Cytogenetic Screening on Mediterranean Italian River Buffalo Males Intended for Reproduction and Females with Fertility Issues—A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cytogenetics Analyses
2.3. CBA-Banding
2.4. RBA-Banding
2.5. FISH-Mapping
2.6. Gynecological and Histopathological Examination
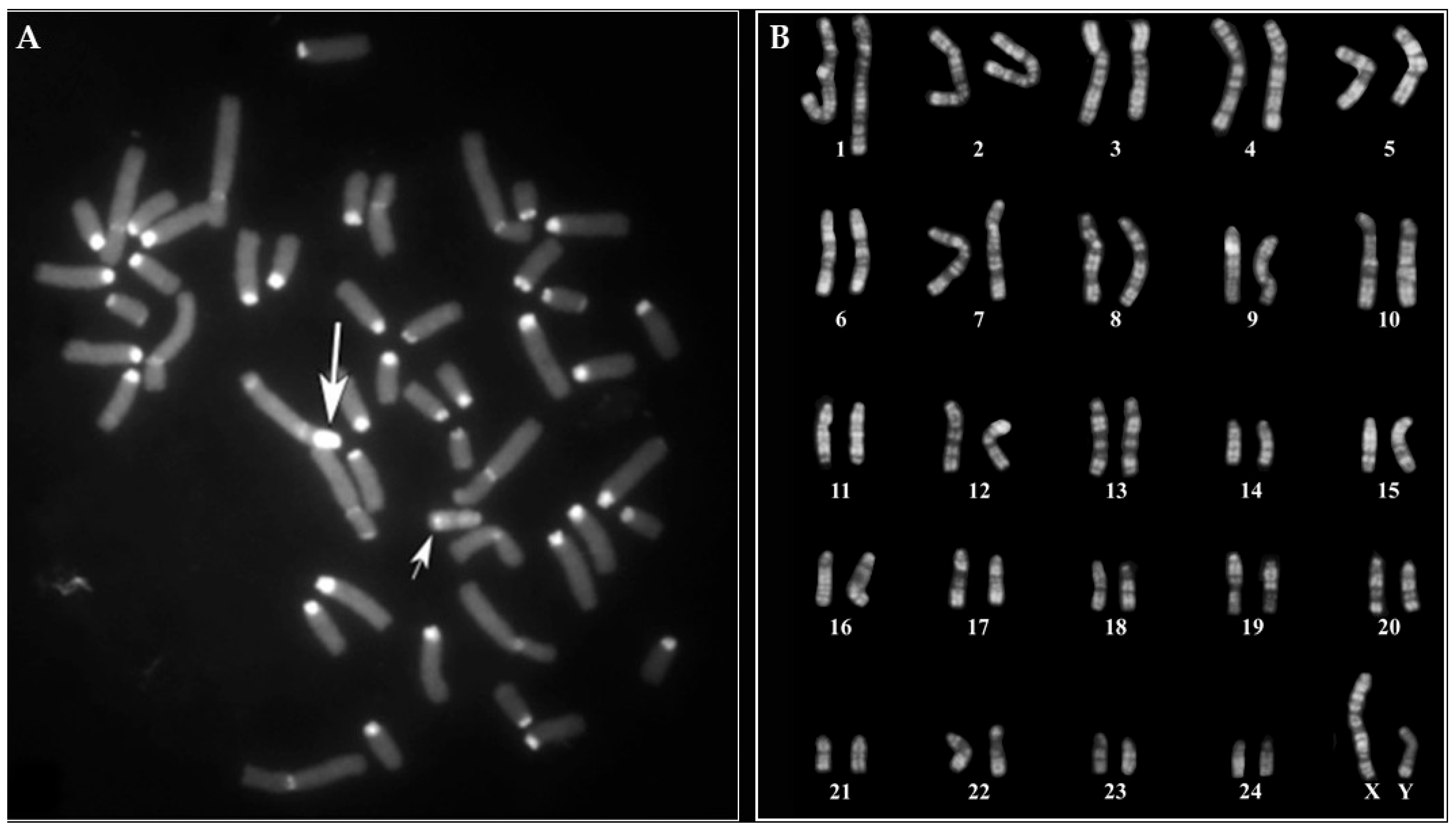
3. Results
3.1. Young MIRB Males
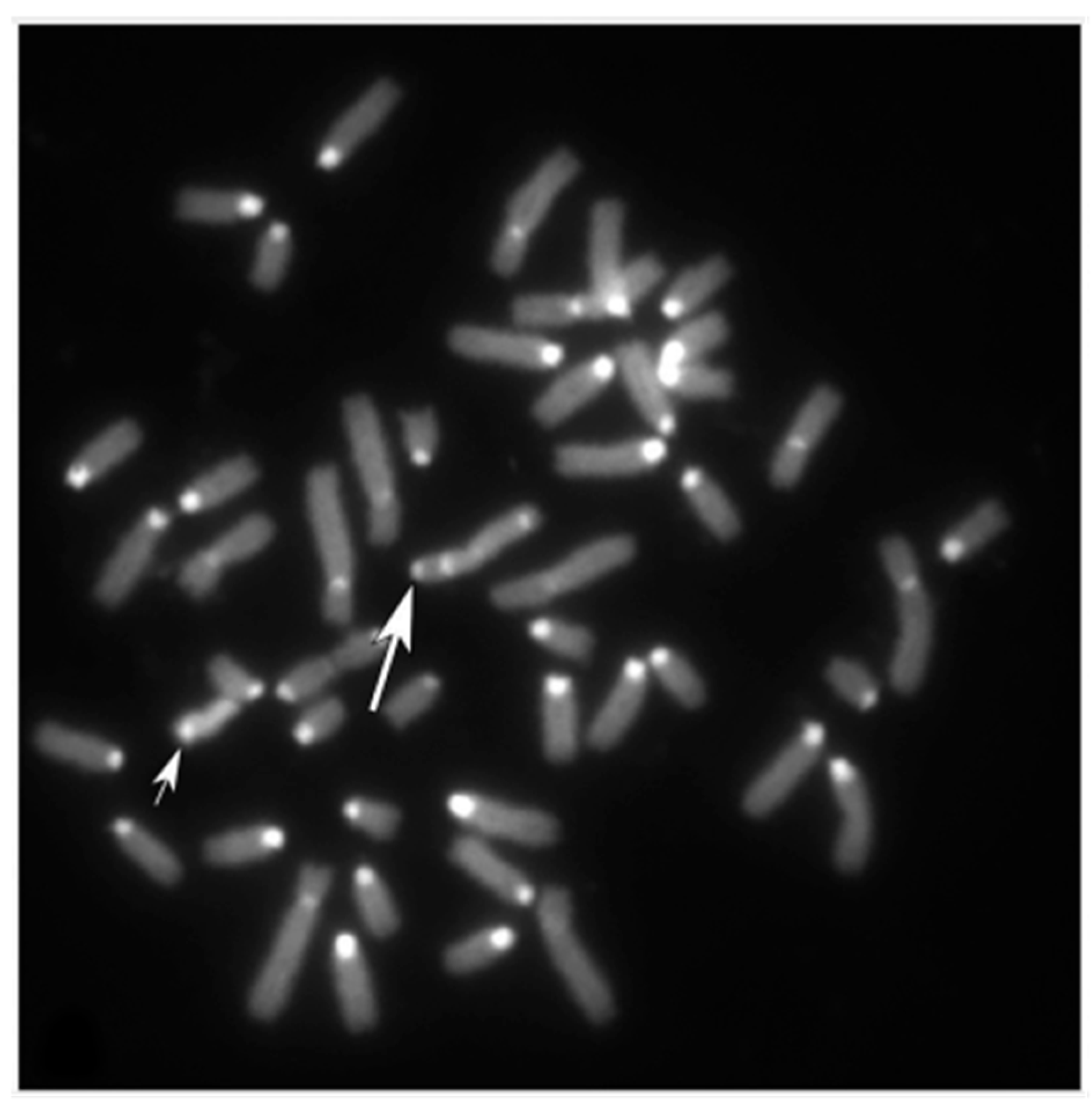
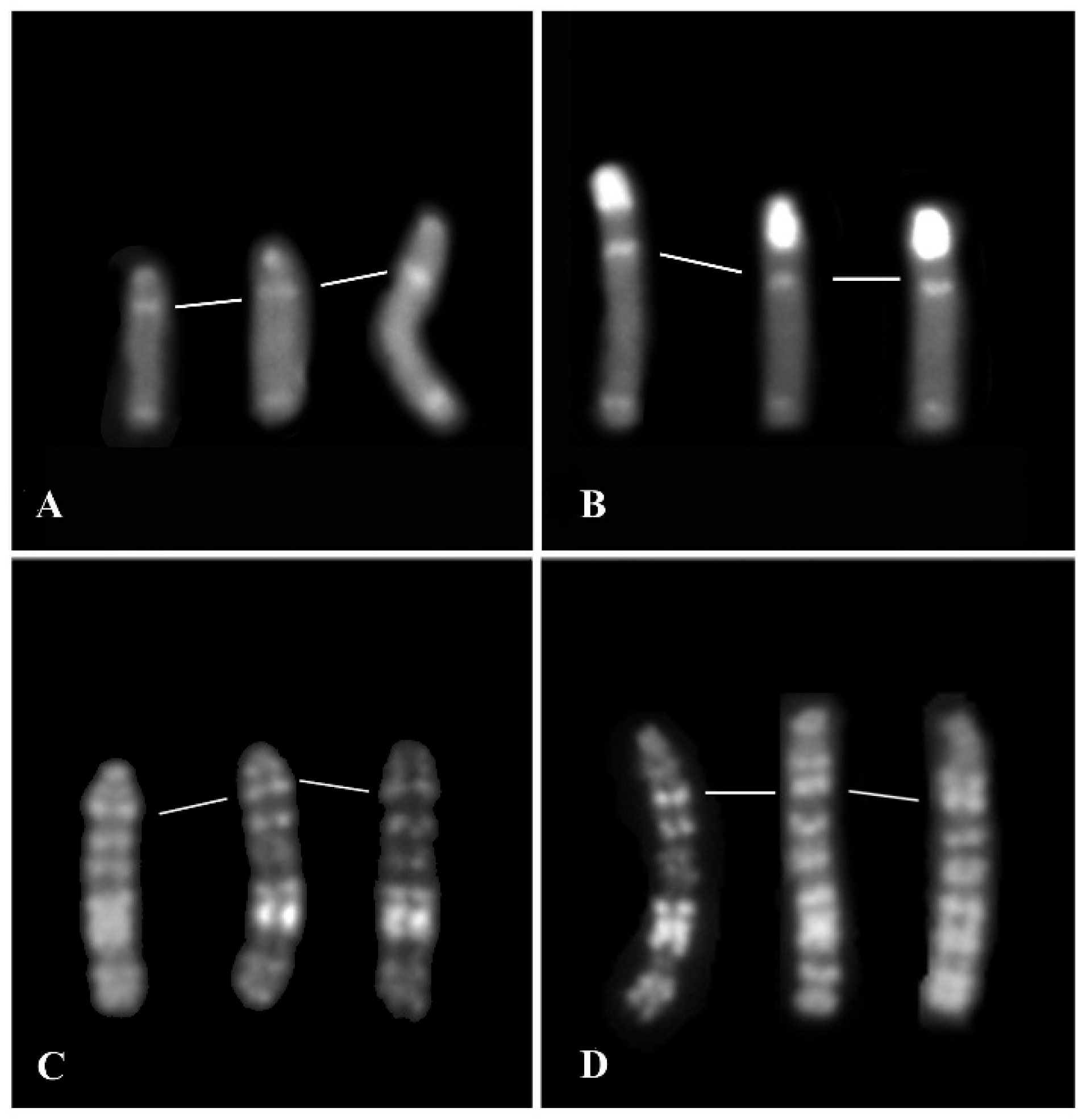
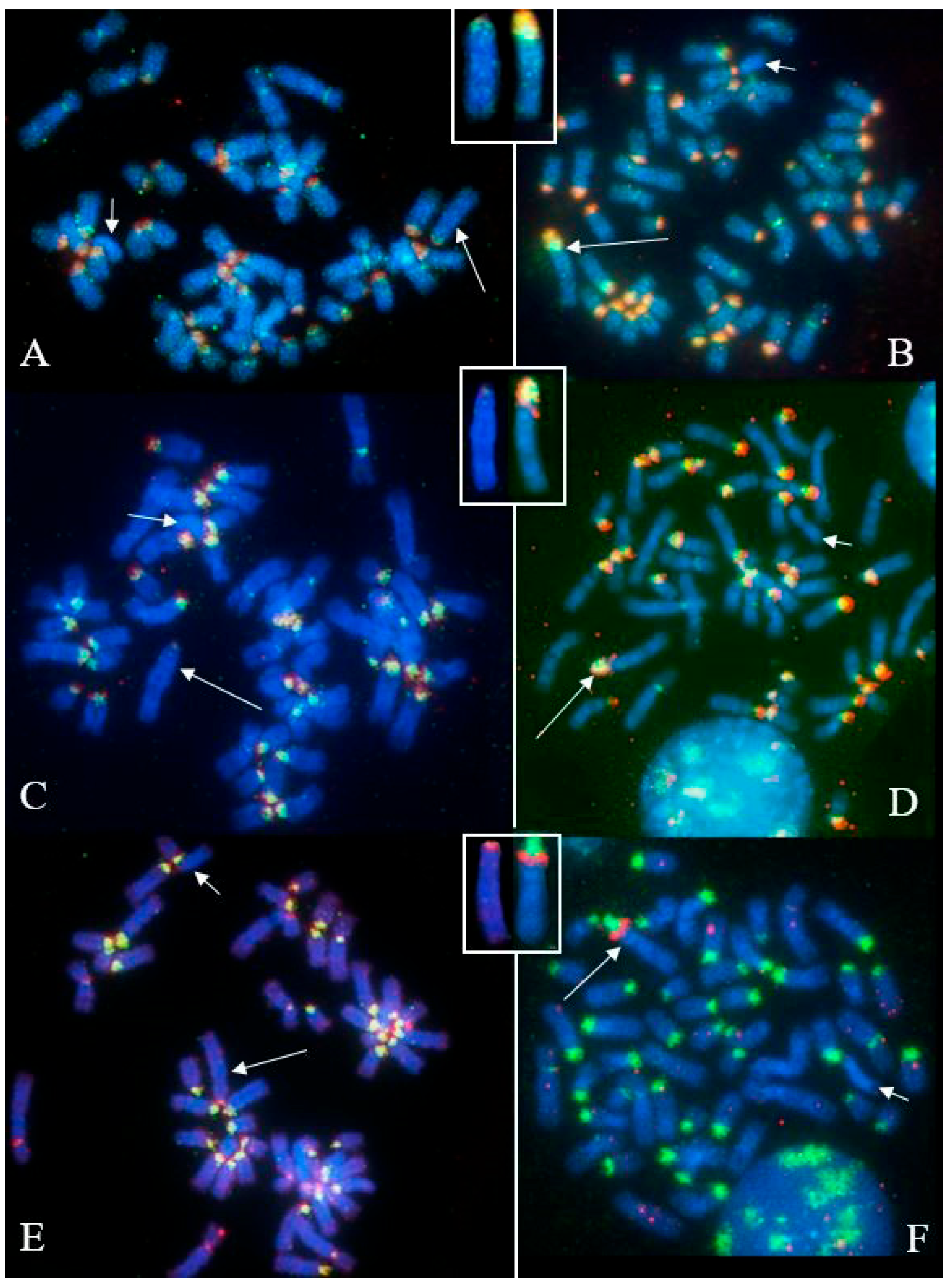
3.2. Females with Fertility Issues
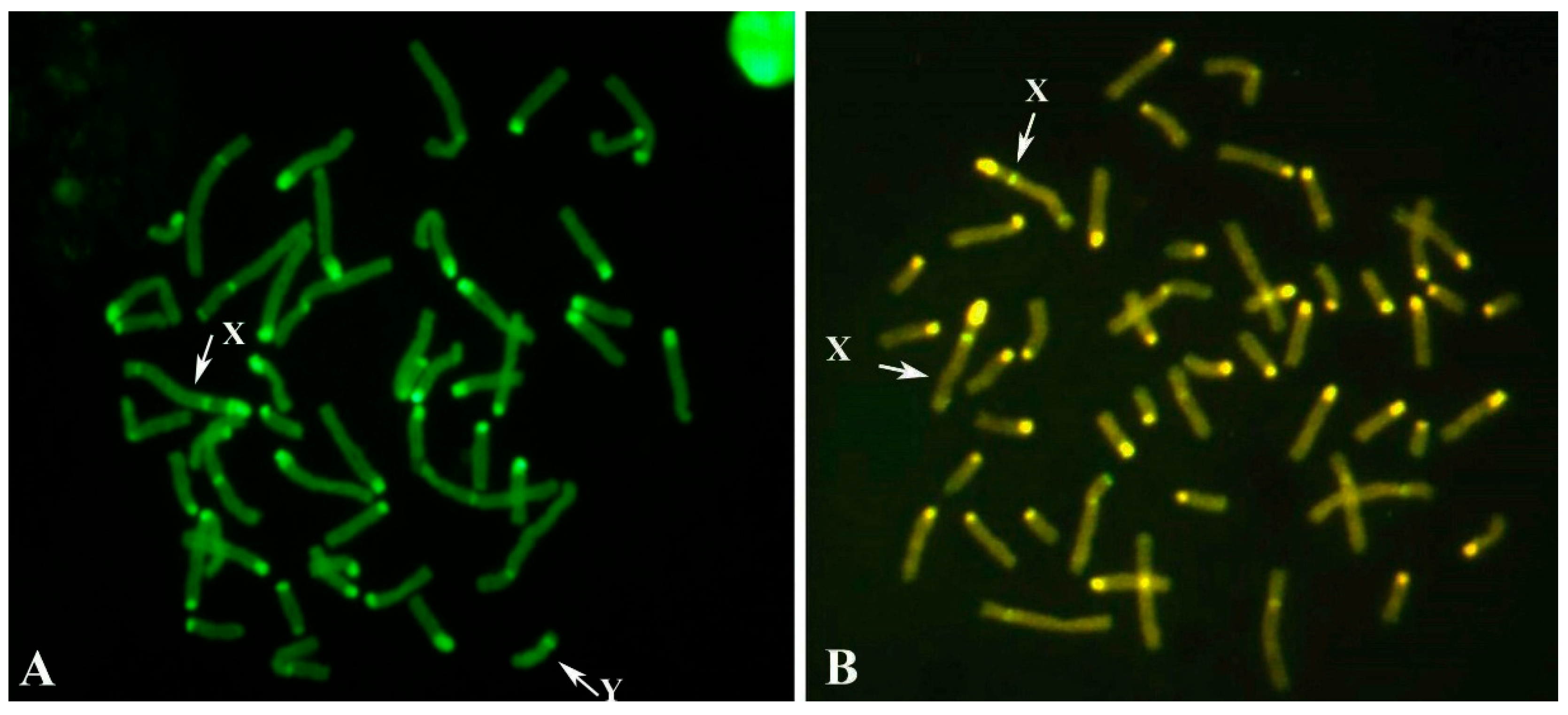
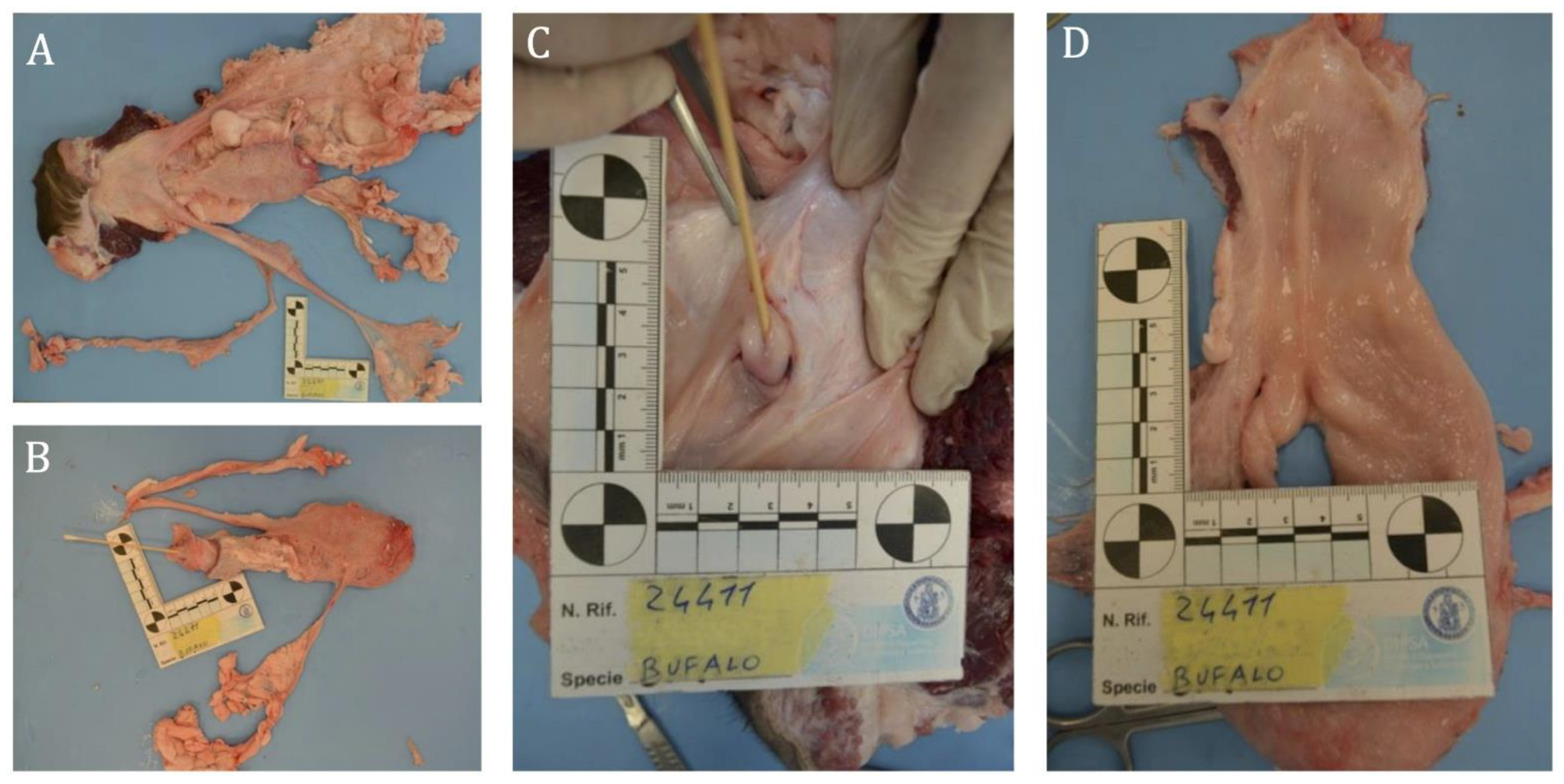
3.2.1. Case B2250
3.2.2. Case B202
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| CBA | C-banding by Acridine Orange Staining |
| RBA | R-banding by late BrdU-incorporation and Acridine Orange Staining |
| BAC | Bacterial Artificial Chromosome |
| FISH | Fluorescence In Situ Hybridization |
| SAT | Satellite DNA |
| BBU | River Buffalo Chromosome (Bubalus bubalis) |
| BrdU | 5-bromo-2′-deoxyuridine |
