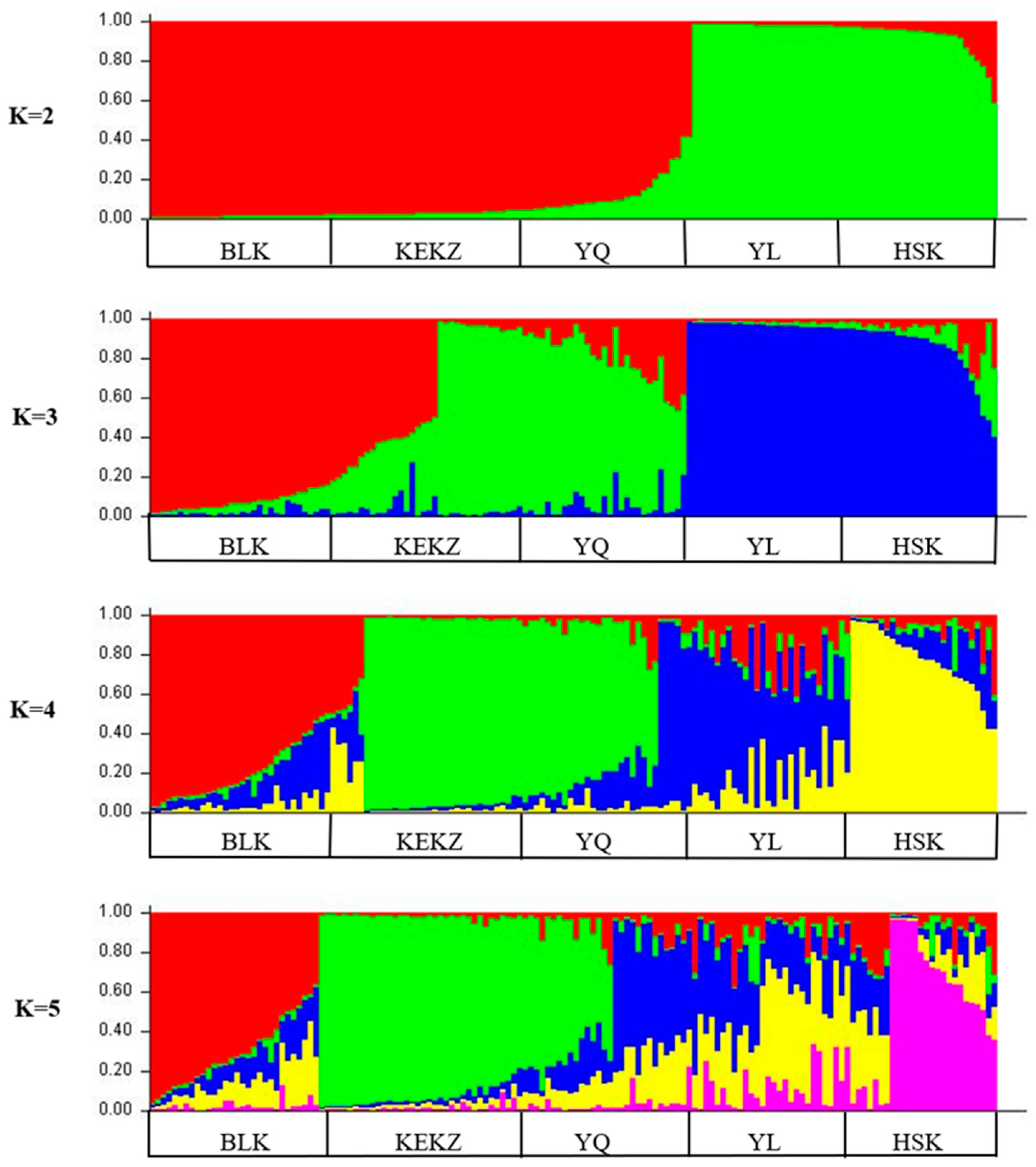
3.3. Genetic Distances and Phylogenetic Relationships Among Five Horse Races in Xinjiang, China
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fages, A.; Hanghøj, K.; Khan, N.; Gaunitz, C.; Seguin-Orlando, A.; Leonardi, M.; McCrory Constantz, C.; Gamba, C.; Al-Rasheid, K.A.S.; Albizuri, S.; et al. Tracking five millennia of horse management with extensive ancient genome time Series. Cell 2019, 177, 1419–1435. [Google Scholar] [CrossRef]
- Ellegren, H.; Galtier, N. Determinants of genetic diversity. Nat. Rev. Genet. 2016, 17, 422–433. [Google Scholar] [CrossRef]
- Achmann, R.; Curik, I.; Dovc, P.; Kavar, T.; Bodo, I.; Habe, F.; Marti, E.; Sölkner, J.; Brem, G. Microsatellite diversity, population subdivision and gene flow in the Lipizzan horse. Anim. Genet. 2004, 35, 285–292. [Google Scholar] [CrossRef]
- Arranz, J.J.; Bayón, Y.; San Primitivo, F. Differentiation among Spanish sheep breeds using microsatellites. Genet. Sel. Evol. 2001, 33, 529–542. [Google Scholar] [CrossRef]
- Mateus, J.C.; Penedo, M.C.; Alves, V.C.; Ramos, M.; Rangel-Figueiredo, T. Genetic diversity and differentiation in Portuguese cattle breeds using microsatellites. Anim. Genet. 2004, 35, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Tautz, D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989, 17, 6463–6471. [Google Scholar] [CrossRef]
- Duderstadt, S.; Distl, O. Genetic diversity and population structure of Dülmen Wild, Liebenthal and Polish Konik Horses in comparison with Przewalski, Sorraia, German Draught and Riding Horses. Animals 2024, 14, 2221. [Google Scholar] [CrossRef]
