A Preliminary Study on Productive Performance and Environmental Requirements of a Newly Established Breed: Nero di Lomellina Pig
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Farm and Animals
2.1.1. Farrowing Unit
- Farrowing pen: 2.78 × 1.64 m;
- Farrowing crate: 2.00 × 0.65 m;
- Feeder trough: 0.50 × 0.43 m.
2.1.2. Post-Weaning and Growing Units
2.1.3. Fattening Unit
2.2. Monitoring Seasons
2.2.1. Animals Productive Performances
2.2.2. Environmental Monitoring
Thermal Environmental Classification for the THI and the Thermoneutral Zone for the Nero di Lomellina Pig
- T is the environmental temperature (°C);
- RH is the relative humidity (%) as a proportion.
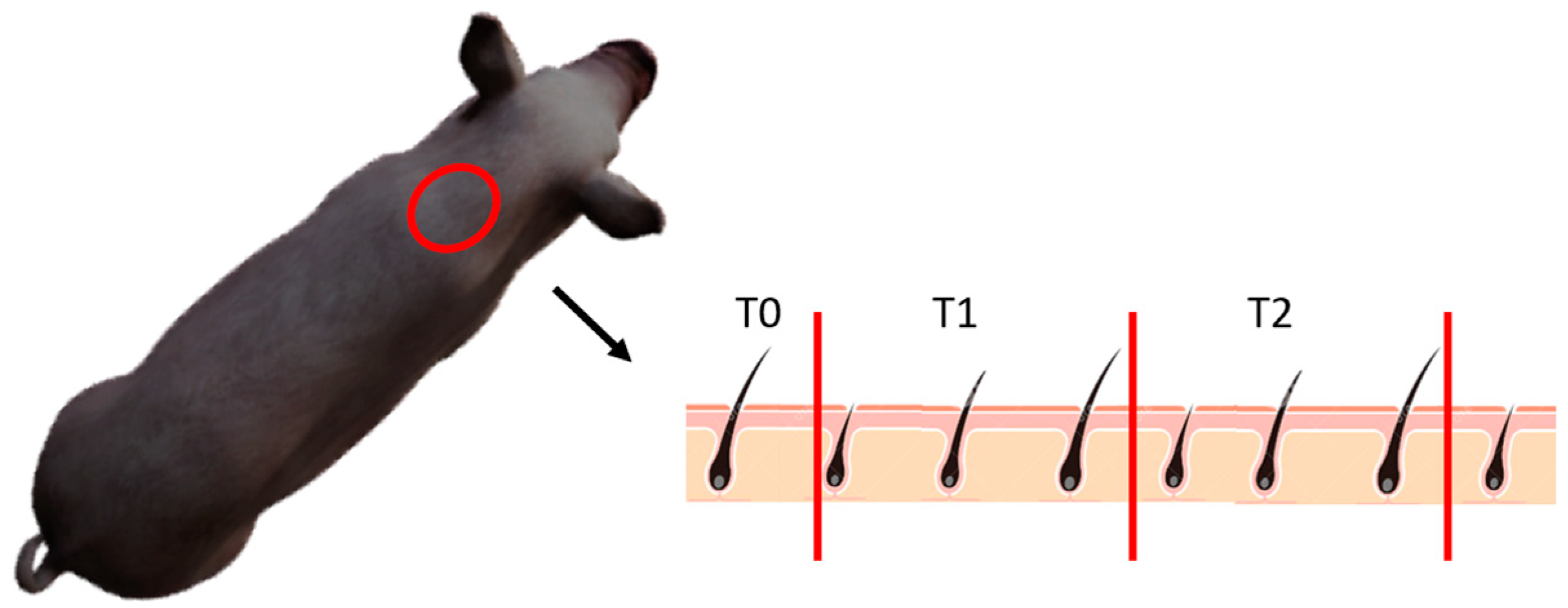
2.3. Hair Cortisol Concentration
2.3.1. Sampling Procedure
2.3.2. Cortisol Extraction Protocol
2.4. Statistical Analysis
- (a)(b) represents the weight estimate at time 0 (birth weight);
- (a) represents the asymptotic weight;
- (c) is a parameter indicating the growth rate over time;
- t is the time considered in the model (days, 0–500).
