Spatiotemporal Patterns of Fish Diversity in the Waters Around the Five West Sea Islands of South Korea: Integrating Bottom Trawl and Environmental DNA (eDNA) Methods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
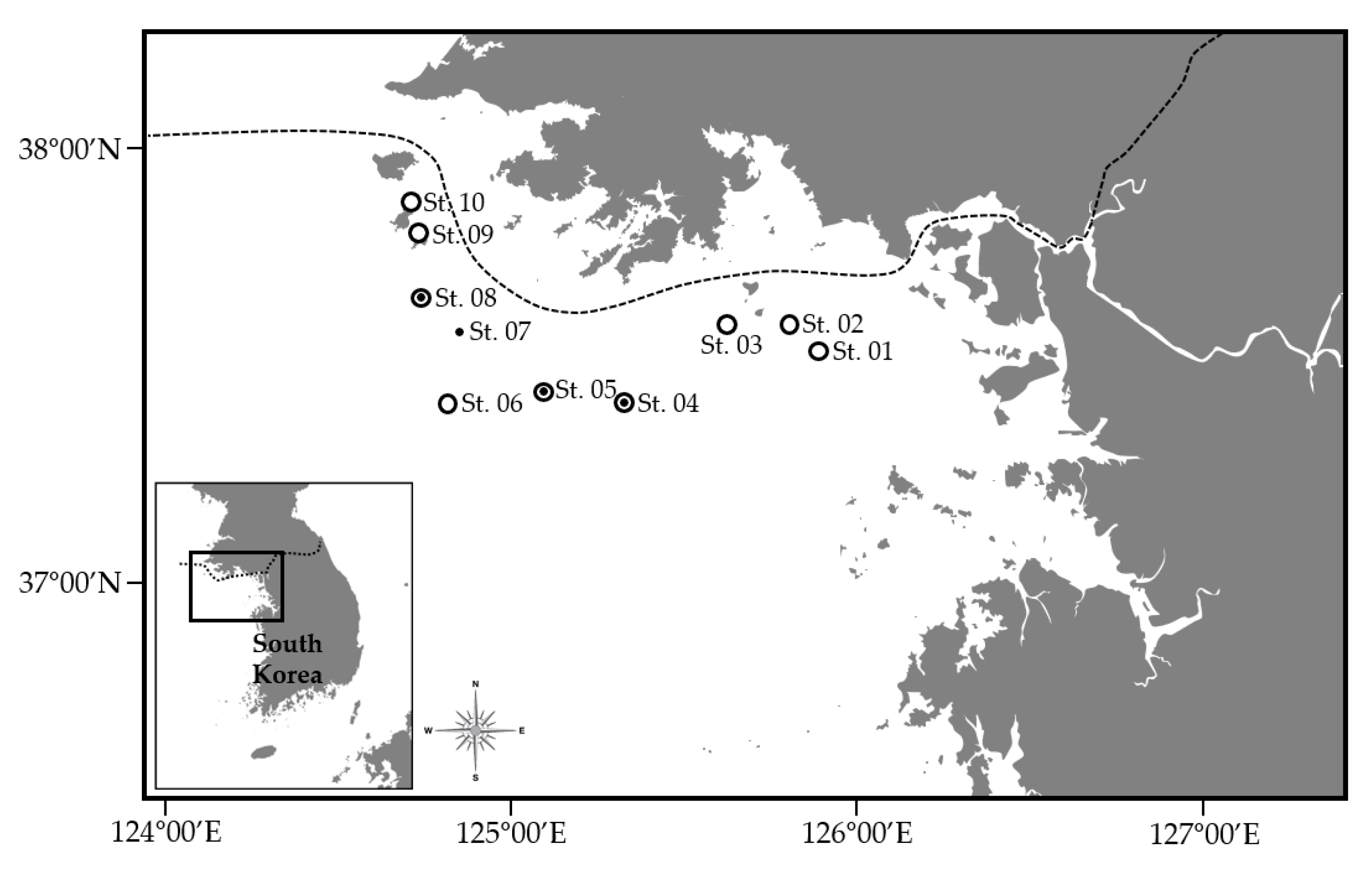
2.1. Station Designation and Environmental Surveys