| FITC | Fluorescein Isothiocyanate |
| RODH | Rhodamine |
| DAPI | 4′,6-diamidin-2-fenilindol |
References
- Iannuzzi, A.; Parma, P.; Iannuzzi, L. The Cytogenetics of the Water Buffalo: A Review. Animals 2021, 11, 3109. [Google Scholar] [CrossRef]
- Albarella, S.; Ciotola, F.; Coletta, A.; Genualdo, V.; Iannuzzi, L.; Peretti, V. A new translocation t(1p;18) in an Italian Mediterranean river buffalo (Bubalus bubalis, 2n = 50) bull: Cytogenetic, fertility and inheritance studies. Cytogenet. Genome Res. 2013, 139, 17–21. [Google Scholar] [CrossRef]
- Di Meo, G.P.; Perucatti, A.; Genualdo, V.; Iannuzzi, A.; Sarubbi, F.; Caputi-Jambrenghi, A.; Incarnato, D.; Peretti, V.; Vonghia, G.; Iannuzzi, L. A rare case of centric fission and fusion in a river buffalo (Bubalus bubalis, 2n = 50) cow with reduced fertility. Cytogenet. Genome Res. 2011, 132, 26–30. [Google Scholar] [CrossRef]
- Iannuzzi, L.; Di Berardino, D. Tools of the trade: Diagnostics and research in domestic animal cytogenetics. J. Appl. Genet. 