Additional Valine and Isoleucine Impact Growth Performance, Intestinal Health, and Muscle Growth in Broilers Under Necrotic Enteritis Challenges
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chickens, Experimental Design, and Necrotic Enteritis Challenge Model
2.2. Intestinal Permeability and Jejunal Lesion Scores
2.3. Jejunal C. perfringens Colony Count and Fecal E. maxima Oocyst Count
2.4. Intestinal Villus Height: Crypt Depth Ratio and Goblet Cell Count
2.5. Breast Muscle Yield and Body Composition Analysis Using DEXA
2.6. Jejunum and Breast Muscle Gene Expression Using qRT-PCR
2.7. Cecal Microbiome Analysis
2.8. Statistical Analysis
3. Results
3.1. Growth Performance
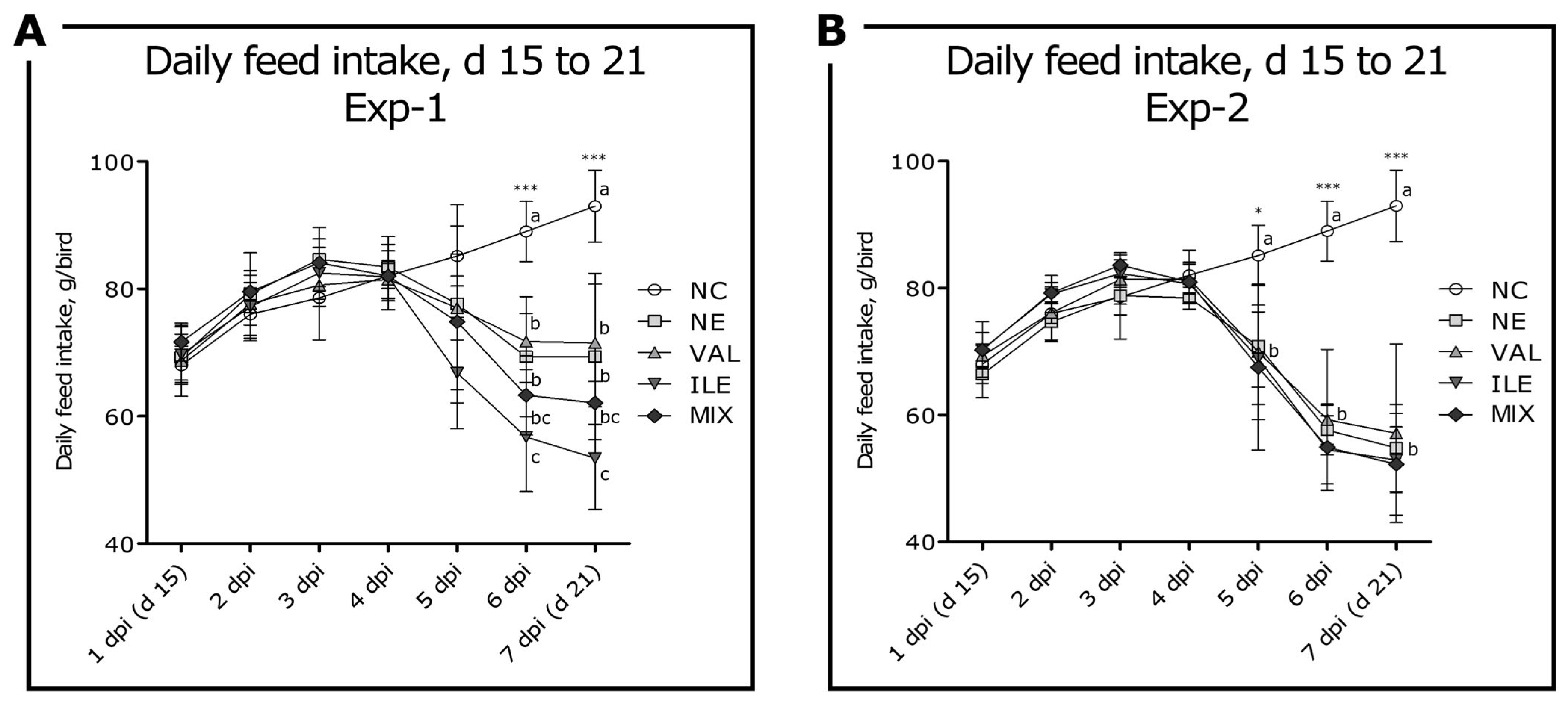
3.2. Daily Feed Intake
3.3. Intestinal Permeability and Jejunal NE Lesion Scores
3.4. Jejunal C. perfringens Colony Counts and Fecal E. maxima Oocyst Counts
3.5. Jejunal Villus Height: Crypt Depth Ratio and Goblet Cell Count
3.6. Breast Muscle Weight and Body Fat, Lean, and Mineral Compositions
3.7. Jejunal Gene Expression Levels
3.8. Breast Muscle Gene Expression Levels
3.9. Taxonomic Composition of Cecal Microbial Communities
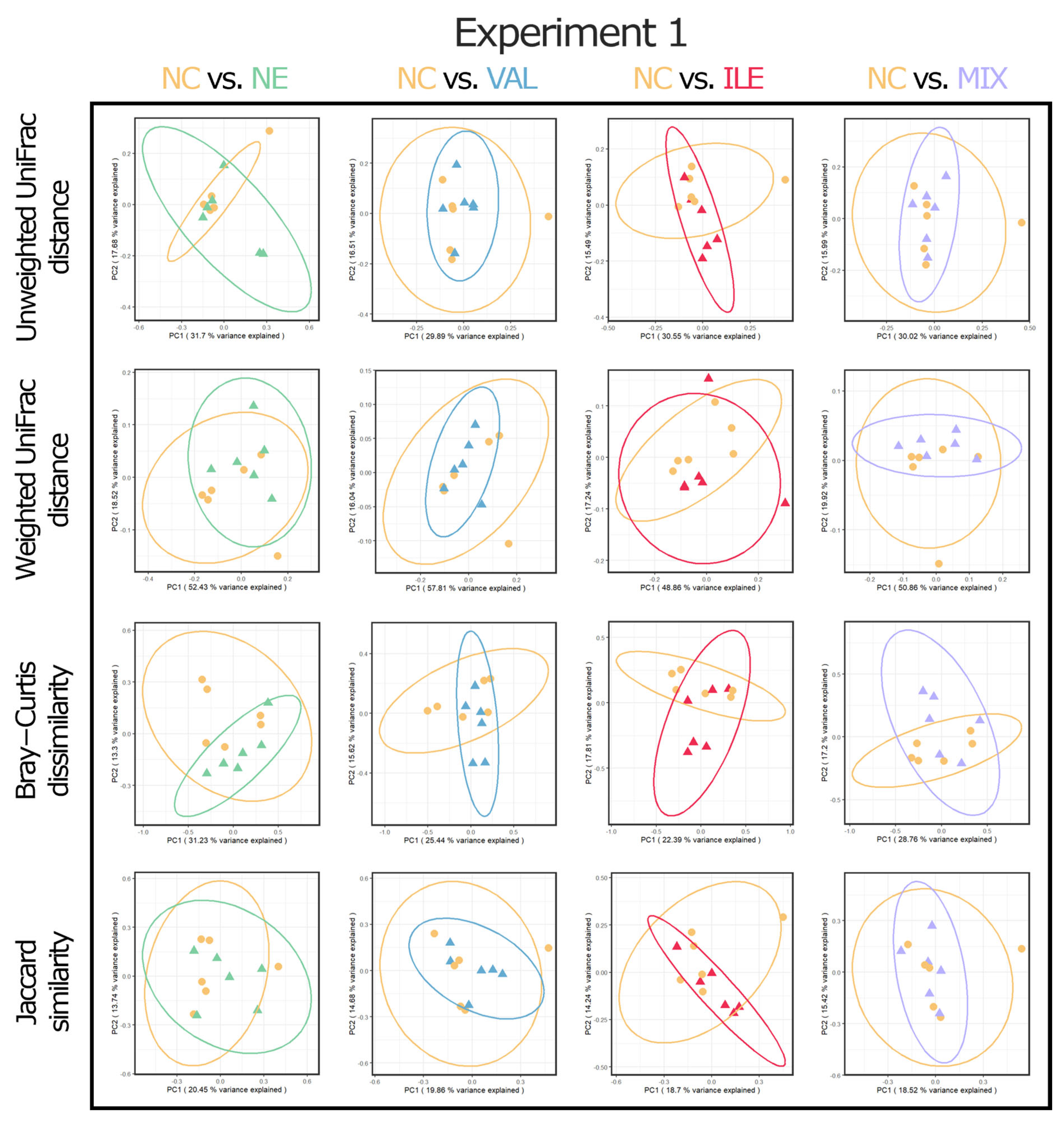
3.10. Alpha and Beta Diversities of Cecal Microbial Communities
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moore, R.J. Necrotic enteritis and antibiotic-free production of broiler chickens: Challenges in testing and using alternative products. Anim. Nutr. 2024, 16, 288–298. [Google Scholar] [CrossRef]
- Timbermont, L.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Necrotic enteritis in broilers: An updated review on the pathogenesis. Avian Pathol. 2011, 40, 341–347. [Google Scholar] [CrossRef]
- Shojadoost, B.; Vince, A.R.; Prescott, J.F. The successful experimental induction of necrotic enteritis in chickens by Clostridium perfringens: A critical review. Vet. Res. 2012, 43, 74. [Google Scholar] [CrossRef] [PubMed]
- Shamshirgaran, M.A.; Golchin, M.A. Comprehensive review of experimental models and induction protocols for avian necrotic enteritis over the past 2 decades. Front. Vet. Sci. 2024, 11, 1429637. [Google Scholar]
- Keyburn, A.L.; Boyce, J.D.; Vaz, P.; Bannam, T.L.; Ford, M.E.; Parker, D.; Di Rubbo, A.; Rood, J.I.; Moore, R.J. NetB, a new toxin that is associated with avian necrotic enteritis caused by Clostridium perfringens. PLoS Pathog. 2008, 4, e26. [Google Scholar] [CrossRef]
- Smyth, J.A.; Martin, T.G. Disease producing capability of netB positive isolates of C. perfringens recovered from normal chickens and a cow, and netB positive and negative isolates from chickens with necrotic enteritis. Vet. Microbiol. 2010, 146, 76–84. [Google Scholar]
- Rood, J.I.; Adams, V.; Lacey, J.; Lyras, D.; McClane, B.A.; Melville, S.B.; Moore, R.J.; Popoff, M.R.; Sarker, M.R.; Songer, J.G.; et al. Expansion of the Clostridium perfringens toxin-based typing scheme. Anaerobe 2018, 53, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.J. Necrotic enteritis predisposing factors in broiler chickens. Avian Pathol. 2016, 45, 275–281. [Google Scholar] [CrossRef]
- Paiva, D.; McElroy, A. Necrotic enteritis: Applications for the poultry industry. J. Appl. Poult. Res. 2014, 23, 557–566. [Google Scholar] [CrossRef]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: What we know and what we need to know: A review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Gharib-Naseri, K.; Kheravii, S.K.; Keerqin, C.; Morgan, N.; Swick, R.A.; Choct, M.; Wu, S.B. Two different Clostridium perfringens strains produce different levels of necrotic enteritis in broiler chickens. Poult. Sci. 2019, 98, 6422–6432. [Google Scholar] [CrossRef]
- Mot, D.; Timbermont, L.; Delezie, E.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F. Day-of-hatch vaccination is not protective against necrotic enteritis in broiler chickens. Avian Pathol. 2013, 42, 179–184. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.Q.; Jia, S.C.; Chen, Y.K.; Wang, J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018, 97, 477–484. [Google Scholar] [CrossRef]
- Tsiouris, V.; Georgopoulou, I.; Batzios, C.; Pappaioannou, N.; Ducatelle, R.; Fortomaris, P. Heat stress as a predisposing factor for necrotic enteritis in broiler chicks. Avian Pathol. 2018, 47, 616–624. [Google Scholar] [CrossRef]
- Dierick, E.; Hirvonen, O.P.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. Rapid growth predisposes broilers to necrotic enteritis. Avian Pathol. 2019, 48, 416–422. [Google Scholar] [CrossRef]
- Adhikari, P.; Kiess, A.; Adhikari, R.; Jha, R. An approach to alternative strategies to control avian coccidiosis and necrotic enteritis. J. Appl. Poult. Res. 2020, 29, 515–534. [Google Scholar] [CrossRef]