3. Results
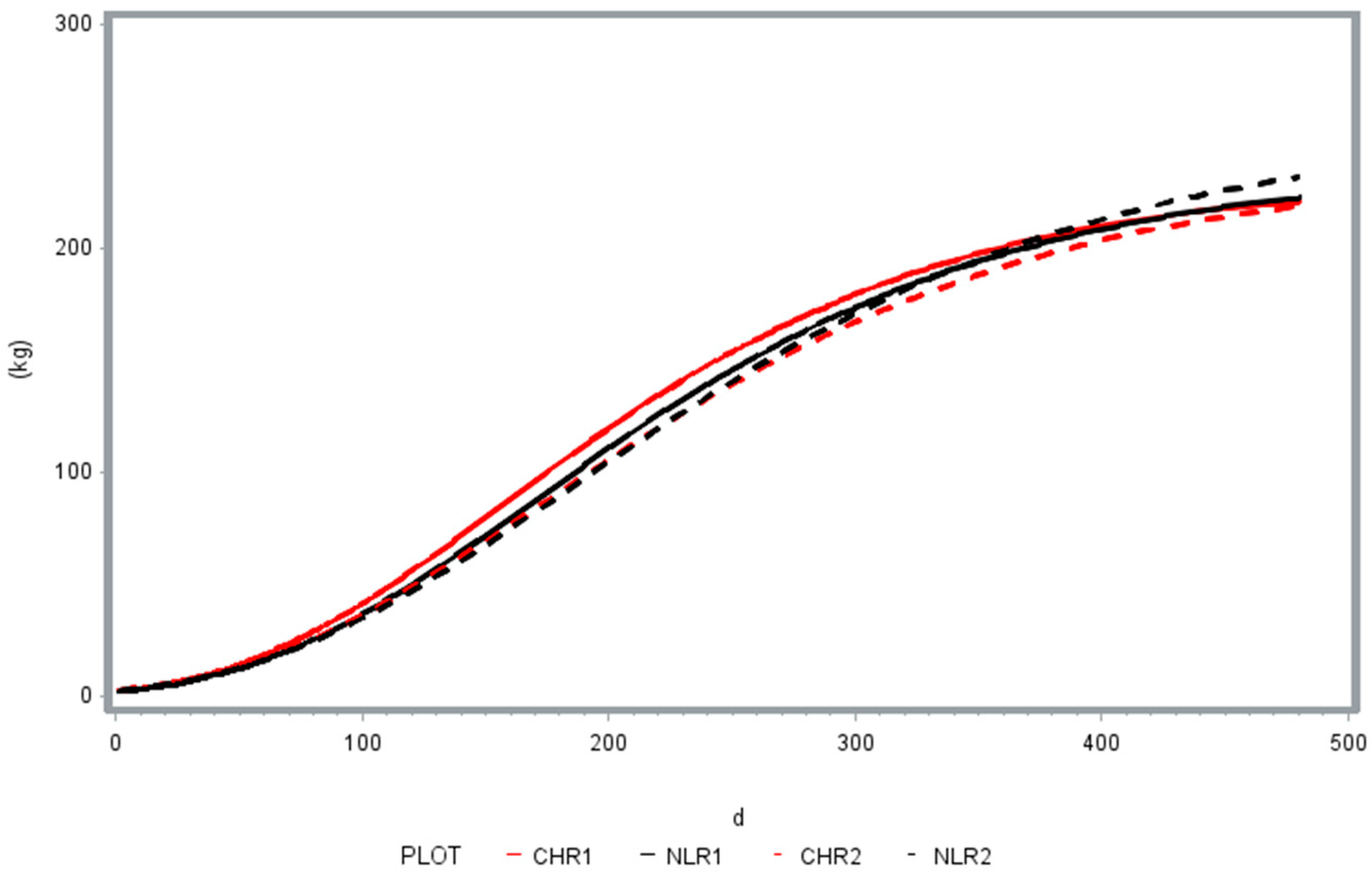
3.1. Animal Growth Performance
3.2. Monitoring Survey for Air Quality in the Pig Houses
3.3. Environmental Data
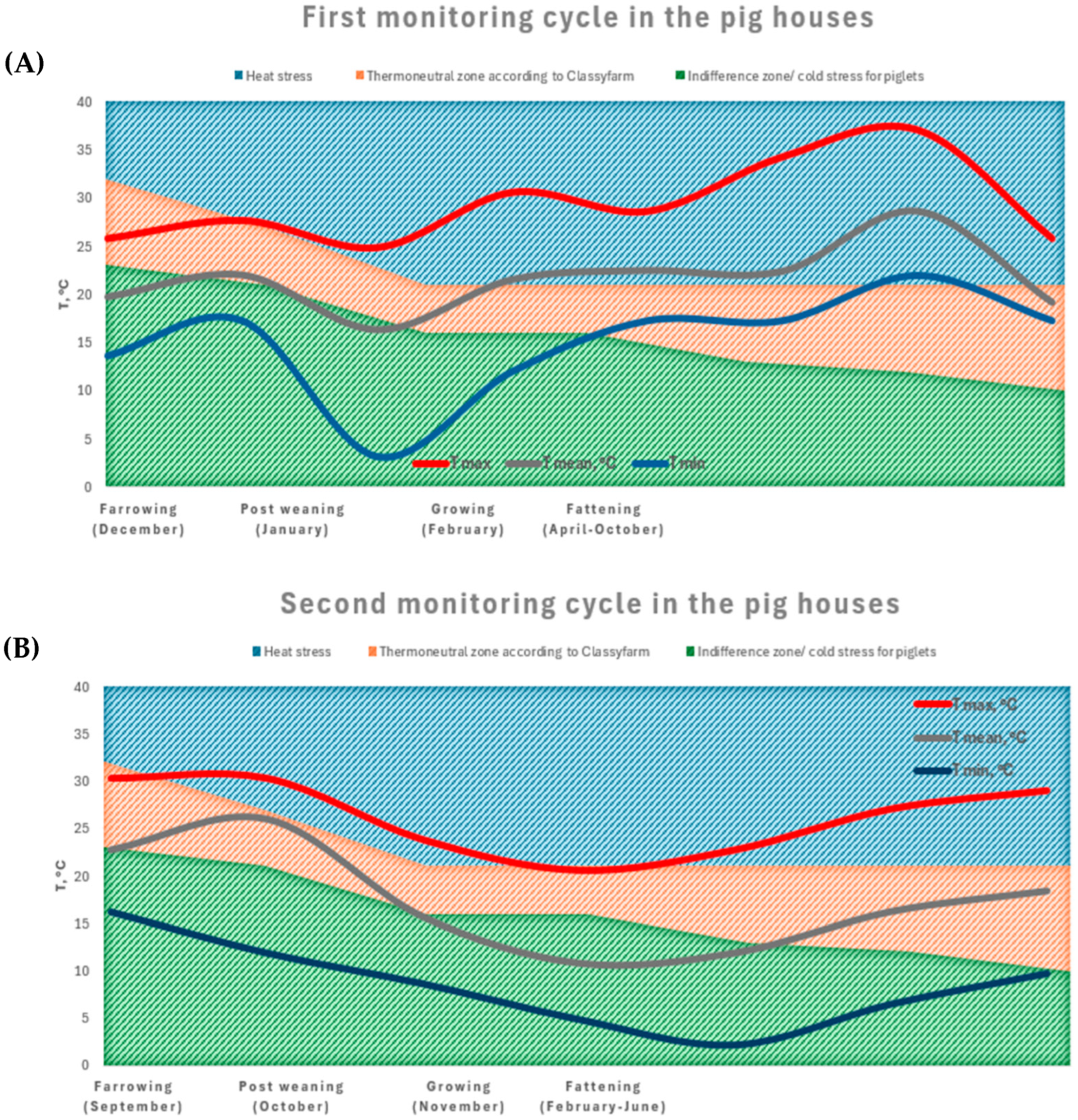
3.3.1. Thermal Environmental Classification: Thermoneutral Zone and THI
The Farrowing Phase
The Post-Weaning Phase
The Growing Phase
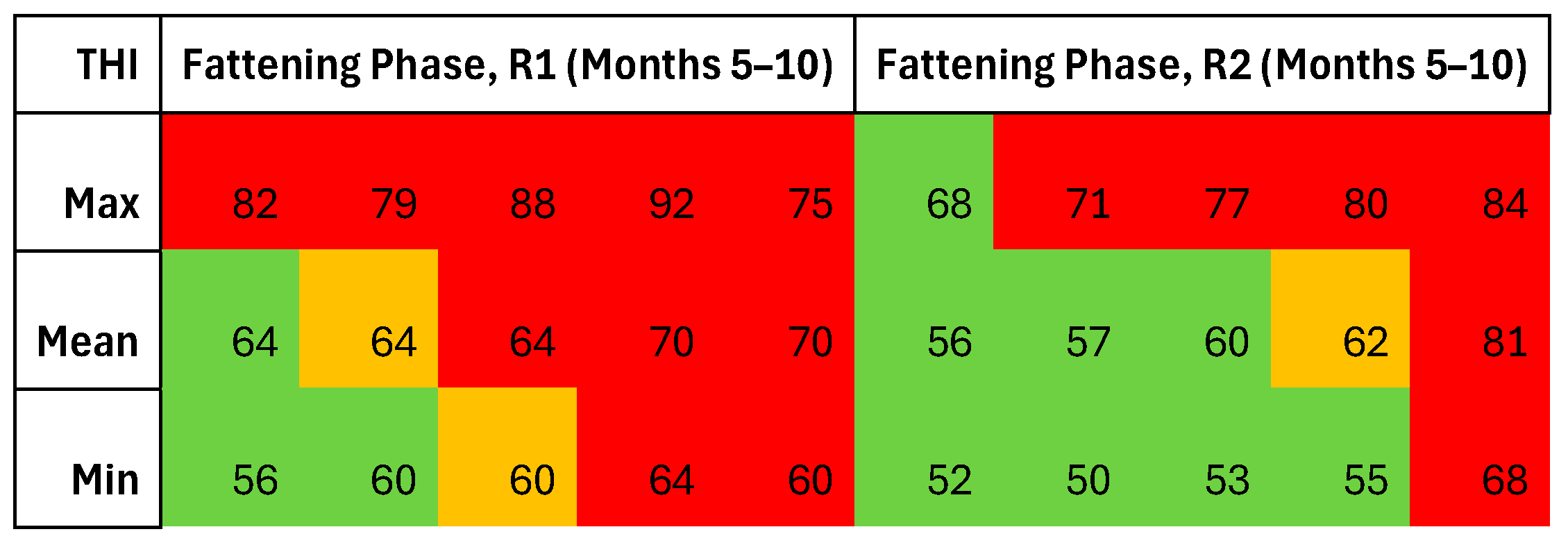
The Fattening Phase
The THI Definition: Thermoneutrality, Comfort, and Discomfort Zones
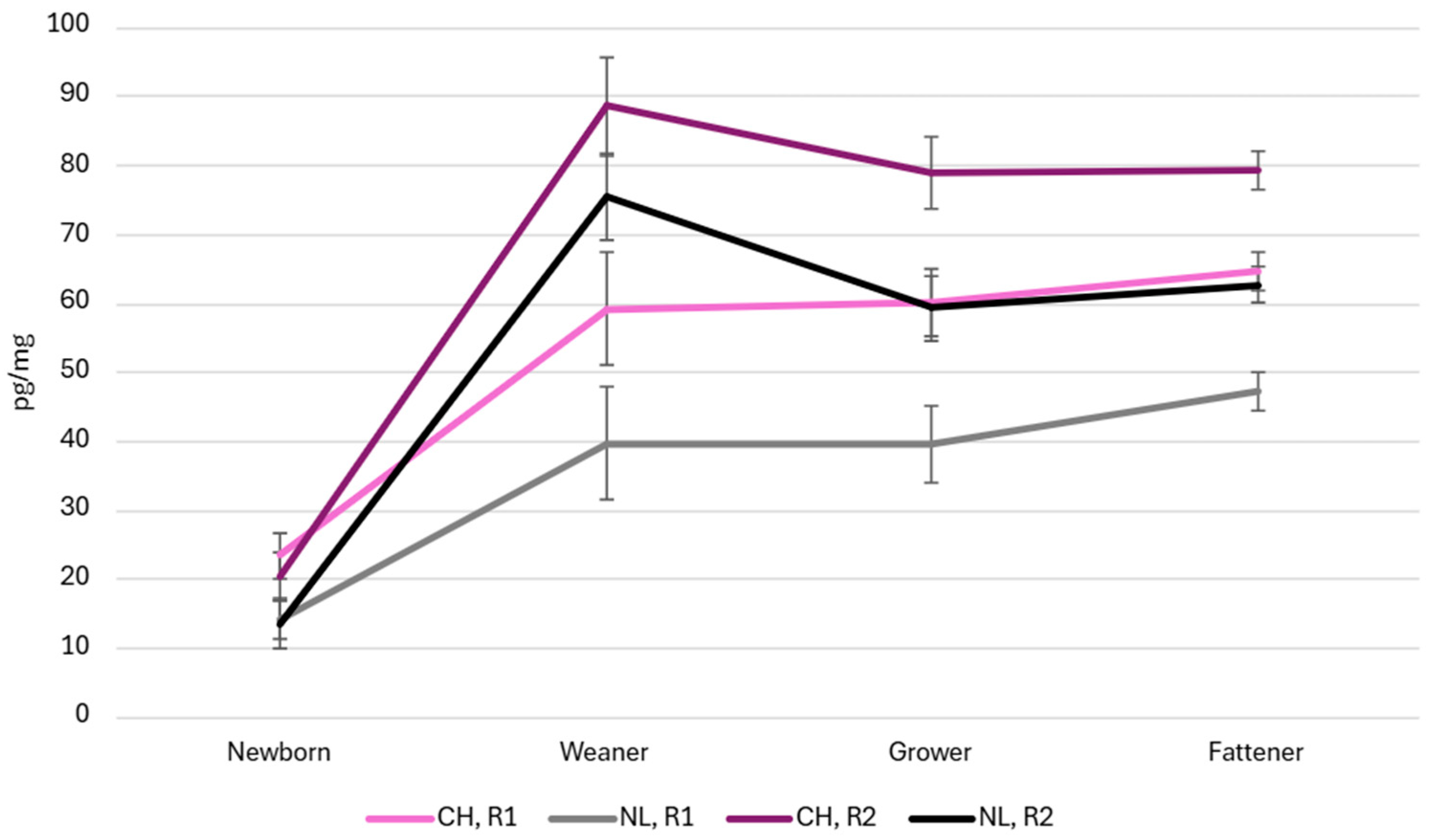
3.4. Hair Cortisol Concentration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NL | Nero di Lomellina |
| CH | Commercial hybrid |
| HCC | Hair cortisol concentration |
| NH3 | Ammonia |
| CO2 | Carbon dioxide |
| N2O | Nitrous oxide |
| CH4 | Methane |
| H2S | Hydrogen sulfide |
| TNZ | Thermoneutral zone |
| THI | Temperature–humidity index |
| RH | Relative humidity |