2008, 49, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Iannuzzi, L. Standard karyotype of the river buffalo (Bubalus bubalis L., 2n = 50). Report of the committee for the standardization of banded karyotypes of the river buffalo. Cytogenet. Cell Genet. 1994, 67, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.; Adega, F.; Heslop-Harrison, J.S.; Guedes-Pinto, H.; Wienberg, J. Complex satellite DNA reshuffling in the polymorphic t(1;29) Robertsonian translocation and evolutionarily derived chromosomes in cattle. Chromosome Res. 2003, 11, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Chaves, R.; Adega, F.; Santos, S.; Guedes-Pinto, H.; Heslop-Harrison, J.S. In situ hybridization and chromosome banding in mammalian species. Cytogenet. Genome Res. 2002, 96, 113–116. [Google Scholar] [CrossRef]
- Piegari, G.; Iovane, V.; Carletti, V.; Fico, R.; Costagliola, A.; De Biase, D.; Prisco, F.; Paciello, O. Assessment of Google Glass for Photographic Documentation in Veterinary Forensic Pathology: Usability Study. JMIR Mhealth Uhealth 2018, 6, e180. [Google Scholar] [CrossRef]
- Piegari, G.; Prisco, F.; De Biase, D.; Meomartino, L.; Fico, R.; Paciello, O. Cardiac laceration following non-penetrating chest trauma in dog and cat. Forensic Sci. Int. 2018, 290, e5–e8. [Google Scholar] [CrossRef]
- Piegari, G.; D’Anza, E.; Costanza, D.; Prisco, F.; Meomartino, L.; d’Aquino, I.; Albarella, S.; Paciello, O.; Ciotola, F. Perosomus Elumbis in Piglets: Pathological, Radiological and Cytogenetic Findings. Animals 2021, 11, 1132. [Google Scholar] [CrossRef]
- Gustavsson, I.; Rockborn, G. Chromosome abnormality in three cases of lymphatic leukemia in cattle. Nature 1964, 203, 990. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, I. Cytogenetics, distribution and phenotypic effects of a translocation in Swedish cattle. Hereditas 1969, 63, 68–169. [Google Scholar] [CrossRef] [PubMed]
- Gustavsson, I. Chromosome aberrations and their influence on the reproductive performance of domestic animals Y: A review. Z. Tierzüchtung Züchtungsbiologie 1980, 97, 76–195. [Google Scholar] [CrossRef]