- Zaytsoff, S.J.; Boras, V.F.; Uwiera, R.R.; Inglis, G.D. A stress-induced model of acute necrotic enteritis in broiler chickens using dietary corticosterone administration. Poult. Sci. 2022, 101, 101726. [Google Scholar] [CrossRef]
- Goo, D.; Ko, H.; Sharma, M.K.; Choppa, V.S.R.; Paneru, D.; Shi, H.; Kim, W.K. Comparison of necrotic enteritis effects on growth performance and intestinal health in two different meat-type chicken strains ACRB and Cobb 500. Poult. Sci. 2024, 103, 103599. [Google Scholar] [CrossRef]
- Shimizu, T.; Ohtani, K.; Hirakawa, H.; Ohshima, K.; Yamashita, A.; Shiba, T.; Ogasawara, N.; Hattori, M.; Kuhara, S.; Hayashi, H. Complete genome sequence of Clostridium perfringens, an anaerobic flesh-eater. Proc. Natl. Acad. Sci. USA 2002, 99, 996–1001. [Google Scholar] [CrossRef]
- Hilliar, M.; Keerqin, C.; Girish, C.K.; Barekatain, R.; Wu, S.B.; Swick, R.A. Reducing protein and supplementing crystalline amino acids, to alter dietary amino acid profiles in birds challenged for subclinical necrotic enteritis. Poult. Sci. 2020, 99, 2048–2060. [Google Scholar] [CrossRef]
- Lee, D.T.; Rochell, S.J. Precision intestinal nutrition: Knowledge and gaps regarding the role of amino acids during an enteric challenge. Poult. Sci. 2022, 101, 101674. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.D.; Wu, S.B.; Choct, M.; Swick, R.A. The role of supplemental glycine in establishing a subclinical necrotic enteritis challenge model in broiler chickens. Anim. Nutr. 2017, 3, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Lin, Y.; Zeng, D.; Zhou, M.; Zeng, Y.; Wang, H.; Zhou, Y.; Zhu, H.; Pan, K.; Jing, B.; et al. Bacillus licheniformis normalize the ileum microbiota of chickens infected with necrotic enteritis. Sci. Rep. 2018, 8, 1744. [Google Scholar] [CrossRef]
- Keerqin, C.; Wu, S.B.; Svihus, B.; Swick, R.; Morgan, N.; Choct, M. An early feeding regime and a high-density amino acid diet on growth performance of broilers under subclinical necrotic enteritis challenge. Anim. Nutr. 2017, 3, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Kidd, M.T. Nutritional modulation of immune function in broilers. Poult. Sci. 2004, 83, 650–657. [Google Scholar] [CrossRef]
- Kim, W.K.; Singh, A.K.; Wang, J.; Applegate, T. Functional role of branched chain amino acids in poultry: A review. Poult. Sci. 2022, 101, 101715. [Google Scholar] [CrossRef]
- Wang, C.; Peng, Y.; Zhang, Y.; Xu, J.; Jiang, S.; Wang, L.; Yin, Y. The biological functions and metabolic pathways of valine in swine. J. Anim. Sci. Biotechnol. 2023, 14, 135. [Google Scholar] [CrossRef]
- Neinast, M.; Murashige, D.; Arany, Z. Branched chain amino acids. Annu. Rev. Physiol. 2019, 81, 139–164. [Google Scholar] [CrossRef]
- Corrent, E.; Bartelt, J. Valine and isoleucine: The next limiting amino acids in broiler diets. Lohmann Inform. 2011, 46, 59–67. [Google Scholar]
- Brosnan, J.T.; Brosnan, M.E. Branched-chain amino acids: Enzyme and substrate regulation. J. Nutr. 2006, 136, 207S–211S. [Google Scholar] [CrossRef]
- Wiltafsky, M.K.; Pfaffl, M.W.; Roth, F.X. The effects of branched-chain amino acid interactions on growth performance, blood metabolites, enzyme kinetics and transcriptomics in weaned pigs. Br. J. Nutr. 2010, 103, 964–976. [Google Scholar] [CrossRef]
- Thornton, S.A.; Corzo, A.; Pharr, G.T.; Dozier, W.A., III; Miles, D.M.; Kidd, M.T. Valine requirements for immune and growth responses in broilers from 3 to 6 weeks of age. Br. Poult. Sci. 2006, 47, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Adabi, S.G.; Ceylan, N.; Ciftci, I.; Ceylan, A. Response of growing chicks to supplementation of low protein diets with leucine, valine and glycine-glutamic acid. S. Afr. J. Anim. Sci. 2019, 49, 1047–1062. [Google Scholar]
- Allameh, S.; Toghyani, M. Effect of dietary valine supplementation to low protein diets on performance, intestinal morphology and immune responses in broiler chickens. Livest. Sci. 2019, 229, 137–144. [Google Scholar] [CrossRef]
- Jian, H.; Miao, S.; Liu, Y.; Wang, X.; Xu, Q.; Zhou, W.; Li, H.; Dong, X.; Zou, X. Dietary valine ameliorated gut health and accelerated the development of nonalcoholic fatty liver disease of laying hens. Oxid. Med. Cell. Longev. 2021, 2021, 4704771. [Google Scholar]
- Ruan, D.; Fan, Q.L.; Zhang, S.; Ei-Senousey, H.K.; Fouad, A.M.; Lin, X.J.; Dong, X.L.; Deng, Y.F.; Yan, S.J.; Zheng, C.T.; et al. Dietary isoleucine supplementation enhances growth performance, modulates the expression of genes related to amino acid transporters and protein metabolism, and gut microbiota in yellow-feathered chickens. Poult. Sci. 2023, 102, 102774. [Google Scholar] [CrossRef]
- Goo, D.; Choi, J.; Ko, H.; Choppa, V.S.R.; Liu, G.; Lillehoj, H.S.; Kim, W.K. Effects of Eimeria maxima infection doses on growth performance and gut health in dual-infection model of necrotic enteritis in broiler chickens. Front. Physiol. 2023, 14, 1269398. [Google Scholar] [CrossRef] [PubMed]