| T | Temperature |
| LW | Live weight |
References
- Sponenberg, D.P.; Martin, M.; Couch, C.; Beranger, J. Conservation strategies for local breed biodiversity. Diversity 2019, 11, 177. [Google Scholar] [CrossRef]
- Sarmiento-García, A.; Vieira-Aller, C. Improving Fatty Acid Profile in Native Breed Pigs Using Dietary Strategies: A Review. Animals 2023, 13, 1696. [Google Scholar] [CrossRef]
- Kušec, G.; Komleni’c, M.; Gvozdanovi’c, K.; Sili, V.; Krvavica, M.; Radiši’c, Ž.; Kušec, I.D. Carcass Composition and Physicochemical Characteristics of Meat from Pork Chains Based on Native and Hybrid Pigs. Processes 2022, 10, 370. [Google Scholar] [CrossRef]
- Lovec, M.; Šumrada, T.; Erjavec, E. New CAP Delivery Model, Old Issues. Intereconomics 2020, 55, 112–119. [Google Scholar] [CrossRef]
- Gourdine, J.L.; Rauw, W.M.; Gilbert, H.; Poullet, N. The Genetics of Thermoregulation in Pigs: A Review. Front. Vet. Sci. 2021, 8, 770480. [Google Scholar] [CrossRef]