- Popescu, C.P.; Pech, A. Une bibliographie sur la translocation 1/29 de bovins dans le monde (1964–1990). Ann. Zootech. 1991, 40, 271–305. [Google Scholar] [CrossRef]
- De Lorenzi, L.; Genualdo, V.; Gimelli, S.; Rossi, E.; Perucatti, A.; Iannuzzi, A.; Zannotti, M.; Malagutti, L.; Molteni, L.; Iannuzzi, L.; et al. Genomic analysis of cattle rob(1;29). Chromosome Res. 2012, 20, 815–823. [Google Scholar] [CrossRef]
- Iannuzzi, A.; Demyda-Peyrás, S.; Pistucci, R.; Morales, R.; Zannotti, M.; Sbarra, F.; Quaglia, A.; Parma, P. A genomic biomarker for the rapid identification of the rob(1;29) translocation in beef cattle breeds. Sci. Rep. 2024, 14, 2951. [Google Scholar] [CrossRef]
- Di Dio, C.; Longobardi, V.; Zullo, G.; Parma, P.; Pauciullo, A.; Perucatti, A.; Higgins, J.; Iannuzzi, A. Analysis of meiotic segregation by triple-color fish on both total and motile sperm fractions in a t(1p;18) river buffalo bull. PLoS ONE 2020, 15, e0232592. [Google Scholar] [CrossRef]