- Cobb Broiler Management Guide; Cobb-Vantress Inc.: Siloam Springs, AR, USA, 2021; Available online: https://www.cobbgenetics.com/assets/Cobb-Files/Broiler-Guide_English-2021-min.pdf (accessed on 15 March 2020).
- Teng, P.Y.; Yadav, S.; de Souza Castro, F.L.; Tompkins, Y.H.; Fuller, A.L.; Kim, W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020, 99, 4203–4216. [Google Scholar] [CrossRef]
- Teng, P.Y.; Choi, J.; Yadav, S.; Tompkins, Y.H.; Kim, W.K. Effects of low-crude protein diets supplemented with arginine, glutamine, threonine, and methionine on regulating nutrient absorption, intestinal health, and growth performance of Eimeria-infected chickens. Poult. Sci. 2021, 100, 101427. [Google Scholar] [PubMed]
- Castro, F.L.; Teng, P.Y.; Yadav, S.; Gould, R.L.; Craig, S.; Pazdro, R.; Kim, W.K. The effects of L-Arginine supplementation on growth performance and intestinal health of broiler chickens challenged with Eimeria spp. Poult. Sci. 2020, 99, 5844–5857. [Google Scholar]
- Goo, D.; Gadde, U.D.; Kim, W.K.; Gay, C.G.; Porta, E.W.; Jones, S.W.; Walker, S.; Lillehoj, H.S. Hyperimmune egg yolk antibodies developed against Clostridium perfringens antigens protect against necrotic enteritis. Poult. Sci. 2023, 102, 102841. [Google Scholar] [CrossRef] [PubMed]
- Goo, D.; Ko, H.; Choi, J.; Lee, J.; White, D.L.; Sharma, M.K.; Kim, W.K. Valine and isoleucine deficiency in necrotic enteritis challenge impact growth performance, intestinal health, and muscle growth in broilers. Poult. Sci. 2025, 104, 105143. [Google Scholar] [PubMed]
- Santos, T.S.D.; Teng, P.Y.; Yadav, S.; Castro, F.L.D.S.; Gould, R.L.; Craig, S.W.; Chen, C.; Fuller, A.L.; Pazdro, R.; Sartori, J.R.; et al. Effects of inorganic Zn and Cu supplementation on gut health in broiler chickens challenged with Eimeria spp. Front. Vet. Sci. 2020, 7, 230. [Google Scholar]
- Choi, J.; Liu, G.; Goo, D.; Wang, J.; Bowker, B.; Zhuang, H.; Kim, W.K. Effects of tannic acid supplementation on growth performance, gut health, and meat production and quality of broiler chickens raised in floor pens for 42 days. Front. Physiol. 2022, 13, 1082009. [Google Scholar] [CrossRef]
- Liu, C.; Radebe, S.M.; Zhang, H.; Jia, J.; Xie, S.; Shi, M.; Yu, Q. Effect of Bacillus coagulans on maintaining the integrity intestinal mucosal barrier in broilers. Vet. Microbiol. 2022, 266, 109357. [Google Scholar]
- Goo, D.; Kim, W.K. Valine deficiency has a greater impact on broiler growth than isoleucine deficiency. Poult. Sci. 2025, 104, 104742. [Google Scholar]
- Goo, D.; Singh, A.K.; Choi, J.; Sharma, M.K.; Paneru, D.; Lee, J.; Katha, H.R.; Zhuang, H.; Kong, N.; Bowker, B.; et al. Different dietary branched-chain amino acid ratios, crude protein levels, and protein sources can affect the growth performance and meat yield in broilers. Poult. Sci. 2024, 103, 104313. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Su, S.; Choi, H.; Koo, E.; Kim, W.K. L-Arginine supplementation enhances growth performance, lean muscle, and bone density but not fat in broiler chickens. Poult. Sci. 2019, 98, 1716–1722. [Google Scholar] [CrossRef]
- Goo, D.; Lee, J.; Paneru, D.; Sharma, M.K.; Rafieian-Naeini, H.R.; Mahdavi, F.S.; Gyawali, I.; Gudidoddi, S.R.; Han, G.; Kim, W.K. Effects of branched-chain amino acid imbalance and dietary valine and isoleucine supplementation in modified corn-soybean meal diets with corn distillers dried grain with soluble on growth performance, carcass quality, intestinal health, and cecal microbiome in Cobb 500. Poult. Sci. 2024, 103, 104483. [Google Scholar]
- Choi, J.; Goo, D.; Sharma, M.K.; Ko, H.; Liu, G.; Paneru, D.; Choppa, V.S.R.; Lee, J.; Kim, W.K. Effects of different Eimeria inoculation doses on growth performance, daily feed intake, gut health, gut microbiota, foot pad dermatitis, and Eimeria gene expression in broilers raised in floor pens for 35 days. Animals 2023, 13, 2237. [Google Scholar] [CrossRef] [PubMed]
- Logue, J.B.; Stedmon, C.A.; Kellerman, A.M.; Nielsen, N.J.; Andersson, A.F.; Laudon, H.; Lindström, E.S.; Kritzberg, E.S. Experimental insights into the importance of aquatic bacterial community composition to the degradation of dissolved organic matter. ISME J. 2016, 10, 533–545. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Jimenez, R. Practical metagenomics: Microbiome tutorial with QIIME 2. F1000Research 2019, 10, 798. [Google Scholar]
- Goo, D.; Ragyari, S.K.R.; Gyawali, I.; Gudidoddi, S.R.; Han, G.; Rafieian-Naeini, H.R.; Neupane, I.; Keshavareddy, V.P.R.; Kim, W.K. Effects of Increasing Dietary Valine Levels on Growth Performance and Branched-Chain Amino Acid Activities in Broilers. University of Georgia: Athens, GA, USA, 2025; to be submitted. [Google Scholar]