- van der Waaij, E. A resource allocation model describing consequences of artificial selection under metabolic stress. J. Anim. Sci. 2004, 82, 973–981. [Google Scholar] [CrossRef]
- Renaudeau, D.; Gourdine, J.L.; St-Pierre, N.R. A meta-analysis of the effects of high ambient temperature on growth performance of growing-finishing pigs. J. Anim. Sci. 2011, 89, 2220–2230. [Google Scholar] [CrossRef]
- Merks, J.W.M.; Mathur, P.K.; Knol, E.F. New phenotypes for new breeding goals in pigs. Animal 2012, 6, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Mascheroni, E. Zootecnia speciale III: Suini. In Nuova Enciclopedia Agraria Italiana (Vol. III); Alpe, V., Zecchini, M., Soave, M., Eds.; Unione Tipografico-Editrice Torinese (UTET): Turin, Italy, 1927. [Google Scholar]
- Mariani, E.; Summer, A.; Ablondi, M.; Sabbioni, A. Genetic Variability and Management in Nero Di Parma Swine Breed toPreserve Local Diversity. Animals 2020, 10, 538. [Google Scholar] [CrossRef] [PubMed]
- Pallaoro, M.; Aidos, L.; Mirra, G.; Sergio, M.; Rossi, R.; Buoio, E.; Costa, A.; Di Giancamillo, M.; Mazzola, S.; Modina, S.; et al. Morphological evaluation of Semimembranosus muscle quality in the Nero di Lomellina pig: A contribution to porcine biodiversity. Ann. Anat. 2025, 260, 152677. [Google Scholar] [CrossRef]
- NRC. Nutrient Requirements of Swine, 11th ed.; The National Academies Press: Washington, DC, USA, 2012.
- Bertocchi, L. Valutazione del Benessere Animale Nella Specie Suina: Manuale Esplicativo Controllo Ufficiale; Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna “Bruno Ubertini”, Centro di Referenza Nazionale per il Benessere Animale; Ministero della Salute: Brescia, Italy, 2024; p. 160.