2.2. Bottom Trawl Surveys
2.3. Seawater Sampling
2.4. eDNA Extraction
2.5. Amplicon Library Preparation and MiSeq Sequencing
2.6. Data Analysis
3. Results
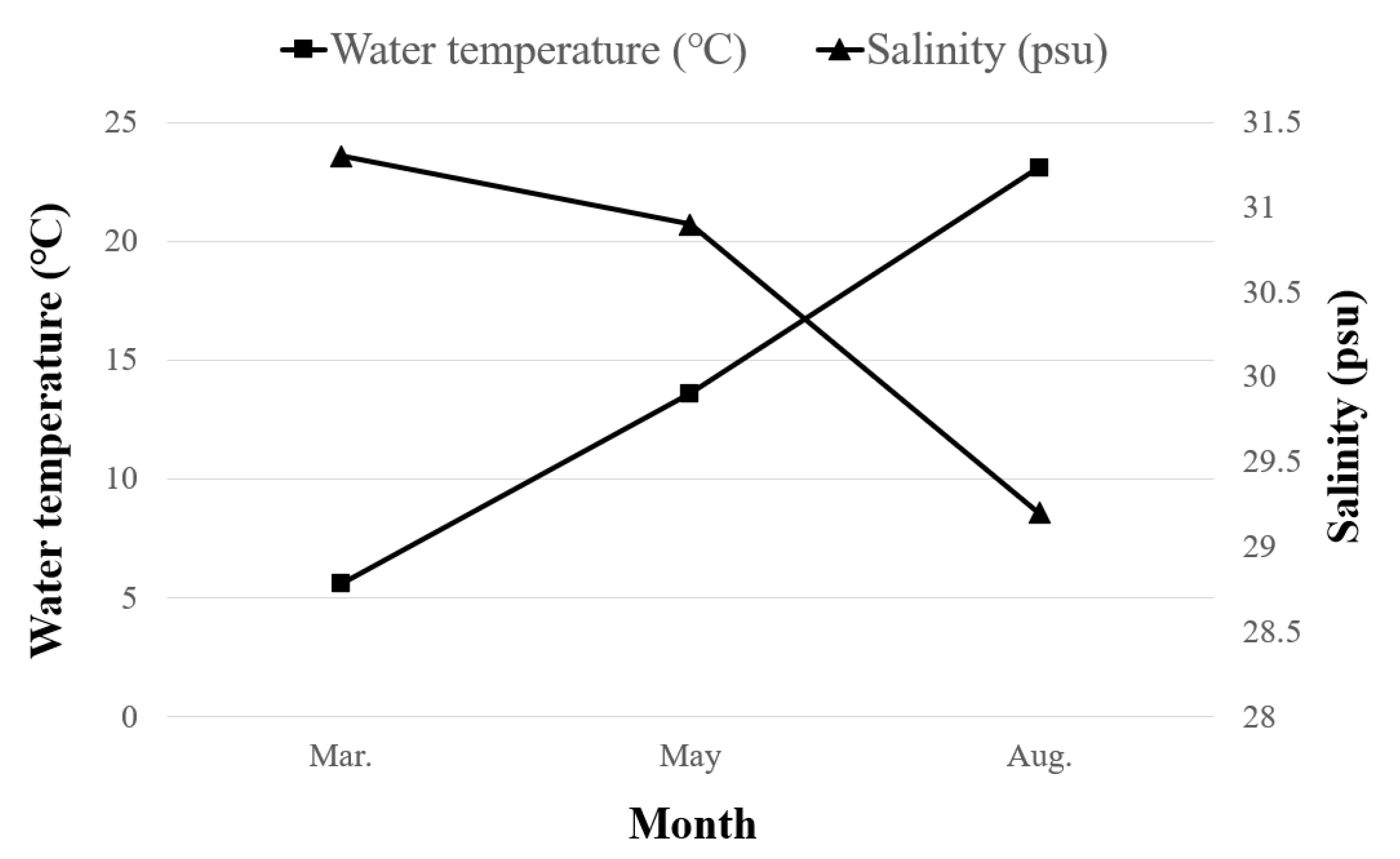
3.1. Oceanographic Characteristics
3.2. Fish Species Composition
3.2.1. Bottom Trawl Surveys