- Lochmiller, R.L.; Deerenberg, C. Trade-offs in evolutionary immunology: Just what is the cost of immunity? Oikos 2000, 88, 87–98. [Google Scholar] [CrossRef]
- Goo, D.; Choi, J.; Lee, J.; Sharma, M.K.; Paneru, D.; Zhuang, H.; Kong, B.; Bowker, B.; Ritz, C.W.; Kim, W.K. Additional Isoleucine Without Valine in an Imbalanced Branched-Chain Amino Acid Diet Further Exacerbates Its Negative Effects in Broilers. J. Appl. Poult. Res. 2025; submitted. [Google Scholar]
- Ospina-Rojas, I.C.; Pozza, P.C.; Rodrigueiro, R.J.B.; Gasparino, E.; Khatlab, A.S.; Murakami, A.E. High leucine levels affecting valine and isoleucine recommendations in low-protein diets for broiler chickens. Poult. Sci. 2020, 99, 5946–5959. [Google Scholar] [CrossRef]
- Kriseldi, R.; Silva, M.; Lee, J.; Adhikari, R.; Williams, C.; Corzo, A. Understanding the interactive effects of dietary leucine with isoleucine and valine in the modern commercial broiler. Poult. Sci. 2022, 101, 102140. [Google Scholar] [CrossRef] [PubMed]
- Tavernari, F.C.; Lelis, G.R.; Vieira, R.A.; Rostagno, H.S.; Albino, L.F.T.; Neto, A.O. Valine needs in starting and growing Cobb (500) broilers. Poult. Sci. 2013, 92, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Maynard, C.W.; Liu, S.Y.; Lee, J.T.; Caldas, J.V.; Diehl, J.J.E.; Rochell, S.J.; Dridi, S.; Kidd, M.T. Determination of digestible valine requirements in male and female Cobb 500 broilers. Anim. Feed Sci. Technol. 2021, 275, 114847. [Google Scholar] [CrossRef]
- McGuckin, M.A.; Lindén, S.K.; Sutton, P.; Florin, T.H. Mucin dynamics and enteric pathogens. Nat. Rev. Microbiol. 2011, 9, 265–278. [Google Scholar] [CrossRef]
- Golder, H.M.; Geier, M.S.; Forder, R.E.A.; Hynd, P.I.; Hughes, R.J. Effects of necrotic enteritis challenge on intestinal micro-architecture and mucin profile. Br. Poult. Sci. 2011, 52, 500–506. [Google Scholar] [CrossRef]
- Celi, P.; Cowieson, A.J.; Fru-Nji, F.; Steinert, R.E.; Kluenter, A.M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar]
- Xue, G.D.; Barekatain, R.; Wu, S.B.; Choct, M.; Swick, R.A. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult. Sci. 2018, 97, 1334–1341. [Google Scholar] [CrossRef]
- Farran, M.T.; Thomas, O.P. Valine deficiency. 2. The effect of feeding a valine-deficient diet during the starter period on performance and leg abnormality of male broiler chicks. Poult. Sci. 1992, 71, 1885–1890. [Google Scholar] [PubMed]
- Foroudi, F.; Rezamand, P. The Effects of Dietary Valine on Performance, Serum Antibody Titre and Bone Mineralization in Broiler Chicks. Iran. J. Appl. Anim. Sci. 2014, 4, 405–409. [Google Scholar]
- Pasare, C.; Medzhitov, R. Toll-like receptors: Linking innate and adaptive immunity. Microbes Infect. 2004, 6, 1382–1387. [Google Scholar] [CrossRef]
- Takeuchi, O.; Akira, S. Pattern recognition receptors and inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Lee, K.W.; Lillehoj, H.S.; Jeong, W.; Jeoung, H.Y.; An, D.J. Avian necrotic enteritis: Experimental models, host immunity, pathogenesis, risk factors, and vaccine development. Poult. Sci. 2011, 90, 1381–1390. [Google Scholar] [CrossRef] [PubMed]
- Rengaraj, D.; Truong, A.D.; Lee, S.H.; Lillehoj, H.S.; Hong, Y.H. Expression analysis of cytosolic DNA-sensing pathway genes in the intestinal mucosal layer of necrotic enteritis-induced chicken. Vet. Immunol. Immunopathol. 2016, 170, 1–12. [Google Scholar] [CrossRef]
- Park, I.; Oh, S.; Nam, H.; Celi, P.; Lillehoj, H.S. Antimicrobial activity of sophorolipids against Eimeria maxima and Clostridium perfringens, and their effect on growth performance and gut health in necrotic enteritis. Poult. Sci. 2022, 101, 101731. [Google Scholar] [CrossRef]
- Liu, G.; Sharma, M.K.; Tompkins, Y.H.; Teng, P.Y.; Kim, W.K. Impacts of varying methionine to cysteine supplementation ratios on growth performance, oxidative status, intestinal health, and gene expression of immune response and methionine metabolism in broilers under Eimeria spp. challenge. Poult. Sci. 2024, 103, 103300. [Google Scholar]
- Park, I.; Nam, H.; Lee, Y.; Smith, A.; Rehberger, T.; Lillehoj, H.S. Effect of β-Alanine Metabolite on Gut Integrity and Immunity in Commercial Broiler Chickens Infected with Eimeria maxima. Animals 2024, 14, 2558. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.K.; Singh, A.K.; Goo, D.; Choppa, V.S.R.; Ko, H.; Shi, H.; Kim, W.K. Graded levels of Eimeria infection modulated gut physiology and temporarily ceased the egg production of laying hens at peak production. Poult. Sci. 2024, 103, 103229. [Google Scholar] [CrossRef]
- Fotiadis, D.; Kanai, Y.; Palacín, M. The SLC3 and SLC7 families of amino acid transporters. Mol. Asp. Med. 2013, 34, 139–158. [Google Scholar] [CrossRef] [PubMed]