- Zimmerman, J.J.; Karriker, L.A.; Ramirez, A.; Schwartz, K.J.; Stevenson, G.W.; Zhang, J.Q. Diseases of Swine; Wiley-Blackwell: Chichester, UK, 2012. [Google Scholar]
- Muirhead, M.R.; Alexander, T.J. Managing Pig Health and Treatment of Disease; 5 M Enterprise: Uckfield, UK, 1997. [Google Scholar]
- Buoio, E.; Cialini, C.; Costa, A. Air Quality Assessment in Pig Farming: The Italian Classyfarm. Animals 2023, 13, 2297. [Google Scholar] [CrossRef]
- Mount, L. The assessment of thermal environment in relation to pig production. Livest. Prod. Sci. 1975, 2, 381–392. [Google Scholar] [CrossRef]
- Rauw, W.M.; de Mercado de la Pena, E.; Gomez-Raya, L.; Garcia-Cortes, L.A.; Ciruelos, J.J.; Gomez-Izquierdo, E. Impact of environmental temperature on production traits in pigs. Sci. Rep. 2020, 10, 2106. [Google Scholar] [CrossRef]
- Lachica, M.; Roman, A.; Fernandez-Figares, I.; Nieto, R. Upper Critical Temperature of Iberian Pigs. Animals 2025, 15, 1374. [Google Scholar] [CrossRef]
- Sterrenburg, P.; Van Ouwerkerk, E.N.J. Rekenmodel Voor de Bepaling van de Thermische Behaaglijkheidszone van Varkens—BEZOVA (Model to Determine the Thermal Comfort Zone in Pigs—BEZOVA); Rapport 78; IMAG: Wageningen, The Netherlands, 1990; 21p. [Google Scholar]
- Payola, F.; Piriou, M. Heat stress is not only an environmental issue. Pig Prog. 2021, 37, 14–15. [Google Scholar]
- Costa, A.; Salvagnini, C.; Buoio, E.; Palmeri, F.; Salvagnini, A.; Mazzola, S.M. The Effect of Lift Crates on Piglet Survival Rate and Sow Stress Level during Farrowing. Animals 2022, 12, 745. [Google Scholar] [CrossRef] [PubMed]
- Casal, N.; Manteca, X.; Peña, L.R.; Bassols, A.; Fàbrega, E. Analysis of cortisol in hair samples as an indicator of stress in pigs. J. Vet. Behav. 2017, 19, 1–6. [Google Scholar] [CrossRef]
- Heimbürge, S.; Kanitz, E.; Otten, W. The use of hair cortisol for the assessment of stress in animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Heimbürge, S.; Kanitz, E.; Tuchscherer, A.; Otten, W. Within a hair’s breadth—Factors influencing hair cortisol levels in pigs and cattle. Gen. Comp. Endocrinol. 2020, 288, 113359. [Google Scholar] [CrossRef]
- Otten, W.; Heimbürge, S.; Kanitz, E.; Tuchscherer, A. It’s getting hairy—External contamination may affect the validity of hair cortisol as an indicator of stress in pigs and cattle. Gen. Comp. Endocrinol. 2020, 295, 113531. [Google Scholar] [CrossRef]
- Otten, W.; Heimbürge, S.; Tuchscherer, A.; Kanitz, E. The age of hair matters—The incorporation of cortisol by external contamination is enhanced in distal hair segments of pigs and cattle. Animal 2022, 16, 100495. [Google Scholar] [CrossRef]
- Wester, V.L.; van der Wulp, N.R.P.; Koper, J.W.; de Rijke, Y.B.; van Rossum, E.F.C. Hair cortisol and cortisone are decreased by natural sunlight. Psychoneuroendocrinology 2016, 72, 94–96. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expression of the law of human mortality, and on a new mode of life contingencies, Philos. Trans. R. Soc. Lond. 1825, 115, 513–583. [Google Scholar]
- Sauvant, D.; Bertrand, D.; Giger, S. Variation and prevision of the in sacco dry matter digestion of concentrates and by-products. Anim. Feed Sci. Technol. 1985, 13, 7–23. [Google Scholar] [CrossRef]
- Hoff, S.J. Airborne dust in livestock buildings. In Air Quality and Livestock Farming Book Chapter; Banhazi, T., Aland, A., Hartung, J., Eds.; Wageningen Academic Publishers: Wageningen, The Netherlands, 2018; pp. 3–11. [Google Scholar]
