1. Introduction
With the development of the economy and the gradual improvement of living standards, the peoples’ demand for pork has changed from the demand for quantity to high quality. The Yuedong black pig, a native breed primarily distributed in Guangdong Province, China, and its unique meat quality, is popular on the market. However, Yuedong black pigs show poorer feed conversion ratio and slower growth compared to commercial crossbred pigs. Therefore, it is critical to improve the growth performance to enhance the market competitiveness and sustainable development of indigenous pig breeds.
Fermented liquid feed (FLF) is a new feed process in which feed or feed ingredients are mixed with micro-organisms and fermented by adding water or other liquids [
1]. FLF can improve feed conversion efficiency and provide specific benefits to the animal body [
2]. In addition to the microbes added by fermentation, feed and feed ingredients may also have other beneficial microbes produced during the fermentation process, which helps to maintain animal gut health and enhance the animal’s body immunity [
3,
4]. In addition, during the process of feed fermentation, microbes can digest antinutritional factors in feed (phytates, glycinin, beta-conglycinin, and trypsin inhibitor activity) and promote the absorption of nutrients [
5,
6]. In summary, FLF has a high potential as a new feed for improving animal productivity and health. However, the effects of FLF on Yuedong black pigs remain unclear.
Therefore, this study aimed to investigate the effects of FLF on the growth performance, carcass traits, meat quality, antioxidant capacity, and intestinal microbiota of Yuedong black pigs. These results could provide a theoretical basis for the improvement of growth performance and high-quality pork production of Yuedong black pigs.
2. Materials and Methods
2.1. Animals and Treatments
A total of 100 healthy 90-day-old Yuedong black pigs (33.79 ± 1.14 kg, castrated male pigs) were randomly selected and divided into 2 treatment groups, each with 5 pens and 10 pigs in each pen. The experiment lasted 105 days. The control group was fed a corn-soybean meal-based diet and the experimental group was fed an FLF. Fermentation of the base diet uses a mixed bacteria pack (
Bacillus subtilis ≥ 1 × 10
8 CFU/g,
Enterococcus faecalis ≥ 1 × 10
8 CFU/g,
Saccharomyces cerevisiae ≥ 1 × 10
8 CFU/g, and
Clostridium butyricum ≥ 1 × 10
8 CFU/g). The preparation method for fermented liquid feed was as follows. After mixing the strain package with the basic feed at a mass ratio of 1:50, mix thoroughly at a water-to-feed ratio of 2.5:1 and ferment at room temperature for 6 h. Fermented liquid feed was prepared daily and delivered from the fermentation tank to the feed trough via an automated feeder pipe. The base diet composition and nutrient levels are shown in
Supplemental Table S1. Body weights were recorded at the beginning and end of the trial. Feed intake was recorded daily. Feed intake in the fermented liquid feed group was calculated on a dry matter basis. Average daily gain (ADG), average daily feed intake (ADFI), and feed-to-gain ratio (FCR) were calculated. Pigs were fed at 7 a.m. and 3 p.m. every day. The pigs were allowed to feed and drink freely. The experimental barn was a semi-open building with natural ventilation. The barns were washed every day and disinfected regularly to keep the barns dry and clean.