- Teng, P.Y.; Choi, J.; Tompkins, Y.; Lillehoj, H.; Kim, W. Impacts of increasing challenge with Eimeria maxima on the growth performance and gene expression of biomarkers associated with intestinal integrity and nutrient transporters. Vet. Res. 2021, 52, 81. [Google Scholar] [CrossRef]
- Ghareeb, A.F.; Schneiders, G.H.; Foutz, J.C.; Milfort, M.C.; Fuller, A.L.; Yuan, J.; Rekaya, R.; Aggrey, S.E. Heat Stress Alters the Effect of Eimeria maxima Infection on Ileal Amino Acids Digestibility and Transporters Expression in Meat-Type Chickens. Animals 2022, 12, 1554. [Google Scholar] [CrossRef]
- Sinclair, L.V.; Rolf, J.; Emslie, E.; Shi, Y.B.; Taylor, P.M.; Cantrell, D.A. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 2013, 14, 500–508. [Google Scholar] [CrossRef]
- Loftus, R.M.; Assmann, N.; Kedia-Mehta, N.; O’Brien, K.L.; Garcia, A.; Gillespie, C.; Hukelmann, J.L.; Oefner, P.J.; Lamond, A.I.; Gardiner, C.M.; et al. Amino acid-dependent cMyc expression is essential for NK cell metabolic and functional responses in mice. Nat. Commun. 2018, 9, 2341. [Google Scholar] [CrossRef]
- Ogbechi, J.; Wright, H.L.; Balint, S.; Topping, L.M.; Kristina, Z.; Huang, Y.S.; Pantazi, E.; Swart, M.; Windell, D.; Marin, E.; et al. LAT1 enables T cell activation under inflammatory conditions. J. Autoimmun. 2023, 138, 103031. [Google Scholar] [CrossRef]
- Song, W.; Li, D.; Tao, L.; Luo, Q.; Chen, L. Solute carrier transporters: The metabolic gatekeepers of immune cells. Acta Pharm. Sin. B 2020, 10, 61–78. [Google Scholar] [CrossRef]
- Cole, J.T. Metabolism of BCAAs. In Branched Chain Amino Acids in Clinical Nutrition; Humana Press: New York, NY, USA, 2015; Volume 1, pp. 13–24. [Google Scholar]
- Zhao, Y.; Zeng, Y.; Zeng, D.; Wang, H.; Sun, N.; Xin, J.; Zhou, M.; Yang, H.; Lei, L.; Ling, H.; et al. Dietary probiotic supplementation suppresses subclinical necrotic enteritis in broiler chickens in a microbiota-dependent manner. Front. Immunol. 2022, 13, 855426. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; You, H.; Li, Y.; Wang, Y.; Hui, P.; Qiao, B.; Lu, J.; Zhang, W.; Zhou, S.; Zheng, Y.; et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci. Rep. 2018, 8, 15809. [Google Scholar] [CrossRef] [PubMed]
- Kamada, R.; Kudoh, F.; Ito, S.; Tani, I.; Janairo, J.I.B.; Omichinski, J.G.; Sakaguchi, K. Metal-dependent Ser/Thr protein phosphatase PPM family: Evolution, structures, diseases and inhibitors. Pharm. Ther. 2020, 215, 107622. [Google Scholar]



| Treatments 1 | ||||
|---|---|---|---|---|
| NC or NE | NE-Challenged | |||
| VAL | ILE | MIX | ||
| Ingredient, % | ||||
| Corn, grain | 65.75 | 65.75 | 65.75 | 65.75 |
| Soybean meal, 46.9% CP | 25.75 | 25.75 | 25.75 | 25.75 |
| Soybean oil | 1.25 | 1.25 | 1.25 | 1.25 |
| Dicalcium phosphate | 1.60 | 1.60 | 1.60 | 1.60 |
| Limestone | 1.20 | 1.20 | 1.20 | 1.20 |
| Sodium bicarbonate | 0.35 | 0.35 | 0.35 | 0.35 |
| Salt | 0.22 | 0.22 | 0.22 | 0.22 |
| DL-Methionine | 0.45 | 0.45 | 0.45 | 0.45 |
| L-Lysine HCl | 0.42 | 0.42 | 0.42 | 0.42 |
| L-Threonine | 0.28 | 0.28 | 0.28 | 0.28 |
| L-Arginine | 0.33 | 0.33 | 0.33 | 0.33 |
| L-Valine | 0.21 | 0.21 | 0.21 | 0.48 |
| L-Isoleucine | 0.11 | 0.11 | 0.11 | 0.36 |
| Glycine | 1.55 | 1.37 | 1.40 | 1.23 |
| Sand | 0.35 | 0.26 | 0.25 | 0.15 |
| Vitamin premix 2 | 0.10 | 0.10 | 0.10 | 0.10 |
| Mineral premix 3 | 0.08 | 0.08 | 0.08 | 0.08 |
| Total | 100.0 | 100.0 | 100.0 | 100.0 |
| Calculated value, % | ||||
| ME, kcal/kg | 3000 | 3000 | 3000 | 3000 |
| Crude protein | 20.0 | 20.0 | 20.0 | 20.0 |
| Total calcium | 0.88 | 0.88 | 0.88 | 0.88 |
| Available phosphorus | 0.44 | 0.44 | 0.44 | 0.44 |
| Arginine | 1.29 | 1.29 | 1.29 | 1.29 |
| Lysine | 1.17 | 1.17 | 1.17 | 1.17 |
| Methionine | 0.68 | 0.68 | 0.68 | 0.68 |
| TSAA | 0.89 | 0.89 | 0.89 | 0.89 |
| Threonine | 0.80 | 0.80 | 0.80 | 0.80 |
| Tryptophan | 0.18 | 0.18 | 0.18 | 0.18 |
| Leucine | 1.31 | 1.31 | 1.31 | 1.31 |
| Valine | 0.89 | 1.15 | 0.89 | 1.15 |
| Isoleucine | 0.76 | 0.76 | 0.98 | 0.98 |
| BCAA/lysine ratio | ||||
| Leucine/lysine | 1.12 | 1.12 | 1.12 | 1.12 |
| Valine/lysine | 0.76 | 0.98 | 0.76 | 0.98 |
| Isoleucine/lysine | 0.65 | 0.65 | 0.84 | 0.84 |
| Treatments 1 | ||||
|---|---|---|---|---|
| NC or NE | NE-Challenged | |||
| VAL | ILE | MIX | ||
| Analyzed value, % | ||||
| Alanine | 0.79 | 0.87 | 0.94 | 0.85 |
| Arginine | 1.32 | 1.42 | 1.43 | 1.37 |
| Aspartic Acid | 1.56 | 1.78 | 1.94 | 1.72 |
| Cysteine | 0.23 | 0.28 | 0.30 | 0.26 |
| Glutamic Acid | 2.77 | 3.16 | 3.43 | 3.04 |
| Glycine | 1.85 | 1.63 | 1.61 | 1.40 |
| Histidine | 0.39 | 0.44 | 0.48 | 0.42 |
| Isoleucine | 0.83 | 0.92 | 1.19 | 1.16 |
| Leucine | 1.36 | 1.50 | 1.62 | 1.50 |
| Lysine | 1.18 | 1.28 | 1.30 | 1.23 |
| Methionine | 0.57 | 0.67 | 0.64 | 0.59 |
| Phenylalanine | 0.78 | 0.87 | 0.94 | 0.86 |
| Proline | 0.88 | 0.97 | 1.05 | 0.96 |
| Serine | 0.63 | 0.73 | 0.78 | 0.70 |