- Costa, A. Ammonia Concentrations and Emissions from Finishing Pigs Reared in Different Growing Rooms. J. Environ. Qual. 2017, 46, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Domeneghini, C. Pollutants in livestock buildings: Ammonia and dust interplay with the respiratory tract. In “Air Quality and Livestock Farming”; Banhazi, T., Bidan, F., Costa, A., Dorenlor, V., Domeneghini, C., Donham, K.J., Jan van Dooren, H., Eono, F., Eveno, E., Fablet, C., et al., Eds.; CRC Press: London, UK, 2018; pp. 49–58. ISBN 9781138027039. [Google Scholar]
- Huynh, T.T.T.; Aarnink, A.J.A.; Verstegen, M.W.A.; Gerrits, W.J.J.; Heetkamp, M.J.W.; Kemp, B.; Canh, T.T. Effects of increasing temperatures on physiological changes in pigs at different relative humidities. J. Anim. Sci. 2005, 83, 1385–1396. [Google Scholar] [CrossRef]
- Ross, J.W.; Gabler, N.K.; Hale, B.J.; Rhoads, R.P.; Keating, A.F.; Baumgard, L.H. Physiological consequences of heat stress in pigs. Anim. Prod. Sci. 2015, 55, 1381. [Google Scholar] [CrossRef]
- Johnson, J.S. Heat stress: Impact on livestock well-being and productivity and mitigation strategies to alleviate the negative effects. Anim. Prod. Sci. 2018, 58, 1404. [Google Scholar] [CrossRef]
- Boon, C.R. The effect of departures from lower critical temperature on the group postural behaviour of pigs. Anim. Prod. 1981, 33, 71–79. [Google Scholar] [CrossRef]
- Muns, R.; Nuntapaitoon, M.; Tummaruk, P. Non-infectious causes of pre-weaning mortality in piglets. Livest. Sci. 2016, 184, 46–57. [Google Scholar] [CrossRef]
- Salak-Johnson, J.L.; Webb, S.R. Pig social status and chronic cold or crowd stressors differentially impacted immune response. Open J. Anim. Sci. 2018, 8, 280–293. [Google Scholar] [CrossRef][Green Version]
- Escribano, D.; Contreras-Jodar, A.; López-Arjona, M.; Cerón, J.J.; Fàbrega, E.; Aymerich, P.; Dalmau, A. Changes in cortisol and cortisone in hair of pigs reared under heat stress conditions. Front. Vet. Sci. 2023, 10, 1156480. [Google Scholar] [CrossRef]
- Bergamin, C.; Comin, A.; Corazzin, M.; Faustini, M.; Peric, T.; Scollo, A.; Gottardo, F.; Montillo, M.; Prandi, A. Cortisol, DHEA, and sexual steroid concentrations in fattening pigs’ hair. Animals 2019, 9, 345. [Google Scholar] [CrossRef]
- Otten, W.; Kanitz, E.; Tuchscherer, M.; Gräbner, M.; Nürnberg, G.; Bellmann, O.; Henning, U.; Rehfeldt, C.; Metges, C.C. Effects of low and high protein:carbohydrate ratios in the diet of pregnant gilts on maternal cortisol concentrations and the adrenocortical and sympathoadrenal reactivity in their offspring. J. Anim. Sci. 2013, 91, 2680–2692. [Google Scholar] [CrossRef]
- Johnson, J.S.; Baumgard, L.H. Physiology Symposium: Postnatal consequences of in utero heat stress in pigs. J. Anim. Sci. 2018, 97, 962–971. [Google Scholar] [CrossRef]
- Martínez-Miró, S.; Tecles, F.; Ramón, M.; Escribano, D.; Hernández, F.; Madrid, J.; Orengo, J.; Martínez-Subiela, S.; Manteca, X.; Cerón, J.J. Causes, consequences and biomarkers of stress in swine: An update. BMC Vet. Res. 2016, 12, 171. [Google Scholar] [CrossRef]
- Bacci, M.L.; Nannoni, E.; Govoni, N.; Scorrano, F.; Zannoni, A.; Forni, M.; Martelli, G.; Sardi, L. Hair cortisol determination in sows in two consecutive reproductive cycles. Reprod. Biol. 2014, 14, 218–223. [Google Scholar] [CrossRef]
- Nadlučnik, E.; Vake, T.; Šket, A.; Žižek, A.; Snoj, T.; Štukelj, M. Hair cortisol of pigs in mixed organic farms: The influence of season, breeding system and sex. Front. Vet. Sci. 2024, 11, 1491785. [Google Scholar] [CrossRef]
- López-Arjona, M.; Tecles, F.; Mateo, S.V.; Contreras-Aguilar, M.D.; Martínez-Miró, S.; Cerón, J.J.; Martínez-Subiela, S. Measurement of cortisol, cortisone and 11β-hydroxysteroid dehydrogenase type 2 activity in hair of sows during different phases of the reproductive cycle. Vet. J. 2020, 260, 105458. [Google Scholar] [CrossRef] [PubMed]