2.2. Sample Collection
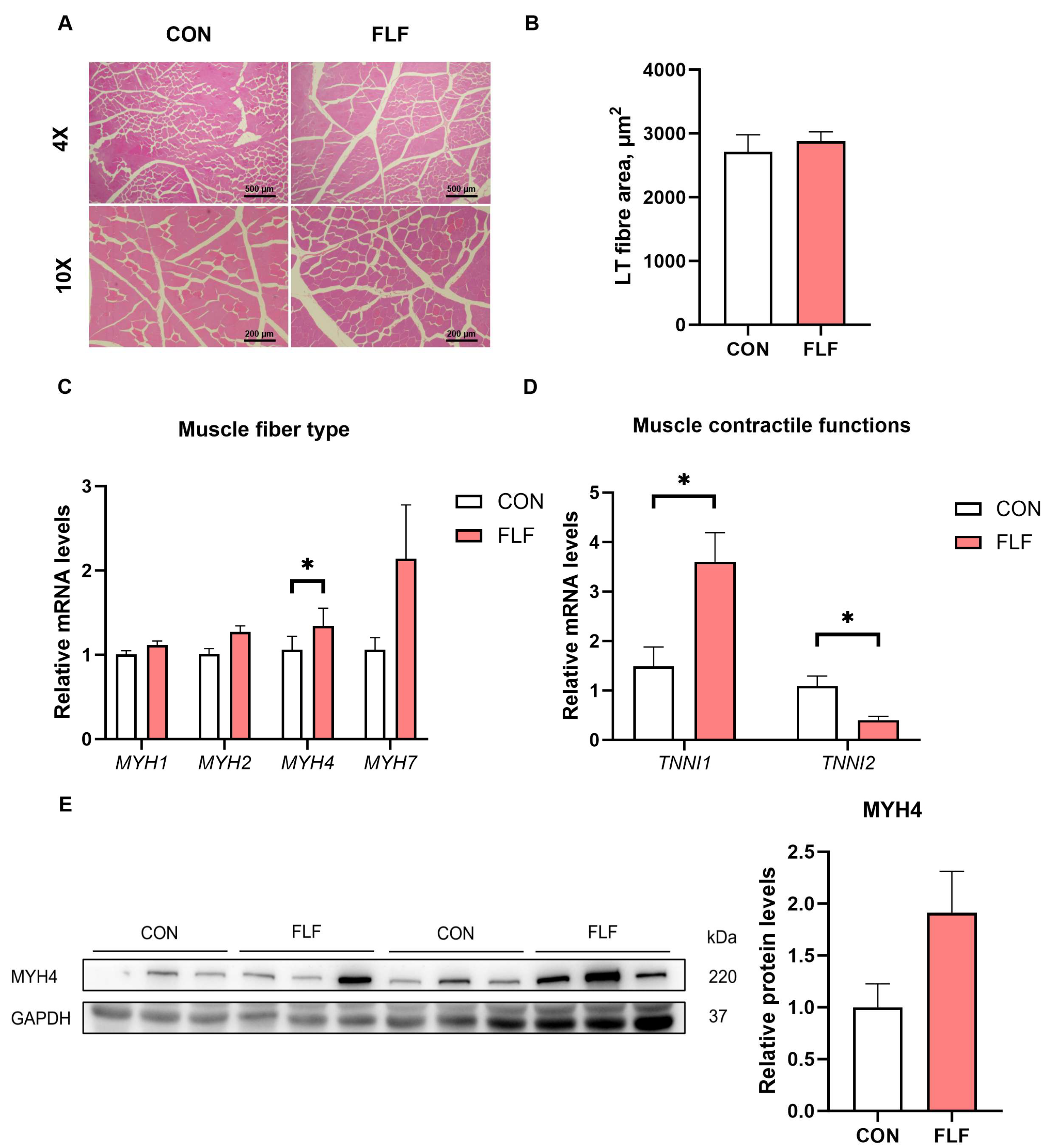
At the end of the trial, 1 or 2 pigs per pen near the per-pen average weight were selected (6 slaughtered in each group) and transferred in treatment groups to the nearest abattoir for blood collection and slaughter sampling. After blood collection, the supernatant was centrifuged at 3000× g for 15 min at 4 °C and stored at −80 °C. Longissimus thoracis (LT) were excised from the 10th–13th ribs on the left side of each carcass to measure meat quality and gene expression analysis. Additional LT samples were collected from the left side of the carcass to measure moisture, intramuscular fat (IMF), and amino acids. After carcass division, a small sample of the LT was taken and fixed using paraformaldehyde, and another sample was placed in liquid nitrogen and frozen, then transferred to a −80 °C refrigerator for storage. The colon contents were collected and stored at −80 °C. A tape measure was used to measure the straight length and the oblique length of the carcass. Skin thickness and backfat thickness were measured using vernier calipers. A pencil was used to trace the outline of the LT on butter paper and measure the area.