| Threonine | 0.86 | 0.87 | 0.92 | 0.80 |
| Tryptophan | 0.20 | 0.19 | 0.21 | 0.20 |
| Tyrosine | 0.53 | 0.59 | 0.63 | 0.62 |
| Valine | 0.97 | 1.37 | 1.09 | 1.28 |
| Crude protein, % | 19.6 | 20.1 | 20.1 | 20.4 |
| BCAA/lysine ratio | ||||
| Leucine/lysine | 1.15 | 1.17 | 1.25 | 1.22 |
| Valine/lysine | 0.82 | 1.07 | 0.84 | 1.04 |
| Isoleucine/lysine | 0.70 | 0.72 | 0.92 | 0.94 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Body weight, g | |||||||
| d 7 | 160 | 159 | 159 | 159 | 159 | 0.49 | 0.988 |
| d 14 (0 dpi 3) | 450 | 461 | 452 | 447 | 454 | 7.8 | 0.770 |
| d 21 (7 dpi) | 864 a | 813 ab | 796 bc | 749 c | 788 bc | 14.1 | <0.001 |
| Body weight gain, g | |||||||
| d 7–14 | 291 | 302 | 293 | 287 | 295 | 7.7 | 0.750 |
| d 15–21 | 414 a | 353 b | 344 bc | 302 c | 333 bc | 11.3 | <0.001 |
| d 7–21 | 704 a | 654 ab | 637 bc | 589 c | 628 bc | 14.0 | <0.001 |
| Feed intake, g | |||||||
| d 7–14 | 343 | 354 | 354 | 351 | 350 | 5.5 | 0.661 |
| d 15–21 | 572 a | 533 ab | 529 ab | 488 b | 517 b | 12.5 | <0.01 |
| d 7–21 | 915 a | 887 ab | 882 ab | 839 b | 867 ab | 16.0 | <0.05 |
| Feed conversion ratio | |||||||
| d 7–14 | 1.18 | 1.17 | 1.21 | 1.22 | 1.19 | 0.017 | 0.270 |
| d 15–21 | 1.39 a | 1.51 ab | 1.54 ab | 1.63 b | 1.55 ab | 0.044 | <0.05 |
| d 7–21 | 1.30 a | 1.36 ab | 1.39 b | 1.43 b | 1.38 b | 0.019 | <0.01 |
| Experiment 2 | |||||||
| Body weight, g | |||||||
| d 7 | 160 | 159 | 159 | 159 | 159 | 0.49 | 0.992 |
| d 14 (0 dpi) | 450 | 450 | 459 | 449 | 457 | 5.8 | 0.637 |
| d 21 (7 dpi) | 864 a | 746 b | 767 b | 745 b | 742 b | 13.6 | <0.001 |
| Body weight gain, g | |||||||
| d 7–14 | 291 | 290 | 300 | 290 | 298 | 5.7 | 0.620 |
| d 15–21 | 414 a | 296 b | 308 b | 296 b | 286 b | 12.3 | <0.001 |
| d 7–21 | 704 a | 586 b | 607 b | 586 b | 583 b | 13.5 | <0.001 |
| Feed intake, g | |||||||
| d 7–14 | 343 | 339 | 352 | 349 | 352 | 3.6 | 0.062 |
| d 15–21 | 572 a | 482 b | 495 b | 489 b | 489 b | 9.5 | <0.001 |
| d 7–21 | 915 a | 821 b | 847 b | 838 b | 841 b | 11.0 | <0.001 |
| Feed conversion ratio | |||||||
| d 7–14 | 1.18 | 1.17 | 1.18 | 1.21 | 1.18 | 0.016 | 0.566 |
| d 15–21 | 1.39 a | 1.63 b | 1.62 b | 1.67 b | 1.72 b | 0.046 | <0.001 |
| d 7–21 | 1.30 a | 1.40 b | 1.40 b | 1.43 b | 1.44 b | 0.019 | <0.001 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Intestinal permeability, ng/mL | 14 b | 183 ab | 254 ab | 263 ab | 404 a | 67.4 | <0.01 |
| Jejunal NE lesion score, 0 to 3 scale | 0 b | 1.5 a | 1.3 a | 1.5 a | 1.0 a | 0.16 | <0.01 † |
| Jejunal C. perfringens colony count, log10 cfu/g | 6.8 b | 8.3 a | 8.2 a | 8.4 a | 8.1 a | 0.25 | <0.001 |
| Fecal E. maxima oocyst count, log10/g | 0 b | 5.6 a | 5.6 a | 5.8 a | 5.7 a | 0.07 | <0.001 |
| Experiment 2 | |||||||
| Intestinal permeability, ng/mL | 14 b | 273 a | 242 a | 483 a | 287 a | 61.5 | <0.001 |
| Jejunal NE lesion score, 0 to 3 scale | 0 b | 1.5 a | 1.3 a | 1.5 a | 1.5 a | 0.23 | <0.01 † |
| Jejunal C. perfringens colony count, log10 cfu/g | 6.8 b | 8.6 a | 8.3 a | 8.7 a | 8.5 a | 0.30 | <0.001 |
| Fecal E. maxima oocyst count, log10/g | 0 b | 5.7 a | 5.7 a | 5.7 a | 5.9 a | 0.08 | <0.001 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Villus height, μm | 1205 a | 863 b | 923 ab | 880 ab | 851 b | 78.3 | <0.05 |
| Crypt depth, μm | 168 b | 261 a | 261 a | 266 a | 310 a | 17.8 | <0.001 |
| VH:CD 3 | 7.5 a | 3.4 b | 3.5 b | 3.3 b | 2.8 b | 0.35 | <0.001 |
| Goblet cells, cells/villus | 152 a | 94 b | 96 b | 99 b | 83 b | 10.5 | <0.001 |
| Experiment 2 | |||||||
| Villus height, μm | 1205 a | 733 b | 913 b | 824 b | 856 b | 57.9 | <0.001 |
| Crypt depth, μm | 168 b | 272 a | 307 a | 277 a | 293 a | 18.5 | <0.001 |
| VH:CD | 7.5 a | 2.8 b | 3.0 b | 3.0 b | 2.9 b | 0.33 | <0.001 |
| Goblet cells, cells/villus | 152 a | 78 b | 109 b | 93 b | 104 b | 7.8 | <0.001 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Breast muscle weight, g | 124.0 | 114.7 | 121.3 | 108.7 | 122.0 | 8.78 | 0.720 |
| Breast muscle weight, % | 14.3 | 14.3 | 15.2 | 14.6 | 14.7 | 0.68 | 0.885 |
| Body composition | |||||||
| Total tissue weight, g | 876 a | 784 ab | 780 ab | 692 b | 756 ab | 40.3 | <0.05 |
| Lean, g | 782 a | 716 ab | 697 ab | 630 b | 680 ab | 34.6 | <0.05 |
| Lean, % | 89.3 | 91.3 | 89.6 | 91.1 | 90.1 | 0.89 | 0.341 |
| Fat, g | 94.4 | 68.3 | 82.2 | 61.0 | 75.5 | 9.06 | 0.099 |
| Fat, % | 10.7 | 8.7 | 10.4 | 8.9 | 9.9 | 0.89 | 0.341 |
| Body mineral composition 3 | |||||||
| BMD, mg/cm2 | 141.6 | 131.0 | 133.7 | 125.3 | 130.5 | 5.54 | 0.319 |
| BMC, g | 13.3 a | 10.1 ab | 10.9 ab | 8.8 b | 10.0 ab | 0.86 | <0.05 |
| BMCR, % | 1.52 | 1.29 | 1.39 | 1.27 | 1.32 | 0.087 | 0.211 |
| Experiment 2 | |||||||
| Breast muscle weight, g | 124.0 | 118.7 | 124.0 | 101.3 | 117.3 | 6.79 | 0.146 |
| Breast muscle weight, % | 14.3 | 15.4 | 15.2 | 14.9 | 15.4 | 0.64 | 0.710 |