- Poklukar, K.; Čandek-Potokar, M.; Batorek Lukač, N.; Tomažin, U.; Škrlep, M. Lipid Deposition and Metabolism in Local and Modern Pig Breeds: A Review. Animals 2020, 3, 424. [Google Scholar] [CrossRef] [PubMed]

| Rearing Cycle | Farrowing Room | Post-Weaning | Growing | Fattening |
|---|---|---|---|---|
| R1 | December | January | February–March | April–October |
| R2 | September | October | November– December | January–June |
| Classes Based on LW | Weight Range (kg) | Critical Lower T (°C) | Critical Upper T (°C) | THI |
|---|---|---|---|---|
| 0 | Newborn | 32 | 35 | 75–81 |
| 1 | 0–8 | 25 | 35 | 62–80 |
| 2 | 9–20 | 22 | 28 | 57–68 |
| 3 | 20–40 | 19 | 26 | 51–64 |
| 4 | 60 | 18 | 24 | 49–63 |
| 5 | 80 | 17 | 23 | 48–61 |
| 6 | 100 | 16 | 22 | 46–60 |
| 7 | 120 | 15 | 21 | 44–59 |
| R1 | R2 | |||
|---|---|---|---|---|
| NL | CH | NL | CH | |
| Weight, kg | ||||
| Birth | 1.01 ± 0.006 | 0.97 ± 0.014 | 1.39 ± 0.022 | 1.38 ± 0.036 |
| Weaning | 8.12 ± 0.19 a | 9.17 ± 0.27 b | 8.64 ± 0.24 | 9.52 ± 0.52 |
| Growing phase | 57.85 ± 2.32 a | 66.71 ± 2.30 b | 56.0 ± 4.39 | 56.28 ± 2.90 |
| Fattening phase | 174.0 ± 4.46 a | 179.85 ± 1.80 b | 171.3 ± 1.23 | 167.3 ± 1.89 |
| Backfat thickness at P2, mm | ||||
| Growing phase | 6.47 ± 0.30 | 6.52 ± 0.24 | 6.30 ± 0.41 | 5.88 ± 0.23 |
| Finishing phase | 16.34 ± 1.41 | 18.74 ± 0.58 | 22.81 ± 0.97 A | 18.37 ± 0.10 B |
| Item | R1 CH | R2 CH | R1 NL | R2 NL |
|---|---|---|---|---|
| a | 231.9 ± 8.2178 A | 237.6 ± 15.9525 A | 237.6 ± 15.141 B | 254.8 ± 21.7049 B |
| b | 0.0119 ± 0.00247 | 0.0107 ± 0.00326 | 0.0107 ± 0.00293 | 0.0121 ± 0.00301 |
| c | 0.9905 ± 0.00057 | 0.9911 ± 0.00087 a | 0.9911± 0.00073 | 0.992 ± 0.00085 b |
| Unit | Air Pollutant Concentrations (mg/m3) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| R1 | R2 | ||||||||
| NH3 | CO2 | N2O | CH4 | NH3 | CO2 | N2O | CH4 | ||
| Farrowing room | Mean | 13.04 | 4191 | 1.85 | 49.70 | 6.2 | 2070.4 | 1.5 | 46.2 |
| Std. dev | 0.56 | 152 | 0.55 | 2.47 | 0.9 | 208.8 | 0.5 | 6.8 | |
| Post-weaning | Mean | 5.37 | 2858.93 | 1.71 | 18.39 | 6 | 2927 | 1.5 | 16.7 |
| Std. dev | 0.18 | 110.11 | 0.34 | 1.82 | 2.11 | 179 | 0.15 | 4.11 | |
| Fattening | Mean | 8.48 | 2554 | 0.61 | 28.60 | 4.9 | 2580 | 1.04 | 16.8 |
| Std. dev | 4.05 | 1260 | 0.09 | 12.17 | 1.9 | 418 | 0.3 | 5.7 | |
| Farrowing | Post-Weaning | Growing | |
|---|---|---|---|
| R1 | 62 (57–75) | 64 (60–77) | 59 (48–75) |
| R2 | 65 (60–81) | 67 (57–82) | 60 (54–74) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, A.; Buoio, E.; Pallaoro, M.; Mainardi, E.; Mirra, G.; Di Giancamillo, A.; Mazzola, S.M.; Rossi, R. A Preliminary Study on Productive Performance and Environmental Requirements of a Newly Established Breed: Nero di Lomellina Pig. Animals 2025, 15, 2655. https://doi.org/10.3390/ani15182655
Costa A, Buoio E, Pallaoro M, Mainardi E, Mirra G, Di Giancamillo A, Mazzola SM, Rossi R. A Preliminary Study on Productive Performance and Environmental Requirements of a Newly Established Breed: Nero di Lomellina Pig. Animals. 2025; 15(18):2655. https://doi.org/10.3390/ani15182655
Chicago/Turabian StyleCosta, Annamaria, Eleonora Buoio, Margherita Pallaoro, Edda Mainardi, Giorgio Mirra, Alessia Di Giancamillo, Silvia Michela Mazzola, and Raffaella Rossi. 2025. "A Preliminary Study on Productive Performance and Environmental Requirements of a Newly Established Breed: Nero di Lomellina Pig" Animals 15, no. 18: 2655. https://doi.org/10.3390/ani15182655
APA StyleCosta, A., Buoio, E., Pallaoro, M., Mainardi, E., Mirra, G., Di Giancamillo, A., Mazzola, S. M., & Rossi, R. (2025). A Preliminary Study on Productive Performance and Environmental Requirements of a Newly Established Breed: Nero di Lomellina Pig. Animals, 15(18), 2655. https://doi.org/10.3390/ani15182655