- Bonnet-Garnier, A.; Lacaze, S.; Beckers, J.F.; Berland, H.M.; Pinton, A.; Yerle, M.; Ducos, A. Meiotic segregation analysis in cows carrying the t(1;29) Robertsonian translocation. Cytogenet. Genome Res. 2008, 120, 91–96. [Google Scholar] [CrossRef]
- Iannuzzi, L.; King, W.A.; Di Berardino, D. Chromosome evolution in domestic bovids as revealed by chromosome banding and FISH-mapping techniques. Cytogenet. Genome Res. 2009, 126, 49–62. [Google Scholar] [CrossRef]
- Padula, A.M. The freemartin syndrome: An update. Anim. Reprod. Sci. 2005, 87, 93–109. [Google Scholar] [CrossRef]
- Ruvinsky, A.; Spicer, L.J. Developmental genetics: Sex determination and differentiation. In The Genetics of Cattle; Fries, R., Ruvinsky, A., Eds.; CABI: Wallingford, UK, 1999; pp. 456–461. [Google Scholar]
- Komisarek, J.; Dorynek, Z. Genetic aspects of twinning in cattle. J. Appl. Genet. 2002, 43, 55–68. [Google Scholar]
- Rocha, L.G.S.; Dos Santos, D.J.A.; Tonhati, H.; Costa, R.B.; de Camargo, G.M.F. Twinning rate in buffaloes: A case report. Reprod. Domest. Anim. = Zuchthyg. 2019, 54, 808–811. [Google Scholar] [CrossRef]