| Body composition | |||||||
| Total tissue weight, g | 876 a | 734 b | 766 b | 749 b | 751 b | 30.0 | <0.05 |
| Lean, g | 782 a | 665 b | 705 ab | 680 ab | 682 ab | 25.2 | <0.05 |
| Lean, % | 89.4 b | 90.7 | 92.0 | 91.1 | 90.8 | 0.83 | 0.249 |
| Fat, g | 94.4 | 69.2 | 61.2 | 68.2 | 69.3 | 7.79 | 0.053 |
| Fat, % | 10.7 | 9.3 | 8.1 | 8.9 | 9.2 | 0.83 | 0.249 |
| Body mineral composition 3 | |||||||
| BMD, mg/cm2 | 141.6 | 134.8 | 129.0 | 131.4 | 126.6 | 4.86 | 0.248 |
| BMC, g | 13.3 a | 9.6 b | 11.0 ab | 9.3 b | 10.2 b | 0.74 | <0.01 |
| BMCR, % | 1.52 | 1.31 | 1.45 | 1.23 | 1.36 | 0.095 | 0.250 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Relative fold change 3 | |||||||
| LAT1 (SLC7A5) | 1.00 b | 11.01 a | 7.42 a | 7.76 a | 6.29 a | 1.182 | <0.001 |
| LAT4 (SLC43A2) | 1.00 | 0.95 | 1.11 | 1.08 | 1.15 | 0.114 | 0.734 |
| SNAT2 (SLC38A2) | 1.00 b | 2.47 ab | 1.94 ab | 2.37 ab | 3.38 a | 0.382 | <0.01 |
| y+LAT1 (SLC7A7) | 1.00 | 1.29 | 1.01 | 1.49 | 1.33 | 0.144 | 0.097 |
| y+LAT2 (SLC7A6) | 1.00 | 1.03 | 1.11 | 1.48 | 1.34 | 0.139 | 0.092 |
| SBAT2 (SLC6A15) | 1.00 | 1.40 | 1.50 | 1.54 | 1.87 | 0.310 | 0.422 |
| BAT1 (SLC7A9) | 1.00 a | 0.32 b | 0.42 b | 0.41 b | 0.28 b | 0.088 | <0.001 |
| IL1β | 1.00 b | 4.86 a | 3.22 ab | 3.16 ab | 3.81 ab | 0.727 | <0.05 |
| IL1RN | 1.00 b | 1.99 a | 1.78 a | 1.98 a | 1.66 a | 0.140 | <0.001 |
| IL6 | 1.00 b | 1.99 ab | 2.50 a | 1.98 ab | 2.12 a | 0.250 | <0.01 |
| IL10 | 1.00 b | 16.76 a | 21.64 a | 10.65 a | 11.54 a | 2.963 | <0.001 |
| IFN-γ | 1.00 b | 13.91 a | 15.60 a | 11.62 a | 14.12 a | 2.376 | <0.001 |
| CCL4 | 1.00 b | 20.31 a | 12.56 a | 13.80 a | 13.30 a | 3.004 | <0.01 |
| CXCL8 | 1.00 b | 5.35 a | 4.13 ab | 3.06 ab | 3.45 ab | 0.823 | <0.05 |
| TLR2 | 1.00 b | 3.03 a | 2.36 ab | 2.88 a | 2.99 a | 0.359 | <0.01 |
| TLR4 | 1.00 b | 2.04 a | 1.49 ab | 1.94 a | 1.76 ab | 0.220 | <0.05 |
| NFκB1 | 1.00 b | 1.47 a | 1.41 a | 1.42 a | 1.48 a | 0.077 | <0.001 |
| Experiment 2 | |||||||
| Relative fold change | |||||||
| LAT1 (SLC7A5) | 1.00 b | 7.18 a | 7.62 a | 4.63 a | 6.31 a | 0.795 | <0.001 |
| LAT4 (SLC43A2) | 1.00 | 1.03 | 0.93 | 1.25 | 1.05 | 0.112 | 0.357 |
| SNAT2 (SLC38A2) | 1.00 b | 2.18 a | 2.26 a | 2.01 a | 2.24 a | 0.239 | <0.01 |
| y+LAT1 (SLC7A7) | 1.00 | 1.43 | 1.31 | 1.54 | 1.98 | 0.225 | 0.054 |
| y+LAT2 (SLC7A6) | 1.00 | 1.09 | 1.25 | 0.81 | 0.95 | 0.140 | 0.259 |
| SBAT2 (SLC6A15) | 1.00 | 1.40 | 1.50 | 1.54 | 1.87 | 0.310 | 0.422 |
| BAT1 (SLC7A9) | 1.00 a | 0.27 b | 0.45 b | 0.20 b | 0.33 b | 0.093 | <0.001 |
| IL1β | 1.00 b | 3.36 ab | 4.43 a | 4.18 a | 3.92 a | 0.701 | <0.05 |
| IL1RN | 1.00 b | 1.88 a | 1.91 a | 1.79 a | 1.82 a | 0.159 | <0.01 |
| IL6 | 1.00 b | 2.09 a | 2.34 a | 1.81 ab | 2.08 a | 0.230 | <0.01 |
| IL10 | 1.00 b | 10.88 a | 13.85 a | 12.96 a | 11.14 a | 1.332 | <0.001 |
| IFN-γ | 1.00 b | 10.98 a | 10.58 a | 12.81 a | 12.44 a | 1.192 | <0.001 |
| CCL4 | 1.00 b | 13.92 a | 14.90 a | 17.47 a | 13.20 a | 3.032 | <0.01 |
| CXCL8 | 1.00 b | 4.01 a | 3.26 ab | 3.99 a | 3.97 a | 0.759 | <0.05 |
| TLR2 | 1.00 b | 2.85 ab | 3.90 a | 2.46 ab | 3.49 a | 0.582 | <0.05 |
| TLR4 | 1.00 | 1.94 | 1.97 | 2.45 | 2.06 | 0.362 | 0.102 |
| NFκB1 | 1.00 b | 1.28 ab | 1.47 a | 1.22 ab | 1.35 a | 0.084 | <0.01 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Relative fold change 3 | |||||||
| mTOR | 1.00 | 0.74 | 0.82 | 0.75 | 0.96 | 0.083 | 0.081 |
| S6K1 | 1.00 a | 0.69 b | 0.80 ab | 0.60 b | 0.76 ab | 0.069 | <0.01 |
| 4EBP1 | 1.00 | 1.40 | 1.12 | 1.42 | 0.88 | 0.262 | 0.449 |
| AMPKα1 | 1.00 | 0.76 | 0.81 | 0.72 | 0.88 | 0.079 | 0.120 |
| AKT1 | 1.00 | 0.81 | 0.84 | 0.73 | 0.89 | 0.077 | 0.110 |
| BCAT1 | 1.00 ab | 0.69 b | 1.01 ab | 0.70 b | 1.09 a | 0.090 | <0.01 |
| BCKDK | 1.00 | 1.07 | 0.97 | 1.01 | 1.03 | 0.092 | 0.945 |
| BCKDHα | 1.00 | 1.10 | 0.96 | 0.96 | 0.93 | 0.062 | 0.340 |
| BCKDHβ | 1.00 | 1.48 | 0.89 | 1.78 | 1.27 | 0.215 | 0.054 |
| PPM1K | 1.00 a | 0.46 b | 0.59 ab | 0.41 b | 0.82 ab | 0.101 | <0.01 |
| LAT1 (SLC7A5) | 1.00 a | 0.61 ab | 1.00 a | 0.38 b | 0.65 ab | 0.111 | <0.01 |
| LAT4 (SLC43A2) | 1.00 | 1.99 | 1.72 | 2.33 | 1.43 | 0.329 | 0.077 |
| SNAT2 (SLC38A2) | 1.00 a | 0.65 ab | 0.72 ab | 0.38 b | 0.68 ab | 0.090 | <0.01 |
| Experiment 2 | |||||||
| Relative fold change | |||||||
| mTOR | 1.00 | 1.08 | 1.02 | 0.98 | 0.77 | 0.086 | 0.106 |
| S6K1 | 1.00 | 0.73 | 0.84 | 0.86 | 0.75 | 0.067 | 0.054 |
| 4EBP1 | 1.00 | 0.96 | 1.40 | 0.99 | 0.99 | 0.173 | 0.279 |
| AMPKα1 | 1.00 | 1.05 | 0.92 | 0.92 | 0.80 | 0.098 | 0.378 |
| AKT1 | 1.00 | 0.98 | 1.02 | 0.81 | 0.84 | 0.069 | 0.115 |
| BCAT1 | 1.00 a | 0.86 ab | 0.99 a | 0.84 ab | 0.55 b | 0.079 | <0.01 |
| BCKDK | 1.00 | 1.06 | 1.29 | 0.97 | 0.98 | 0.128 | 0.375 |
| BCKDHα | 1.00 | 1.05 | 1.21 | 1.06 | 0.91 | 0.081 | 0.166 |