- Ling, Y.H.; Ma, Y.H.; Guan, W.J.; Cheng, Y.J.; Wang, Y.P.; Han, J.L.; Mang, L.; Zhao, Q.J.; He, X.H.; Pu, Y.B.; et al. Evaluation of the genetic diversity and population structure of Chinese indigenous horse breeds using 27 microsatellite markers. Anim. Genet. 2011, 42, 56–65. [Google Scholar] [CrossRef]
- Zeng, L.; Chen, N.; Yao, Y.; Dang, R.; Chen, H.; Lei, C. Analysis of genetic diversity and structure of Guanzhong Horse using microsatellite markers. Anim. Biotechnol. 2019, 30, 95–98. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, C.; Xue, P.; Yang, N.; Sun, X.; Serik, K.; Assanbayer, T.; Shamekova, M.; Kozhanov, Z.; Sapakhova, Z.; et al. Identification of genetic relationships and group structure analysis of Yanqi horses. Genes 2025, 16, 294. [Google Scholar] [CrossRef]
- Li, Z.J.; Liu, W.J.; Qi, J.Z.; Meng, J.; Liu, J.; Geng, M.; Yao, X. Test on identifying different horse breeds with microsatellite polymorphism. Xinjiang Agric. Sci. 2013, 50, 1692–1703. [Google Scholar]
- Wei, Y.F.; Yang, W.K.; Yu, S. Germplasm resource of Kyrgyz horse in Xinjiang and its exploitation and utilization. Contemp. Anim. Husb. 2016, 6, 26–28. [Google Scholar]
- Zhao, T.Z. Barkol horse. Xinjiang Agric. Sci. 1992, 29, 85. [Google Scholar]
- Ding, L.Y.; Dulati, K.; Iskhan, K.; Aiderhan, S.; Gemingguli, M. Study on the characteristics of genetic resources of Kazakh horse. Heilongjiang Anim. Sci. Vet. Med. 2017, 3, 91–94. [Google Scholar]
- Bahmurati, T. A century of changes in the Yili horses. Xinjiang Xumuye 2016, 9, 38–41. [Google Scholar]
- FAO. The State of the World’s Animal Genetic Resources for Food and Agriculture. 2007. Available online: http://dad.fao.org/ (accessed on 4 July 2024).