| Case | Age 1 | Cells 2 | Sex | % XX | % XY | C.I. 95% XY Cells 3 |
|---|---|---|---|---|---|---|
| B2250 | 32 | 100 | F | 89.1 | 9.9 | 4.1–15.7 |
| B202 | 26 | 100 | F | 51.0 | 49.0 | 39.2–58.8 |
| B2019 * | 3 | 30 | M | 99.1 | 0.9 | 2.5–4.3 |
| B2020 * | 3 | 100 | F | 95.8 | 4.2 | 0.3–8.1 |
| B1650 | 3 | 100 | F | 86.2 | 13.8 | 7.0–20.6 |
| B577 | 22 | 100 | F | 68.0 | 32.0 | 22.9–41.1 |
| B475 | 1 | 100 | F | 89.0 | 11.0 | 4.9–17.1 |
| B179 | 30 | 100 | F | 10.0 | 90.0 | 84.1–95.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perucatti, A.; Ciotola, F.; Pistucci, R.; Albarella, S.; Genualdo, V.; Rossetti, C.; Cimmino, R.; Piscopo, N.; Di Napoli, E.; Incarnato, D.; et al. Cytogenetic Screening on Mediterranean Italian River Buffalo Males Intended for Reproduction and Females with Fertility Issues—A Pilot Study. Animals 2025, 15, 2654. https://doi.org/10.3390/ani15182654