| BCKDHβ | 1.00 | 1.51 | 1.75 | 1.14 | 1.20 | 0.232 | 0.173 |
| PPM1K | 1.00 a | 0.47 b | 0.68 ab | 0.59 ab | 0.35 b | 0.105 | <0.01 |
| LAT1 (SLC7A5) | 1.00 a | 0.73 ab | 0.57 b | 0.64 b | 0.56 b | 0.094 | <0.01 |
| LAT4 (SLC43A2) | 1.00 b | 4.91 a | 2.00 b | 2.42 b | 1.84 b | 0.581 | <0.001 |
| SNAT2 (SLC38A2) | 1.00 a | 0.65 ab | 0.61 ab | 0.55 b | 0.55 b | 0.096 | <0.05 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Relative frequency, % | |||||||
| Firmicutes | 96.0 | 92.2 | 93.7 | 90.8 | 91.4 | 2.40 | 0.574 |
| Bacteroidota | 1.87 | 4.52 | 1.83 | 8.05 | 3.18 | 3.166 | 0.625 |
| F:B ratio 3 | 194 | 78 | 53 | 54 | 33 | 66.79 | 0.460 |
| Cyanobacteria | 0.04 | 0.66 | 1.17 | 0.61 | 1.22 | 0.501 | 0.469 |
| Proteobacteria | 1.05 | 1.45 | 1.39 | 1.86 | 1.31 | 0.334 | 0.559 |
| Actinobacteriota | 0.55 | 0.66 | 0.67 | 0.64 | 0.67 | 0.099 | 0.911 |
| Fusobacteriota | 0.156 | 0.149 | 0.201 | 0.195 | 0.187 | 0.034 | 0.756 |
| Verrucomicrobiota | 0.100 | 0.119 | 0.117 | 0.091 | 0.106 | 0.035 | 0.977 |
| Desulfobacterota | 0.148 | 0.118 | 0.102 | 0.084 | 0.130 | 0.035 | 0.736 |
| Campilobacterota | 0.076 | 0.055 | 0.053 | 0.051 | 0.070 | 0.028 | 0.961 |
| Patescibacteria | 0.066 | 0.060 | 0.073 | 0.067 | 0.058 | 0.013 | 0.932 |
| Experiment 2 | |||||||
| Relative frequency, % | |||||||
| Firmicutes | 96.0 | 92.6 | 95.6 | 93.6 | 88.8 | 1.94 | 0.097 |
| Bacteroidota | 1.87 | 4.16 | 2.52 | 3.14 | 9.65 | 1.997 | 0.073 |
| F:B ratio | 194 | 62 | 53 | 52 | 18 | 66.9 | 0.407 |
| Cyanobacteria | 0.04 | 0.04 | 0.03 | 0.61 | 1.09 | 0.338 | 0.127 |
| Proteobacteria | 1.05 | 1.97 | 1.00 | 1.88 | 1.34 | 0.487 | 0.492 |
| Actinobacteriota | 0.55 | 0.84 | 0.56 | 0.74 | 1.22 | 0.163 | 0.054 |
| Fusobacteriota | 0.156 | 0.100 | 0.104 | 0.196 | 0.179 | 0.058 | 0.690 |
| Verrucomicrobiota | 0.100 | 0.112 | 0.069 | 0.140 | 0.135 | 0.033 | 0.570 |
| Desulfobacterota | 0.148 | 0.082 | 0.077 | 0.123 | 0.149 | 0.031 | 0.317 |
| Campilobacterota | 0.076 | 0.017 | 0.013 | 0.028 | 0.033 | 0.016 | 0.060 |
| Patescibacteria | 0.066 | 0.027 | 0.033 | 0.064 | 0.065 | 0.016 | 0.255 |
| NC 1 | NE Challenge | SEM (n = 6) | p-Value | ||||
|---|---|---|---|---|---|---|---|
| NE | VAL | ILE | MIX | ||||
| Experiment 1 2 | |||||||
| Relative frequency, % | |||||||
| Ruminococcaceae | 37.7 | 22.6 | 42.4 | 36.5 | 38.8 | 7.19 | 0.371 |
| Clostridia vadinBB60 group | 29.5 | 23.5 | 21.8 | 16.1 | 17.5 | 6.68 | 0.644 |
| Bacteroidaceae | 0.79 | 3.72 | 0.93 | 7.29 | 2.21 | 3.142 | 0.581 |
| Lachnospiraceae | 14.3 | 22.8 | 12.0 | 17.9 | 17.5 | 3.34 | 0.234 |
| Oscillospiraceae | 5.9 | 11.1 | 7.6 | 7.1 | 6.6 | 1.37 | 0.104 |
| Clostridia UCG-014 | 2.46 | 1.26 | 2.02 | 2.49 | 5.37 | 1.463 | 0.362 |
| Erysipelotrichaceae | 0.46 | 2.98 | 0.37 | 0.45 | 0.58 | 1.040 | 0.345 |
| Enterobacteriaceae | 0.44 | 0.85 | 0.53 | 1.16 | 0.60 | 0.299 | 0.448 |
| Lactobacillaceae | 0.56 | 1.24 | 0.86 | 1.28 | 1.23 | 0.238 | 0.178 |
| Butyricicoccaceae | 0.84 | 1.99 | 1.09 | 0.87 | 0.54 | 0.392 | 0.128 |
| Experiment 2 | |||||||
| Relative frequency, % | |||||||
| Ruminococcaceae | 37.7 | 26.2 | 41.0 | 28.7 | 29.2 | 8.21 | 0.658 |
| Clostridia vadinBB60 group | 29.5 | 29.5 | 27.5 | 26.7 | 29.7 | 7.83 | 0.998 |
| Bacteroidaceae | 0.79 | 3.35 | 1.73 | 2.13 | 8.40 | 2.042 | 0.102 |
| Lachnospiraceae | 14.3 | 21.1 | 13.8 | 18.0 | 13.4 | 4.04 | 0.615 |
| Oscillospiraceae | 5.9 | 5.0 | 6.0 | 7.6 | 5.2 | 1.25 | 0.608 |
| Clostridia UCG-014 | 2.46 | 2.56 | 2.52 | 1.80 | 3.47 | 1.509 | 0.958 |
| Erysipelotrichaceae | 0.46 | 0.75 | 0.44 | 0.63 | 0.39 | 0.200 | 0.687 |
| Enterobacteriaceae | 0.44 | 1.59 | 0.62 | 1.17 | 0.65 | 0.456 | 0.387 |
| Lactobacillaceae | 0.56 | 5.04 | 0.48 | 0.86 | 1.36 | 1.878 | 0.404 |
| Butyricicoccaceae | 0.84 | 0.40 | 1.24 | 0.37 | 0.63 | 0.289 | 0.220 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goo, D.; Kim, W.K. Additional Valine and Isoleucine Impact Growth Performance, Intestinal Health, and Muscle Growth in Broilers Under Necrotic Enteritis Challenges. Animals 2025, 15, 2641. https://doi.org/10.3390/ani15182641
Goo D, Kim WK. Additional Valine and Isoleucine Impact Growth Performance, Intestinal Health, and Muscle Growth in Broilers Under Necrotic Enteritis Challenges. Animals. 2025; 15(18):2641. https://doi.org/10.3390/ani15182641
Chicago/Turabian StyleGoo, Doyun, and Woo Kyun Kim. 2025. "Additional Valine and Isoleucine Impact Growth Performance, Intestinal Health, and Muscle Growth in Broilers Under Necrotic Enteritis Challenges" Animals 15, no. 18: 2641. https://doi.org/10.3390/ani15182641
APA StyleGoo, D., & Kim, W. K. (2025). Additional Valine and Isoleucine Impact Growth Performance, Intestinal Health, and Muscle Growth in Broilers Under Necrotic Enteritis Challenges. Animals, 15(18), 2641. https://doi.org/10.3390/ani15182641