- Peakall, R.; Smouse, P.E. GenAlEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping error increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Excoffier, L.; Laval, G.; Schneider, S. Arlequin (version 3.0): An integrated software package for population genetics data analysis. Evol. Bioinform. Online 2007, 1, 47–50. [Google Scholar] [CrossRef]
- Hall, B.G. Building phylogenetic trees from molecular data with MEGA. Mol. Biol. Evol. 2013, 30, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Falush, D.; Stephens, M.; Pritchard, J.K. Inference of population structure using multilocus genotype data: Dominant markers and null alleles. Mol. Ecol. Notes 2007, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Hubisz, M.J.; Falush, D.; Stephens, M.; Pritchard, J.K. Inferring weak population structure with the assistance of sample group information. Mol. Ecol. Resour. 2009, 9, 1322–1332. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.L.; Liu, J.X. StructureSelector: A web-based software to select and visualize the optimal number of clusters using multiple methods. Mol. Ecol. Resour. 2018, 18, 176–177. [Google Scholar] [CrossRef]
- Yun, J.; Oyungerel, B.; Kong, H.S. Genetic diversity and population structure of Mongolian regional horses with 14 microsatellite markers. Anim. Biosci. 2022, 35, 1121–1128. [Google Scholar] [CrossRef]
- Lopes, M.S.; Mendonça, D.; Rojer, H.; Cabral, V.; Bettencourt, S.X.; da Câmara Machado, A. Morphological and genetic characterization of an emerging Azorean horse breed: The Terceira Pony. Front. Genet. 2015, 6, 62. [Google Scholar] [CrossRef]
- Dell, A.; Curry, M.; Yarnell, K.; Starbuck, G.; Wilson, P.B. Genetic analysis of the endangered Cleveland Bay horse: A century of breeding characterised by pedigree and microsatellite data. PLoS ONE 2020, 15, e0240410. [Google Scholar] [CrossRef]
- Kobayashi, I.; Akita, M.; Takasu, M.; Tozaki, T.; Kakoi, H.; Nakamura, K.; Senju, N.; Matsuyama, R.; Horii, Y. Genetic characteristics of feral Misaki horses based on polymorphisms of microsatellites and mitochondrial DNA. J. Vet. Med. Sci. 2019, 81, 707–711. [Google Scholar] [CrossRef]
- Dorji, J.; Tamang, S.; Tshewang, T.; Dorji, T.; Dorji, T.Y. Genetic diversity and population structure of three traditional horse breeds of Bhutan based on 29 DNA microsatellite markers. PLoS ONE 2018, 13, e0199376. [Google Scholar] [CrossRef]
- An, J.; Tseveen, K.; Oyungerel, B.; Kong, H.S. Analysis of genetic diversity and structure of Mongolian horse using microsatellite markers. J. Anim. Sci. Technol. 2022, 64, 1226–1236. [Google Scholar] [CrossRef]
- Amjadi, M.A.; Yeganeh, H.M.; Sadeghi, M.; Abbas Raza, S.H.; Yang, J.; Najafabadi, H.A.; Batool, U.; Shoorei, H.; Abdelnour, S.A.; Ahmed, J.Z. Microsatellite analysis of genetic diversity and population structure of the Iranian Kurdish horse. J. Equine Vet. Sci. 2021, 98, 103358. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.R.; Zhao, L.F.; Cai, S.D.; You, W.B.; Hali, H. The status and development countermeasures of Barkol horse breeds. Heilongjiang J. Anim. Reprod. 2024, 32, 28–32. [Google Scholar]
- Zhang, Y.; Sun, D.; Yu, Y.; Zhang, Y. Genetic diversity and differentiation of Chinese domestic buffalo based on 30 microsatellite markers. Anim. Genet. 2007, 38, 569–575. [Google Scholar] [CrossRef]
- Yatkın, S.; Özdil, F.; Ünal, E.; Genç, S.; Kaplan, S.; Gürcan, E.K.; Arat, S.; Soysal, M. Genetic characterization of native donkey (Equus asinus) populations of Turkey using microsatellite markers. Animals 2020, 10, 1093. [Google Scholar] [CrossRef]
- Leroy, G.; Callède, L.; Verrier, E.; Mériaux, J.C.; Ricard, A.; Danchin-Burge, C.; Rognon, X. Genetic diversity of a large set of horse breeds raised in France assessed by microsatellite polymorphism. Genet. Sel. Evol. 2009, 41, 5. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.X.; Yang, S.L.; Lin, R.Y.; Yang, H.B.; Li, A.P.; Wan, Q.S. Genetic diversity and population structure of Chinese pony breeds using microsatellite markers. Genet. Mol. Res. 2012, 11, 2629–2640. [Google Scholar] [CrossRef] [PubMed]