2.3. Meat Quality Determination
The meat color of LT was measured with a calibrated D-65 light source colorimeter (NR20XE, Sanenshi Technology, Guangzhou, China), and L* (brightness), a* (redness), and b* (yellowness) were recorded (blooming time for 20 min). The measuring aperture of the colorimeter was 20 mm. The light source was D65. The field of view was the CIE 10° standard observer. Before using the colorimeter, it was calibrated with a white calibration plate. The pH value of the LT at 45 min and 24 h after slaughter was measured using a pH meter (Testo-205, Desto, Lenzkirch, Germany). The pH meter was set for automatic temperature compensation according to the manual. LT marbling was assessed by 10 different assessors with reference to a standard scale for pork marbling (NPPC, 1991). Samples of LT strips were weighed and suspended in aerated polyethylene film bags with fish hooks for 24 h at 2–4 °C before being weighed again to determine drip loss. The samples were cut into cubes with sides measuring 2 cm and weighed and recorded as m1. The samples were put into the measuring tube in the refrigerator (4 °C), and were taken out after 24 h. The samples were treated with filter papers to absorb the residual liquid and weighed and recorded as m2. The calculation formula was as follows: Drip loss, % = (m1 − m2)/m1 × 100. The LT was cut into squares with sides of 2 cm and weighed, and were recorded as m1 determination of cooking loss. All samples were then placed in individual self-sealing bags and heated in a water bath at 85 °C for 30 min before being removed and cooled under running water. Finally, the moisture was dried with filter paper and weighed again and recorded as m2. The calculation formula was as follows: Cooking loss rate, % = (m1 − m2)/m1 × 100.
2.4. Serum Biochemical Indicators and Antioxidant Capacity
The liver function indices (TP, ALB, ALP, AST, and ALT), renal function indices (CRE, UREA), and glucose and blood lipids (GLU, TG, CHO, HDL, and LDL) were measured by an automatic biochemical analyzer according to the manufacturer’s instructions. Serum Malondialdehyde (MDA, A003-1-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), Total antioxidant capacity (T-AOC, A015-2-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China), and Glutathione peroxidase (GPx, A005-1-2, Nanjing Jiancheng Bioengineering Institute, Nanjing, China) were measured using the relevant kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China), according to the specific operations as described in the instruction manual.
2.5. HE Staining of Muscle, and Determination of IMF
Muscle tissue was cut into 1 cm × 1 cm × 1 cm pieces and fixed in 4% paraformaldehyde. Samples were sent to Wuhan Saiweier Biotechnology Co., Ltd. for staining and preparation. HE images of the LT (section) of Yuedong black pig were analyzed and processed by Image Pro Plus 6.0 (Image Pro Plus, Media Cybernetics, Rockville, MD, USA). Moisture and IMF content in the LT were determined using lyophilization and the Soxhlet extractor method according to the previous methods [
7]. Free amino acids were determined according to the previous method [
8]. Briefly, freeze-dried samples were mixed with 10% sulfosalicylic acid, centrifuged to remove the supernatant, and, finally, measured using an automated amino acid analyzer (L-8900, Hitachi Ltd., Tokyo, Japan).
2.6. RT-PCR (qPCR)
Total RNA was extracted from the LT using the kit (R4130-02, Magen, Guangzhou, China). The total RNA (2 μg) was reverse-transcribed into cDNA with random primers using the M-MLV enzyme (Promega, Madison, WI, USA). qPCR was performed on a 7300HT Fast RT-PCR system (Applied Biosystems, Carlsbad, CA, USA) using specific primers and the 2×SYBR Green master mix, following the manufacturer’s instructions (Q711, Vazyme, Nanjing, China). The sequences of the qPCR primers were shown in
Supplemental Table S2. ACTB was a housekeeping gene.
2.7. Western Blot
Proteins were extracted from the LT using RIPA lysis buffer (BB-3101-2, BestBio, Nanjing, China). The samples underwent 10% SDS-PAGE electrophoresis and were then transferred to PVDF membrane (Merck Millipore, Darmstadt, Germany). The membrane was blocked in 6% skim milk. The membranes were then placed at 4 °C and incubated with primary antibodies overnight and then incubated with the secondary antibody for 1.5–2 h. The proteins were visualized using Omni-ECL (Epizyme, Shanghai, China). The band intensity was quantified via Image Pro Plus. The primary antibodies used included anti-MYH4 (1:1000, 10F5, DSHB, Iowa City, IA, USA), anti-FATP4 (1:1000, A2640, Proteintech, Rosemont, IL, USA), and anti-GAPDH (1:50,000, P04406, Bioworld, Nanjing, China).
2.8. DNA Extraction, 16S rRNA Amplification, and Bioinformatics Analysis
A 16s rRNA sequencing of colon contents was commissioned to Hangzhou Lianchuan Biotechnology Co. Briefly, the total DNA of the contents was extracted and then PCR-amplified using primers for the V3-V4 region. The forward primer sequence was 341F (5′-CCTACGGGNGGCWGCAG-3′). The reverse primer sequence was 805R (5′-GACTACHVGGGTATCTAATCC-3′). AMPure XT beads were used to purify the PCR product. Qubit (Invitrogen, Carlsbad, CA, USA) was used to quantitatively purify the PCR product. Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA, USA) and Illumina Library Quantification Kit (Kapa Biosciences, Woburn, Massachusetts, USA) were used to analyze amplification products. Sequencing was performed by the NovaSeq PE250 platform.