Perucatti A, Ciotola F, Pistucci R, Albarella S, Genualdo V, Rossetti C, Cimmino R, Piscopo N, Di Napoli E, Incarnato D, et al. Cytogenetic Screening on Mediterranean Italian River Buffalo Males Intended for Reproduction and Females with Fertility Issues—A Pilot Study. Animals. 2025; 15(18):2654. https://doi.org/10.3390/ani15182654
Chicago/Turabian StylePerucatti, Angela, Francesca Ciotola, Ramona Pistucci, Sara Albarella, Viviana Genualdo, Cristina Rossetti, Roberta Cimmino, Nadia Piscopo, Evaristo Di Napoli, Domenico Incarnato, and et al. 2025. "Cytogenetic Screening on Mediterranean Italian River Buffalo Males Intended for Reproduction and Females with Fertility Issues—A Pilot Study" Animals 15, no. 18: 2654. https://doi.org/10.3390/ani15182654
APA StylePerucatti, A., Ciotola, F., Pistucci, R., Albarella, S., Genualdo, V., Rossetti, C., Cimmino, R., Piscopo, N., Di Napoli, E., Incarnato, D., Paciello, O., Peretti, V., Parma, P., & Iannuzzi, L. (2025). Cytogenetic Screening on Mediterranean Italian River Buffalo Males Intended for Reproduction and Females with Fertility Issues—A Pilot Study. Animals, 15(18), 2654. https://doi.org/10.3390/ani15182654