- Yordanov, G.; Mehandjyiski, I.; Palova, N.; Atsenova, N.; Neov, B.; Radoslavov, G.; Hristov, P. Genetic diversity and structure of the main Danubian horse paternal genealogical lineages based on microsatellite genotyping. Vet. Sci. 2022, 9, 333. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Liu, Z.; Shi, X.; Zhang, Z.; Li, Y.; Huang, B.; Ren, W.; Wang, X.; Wang, C.; Chai, W. An investigation of genetic diversity in three Dezhou donkey original breeding farms. Sci. Rep. 2023, 13, 11203. [Google Scholar] [CrossRef]
- Yang, L.P. Geography of Xinjiang Uygur Autonomous Region; Xinjiang People’s Publishing House: Urumqi, China, 1987; pp. 3–24. [Google Scholar]


| Breed | Abbreviation | Sample Size | Source Region |
|---|---|---|---|
| Barkol | BLK | 30 | Barkol County, Xinjiang |
| Kyrgyz | KEKZ | 50 | Wuqia County, Xinjiang |
| Yanqi | YQ | 30 | Hejing County, Xinjiang |
| Yili | YL | 30 | Zhaosu County, Xinjiang |
| Kazakh | HSK | 30 | Qinghe County, Xinjiang |
| Pop | Sample Size | Na | Ne | Ho | He |
|---|---|---|---|---|---|
| BLK | 30 | 11.308 | 6.330 | 0.792 | 0.816 |
| KEKZ | 50 | 11.231 | 6.025 | 0.737 | 0.810 |
| YQ | 30 | 11.308 | 6.326 | 0.753 | 0.814 |
| YL | 30 | 10.538 | 6.207 | 0.795 | 0.815 |
| HSK | 30 | 10.692 | 6.305 | 0.782 | 0.820 |
| Mean ± SE | 11.015 ± 0.371 | 6.239 ± 0.129 | 0.772 ± 0.256 | 0.815 ± 0.004 |
| Races | BLK | KEKZ | YQ | YL | HSK |
|---|---|---|---|---|---|
| BLK | 0.000 | ||||
| KEKZ | 0.128 | 0.000 | |||
| YQ | 0.156 | 0.125 | 0.000 | ||
| YL | 0.219 | 0.153 | 0.164 | 0.000 | |
| HSK | 0.149 | 0.148 | 0.181 | 0.157 | 0.000 |
| Source of Variation | d.f | Sum of Squares | Variance Component |
|---|---|---|---|
| Among populations | 4 | 39.657 | 0.08341 |
| Within populations | 295 | 1448.400 | 4.90983 |
| Total | 299 | 1488.057 | 4.99324 |
| Pop | Locus | Fis | Fit | Fst |
|---|---|---|---|---|
| All pops | HMS07 | 0.103 | 0.118 | 0.017 |
| ASB17 | 0.052 | 0.075 | 0.024 | |
| HMS06 | 0.050 | 0.079 | 0.031 | |
| HTG04 | 0.260 | 0.281 | 0.028 | |
| HMS03 | 0.117 | 0.136 | 0.022 | |
| HTG06 | −0.053 | −0.023 | 0.028 | |
| AHT04 | −0.135 | −0.121 | 0.012 | |
| ASB23 | 0.064 | 0.102 | 0.041 | |
| ASB2 | 0.032 | 0.052 | 0.020 | |
| HMS2 | 0.007 | 0.031 | 0.023 | |
| HTG07 | 0.199 | 0.231 | 0.040 | |
| UCDEQ425 | −0.052 | −0.023 | 0.028 | |
| VHL20 | 0.031 | 0.053 | 0.022 | |
| Mean ± SE | 0.052 ±0.029 | 0.076 ±0.029 | 0.026 ±0.002 |
| Races | BLK | KEKZ | YQ | YL | HSK |
|---|---|---|---|---|---|
| BLK | 0.000 | ||||
| KEKZ | 0.014 | 0.000 | |||
| YQ | 0.016 | 0.013 | 0.000 | ||
| YL | 0.022 | 0.016 | 0.017 | 0.000 | |
| HSK | 0.016 | 0.016 | 0.019 | 0.016 | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, L.; Sulayman, A.; Zeng, Y.; Zhou, L.; Aimaier, A.; Kader, A.; Shi, L. Analysis of Genetic Diversity and Race Genetic Structure of Major Horse Breeds in Xinjiang, China. Animals 2025, 15, 2690. https://doi.org/10.3390/ani15182690
Hou L, Sulayman A, Zeng Y, Zhou L, Aimaier A, Kader A, Shi L. Analysis of Genetic Diversity and Race Genetic Structure of Major Horse Breeds in Xinjiang, China. Animals. 2025; 15(18):2690. https://doi.org/10.3390/ani15182690
Chicago/Turabian StyleHou, Linlang, Ablat Sulayman, Yaqi Zeng, Lu Zhou, Ainiwan Aimaier, Adiljan Kader, and Lei Shi. 2025. "Analysis of Genetic Diversity and Race Genetic Structure of Major Horse Breeds in Xinjiang, China" Animals 15, no. 18: 2690. https://doi.org/10.3390/ani15182690
APA StyleHou, L., Sulayman, A., Zeng, Y., Zhou, L., Aimaier, A., Kader, A., & Shi, L. (2025). Analysis of Genetic Diversity and Race Genetic Structure of Major Horse Breeds in Xinjiang, China. Animals, 15(18), 2690. https://doi.org/10.3390/ani15182690