The samples were sequenced on the Illumina NovaSeq platform. Data cleaning was performed using fqtrim (v 0.94). Data normalization was performed using SILVA (release 132) classifier. Diagrams were implemented using the R package (v3.5.2). Bioinformatics analysis using the Lianchuan biocaloud platform (v1.0).
2.9. Statistical Analysis
HE images of the LT tissue (section) of Yuedong black pigs were analyzed and processed by Image Pro Plus 6.0 (Image Pro Plus, Media Cybernetics, Rockville, MD, USA). The Shapiro–Wilk test was used to ensure that the data were normally distributed. For growth performance data, each pen was treated as the experimental unit for statistical analysis. The experimental data, excluding 16S rRNA results, were analyzed using Student’s t-test in Graphpad Prism 10.1.2 (GraphPad Software, Inc. San Diego, CA, USA) unless otherwise stated. For bioinformatics analysis of colonic contents, each pig was treated as the experimental unit for statistical analysis. The 16S rRNA results were plotted using the Lianchuan biocaloud platform. The difference between the two groups of microbial data was analyzed using the Wilcoxon rank sum test. Spearman correlation analysis of colonic barrier-related gene levels was conducted with the top 10 differential microbes in genus. Significance was set at p < 0.05.
4. Discussion
The indigenous pig breeds in South China are mainly black- and white-colored, characterized by high meat quality, heat resistance, and rough feed tolerance, but there is a significant gap in growth rate and feed efficiency compared with commercial crossbred pigs [
9,
10]. Therefore, improving the growth performance and increasing the growth rate play a crucial role in enhancing the market competitiveness of Yuedong black pigs.
Liquid fermentation can convert some of the large molecular proteins into small peptides and free amino acids, thus improving nutrient utilization [
5]. A study found that feeding weaned piglets for 82 days with wheat-based FLF significantly increased ADG, ADFI, and feed efficiency [
11]. In another study, the use of an FLF to feed pigs (weight 44.8–86.8 kg) can increase final weight and ADG, but there were no changes to ADFI and feed conversion ratio [
12]. Xin et al. [
13] found that FLF feeding increased ADG and ADFI of commercial crossbred pigs from 8 to 125 kg, but had no significant effect on F/G. We found that FLF feeding increased the ADG of Yuedong black pigs. Although the ADFI was elevated and F/G was reduced, neither of them was different. A meta-analysis showed that fermented feed increased ADG, body weight, and F/G in pigs [
14]. However, some studies have shown that FLF has no significant effect or even reduces the growth performance of pigs [
15,
16]. Thus far, there is no consistent conclusion on the effect of FLF on pig growth performance. Different feedstuffs, fermentation strains, and fermentation temperatures can affect the nutrient content of the fermentation product.
Carcass traits are influenced by a variety of genetic, environmental, and nutrient factors. Nutrients ingested by the organism are supplied preferentially to vital organs and physiological processes, followed by bone and muscle development, and, finally, fat deposition [
17]. Therefore, improving the body’s nutrient digestion and utilization is necessary to increase the rate of growth and development. The FLF can increase carcass weight, kill-out, backfat thickness, and muscle depth [
18]. Fermented mixed feed could increase loin muscle area [
19]. In this study, FLF significantly increased loin muscle area, but had no significant effect on other carcass traits. The improved carcass traits of fermented feeds may be related to their increased feed digestibility, which provides more absorbable nutrients to the organism.
Meat quality mainly includes IMF content, marbling, meat color, and drip loss. Myoglobin is the main protein that determines the meat color [
20]. A study found that fermented mixed feeds can increase the value of meat color a* and improve marbling scores [
19]. In this study, FLF decreased the meat color brightness value (L*) of Yuedong black pigs. Free amino acid content affects the meat taste and flavor [
21]. Tang et al. [
22] showed that fermented solid feeds increased glutamate (umami taste) in the LT of finishing pigs. Tian et al. [
23] showed that the use of fermented feed reduced the content of threonine (bitter) and proline (sweet and bitter) amino acids in the LT of finishing pigs. Our study found that FLF significantly reduced the levels of histidine (bitter), arginine (bitter), and proline (sweet and bitter) in the LT. This suggests that fermented feeds enhance pork taste and flavor by decreasing the content of bitter amino acids, but the exact mechanism needs to be further explored. When muscles are exposed to air, myoglobin oxygenates to form a bright cherry-red color and gradually turns white with increased exposure to oxygen, affecting the senses [
24]. Increasing the antioxidant capacity of the organism slows down the process of oxygenation of the carcass muscles, thereby increasing meat color stability [
25]. Fermented mixed feeds improved meat color and increased both serum and muscle SOD and GSH-Px activities [
19], which is consistent with the results of this study. Previous studies have reported that dietary supplementation with both Bacillus subtilis and Lactobacillus spp. can improve antioxidant capacity in pigs [
26,
27]. This may be related to microbial metabolites during fermentation. It has been suggested that drip loss of meat may be positively correlated with protein oxidation claims [
28]. In this study, cooking loss of LT was reduced in the FLF group, which may be related to the improvement of the antioxidant capacity of the organism. However, it needs to be further explored.
Muscle protein consists of myofibrillar connective tissue and sarcoplasmic proteins. Myofibrillars are the main proteins that make up skeletal muscle [
29]. The main muscle fibers are mainly divided into type I muscle fibers and type II muscle fibers. Type II fibers are thicker than Type I fibers [
30]. In this study, muscle HE-staining results did not observe a large difference in muscle fiber area, but
MYH4 gene was upregulated in the FLF group. In addition, our study found that FLF increased loin muscle area. This implies that FLF has a positive effect on muscle protein deposition and fiber development, but the specific molecular mechanisms need to be further investigated. TNNI1 and TNNI2 are subunits that regulate skeletal muscle contractile function [
31]. The
TNNI1 gene positively correlates with IMF content [
32,
33,
34]. Our study found that FLF significantly increased
TNNI1 gene expression in the LT. Pork with an IMF content of more than 3% will show better palatability and juiciness [
35]. Muscle fiber area is positively correlated with IMF content [
36]. Liu et al. [
37] showed that feeding fermented mixed feed increased IMF content. In this study, there was no significant difference in IMF content between the FLF group and the control group. CEBPα and PPARγ regulate downstream target genes ACACA and FABP4 and promote fat deposition [
38].
FASN,
FATP1, and
FABP3 gene expression positively correlates with IMF deposition [
39]. Currently, there is less information on the molecular mechanisms of fermented feeds on the expression of fat deposition-related genes. Liu et al. [
37] found that fermented mixed feeds up-regulated
CEBPα and
PPARγ gene expression in LT. Our study found that
FABP4,
CEBPα, and
PPARγ were significantly upregulated in the FLF group. In conclusion, the increase in IMF content by liquid fermentation material is associated with the regulation of lipogenesis-related genes.
There is a complex crosstalk between gut microbes-host and diet. The intestinal microbiota is regulated by the host’s diet, while compounds produced by gut microbial metabolism also affect host health [
40]. Studies have shown that FLF reduces the number of pathogenic bacteria in pigs, thereby reducing the incidence of clinical disease [
41]. In this study, FLF improved the biodiversity of colonic microbes in Yuedong black pigs. Gut microbes in finishing pigs can affect pig health, growth performance, and meat quality. Short-chain fatty acids are metabolites of hindgut micro-organisms, which are important for maintaining intestinal barrier integration [
42]. Increasing intestinal short-chain fatty acids improved carcass traits and meat color in pigs [
43]. In our study, we found that FLF increased the abundance of butyric acid-producing Lachnospiraceae-AC2044 groups (genus). It was found that Fibrobacterota (Phylum) is the main bacterium that degrades lignin and cellulose in the mammalian gut [
44]. It has been shown that Fibrobacterota (Phylum), which is enriched in the intestinal tract of Tibetan pigs, is associated with the synthesis of short-chain fatty acids, lactic acid, essential amino acids, and several B vitamins [
45]. This implies that the tolerance of Tibetan pigs in facing the extreme environment of the plateau is related to the enrichment of Fibrobacterota (Phylum). In this study, the abundance of colonic microbes Fibrobacterota (Phylum) was higher in the FLF group. In addition, the FLF increased the colonic barrier gene
CLDN1 expression, which was associated with the altered colony composition by FLF. All in all, FLF had beneficial effects on the intestinal microbes of Yuedong black pigs.