3.2.2. eDNA Surveys
3.2.3. Comparison of the Bottom Trawl and eDNA Surveys
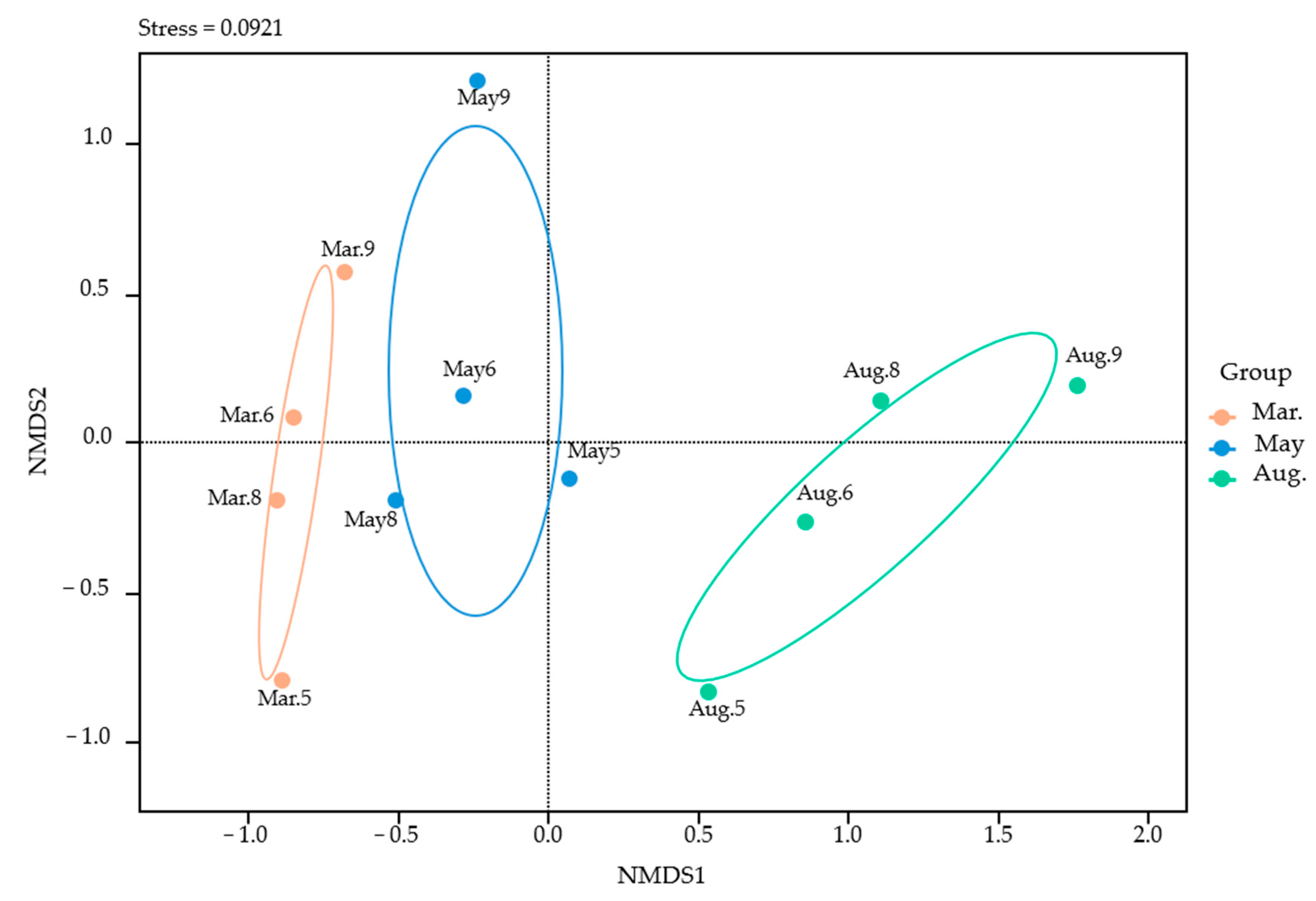
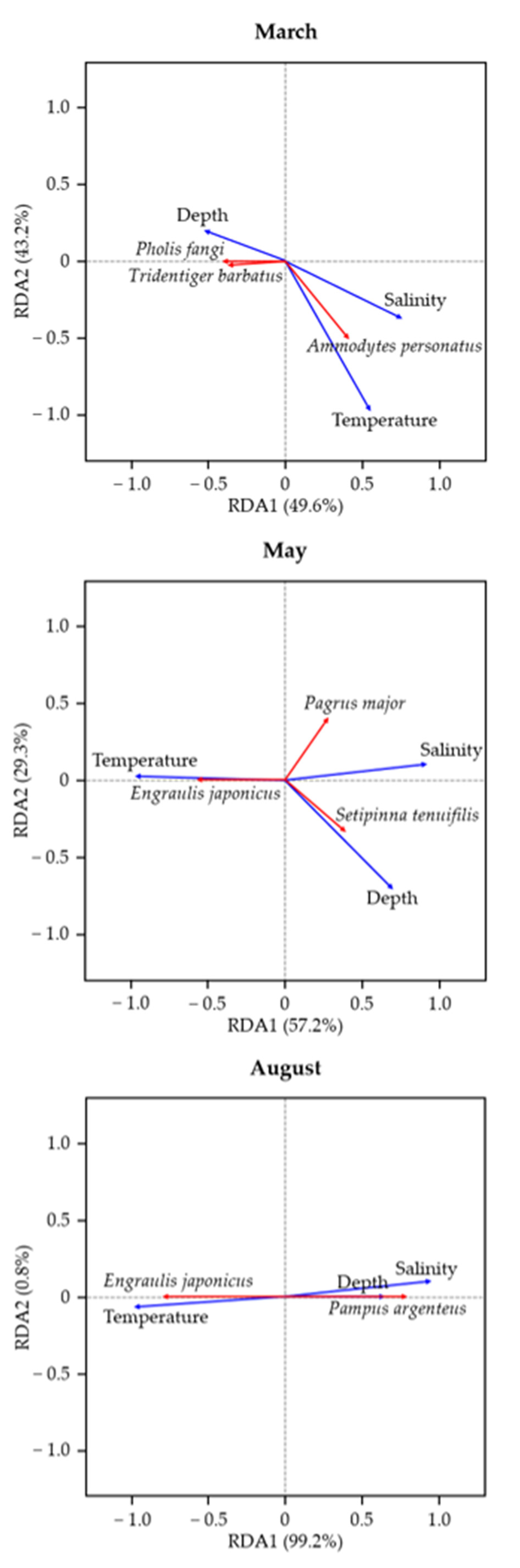
3.3. Relationship Between Fish Community Similarity and Environmental Factors
4. Discussion
4.1. Fish Diversity and Occurrence Patterns
4.2. Comparison of Bottom Trawling and eDNA Survey Results
4.3. Changes in the Fish Community Composition Due to Temperature Variation
4.4. Advantages and Limitations of eDNA Surveys
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Mendoza, R.; Saborido-Rey, F. The potential use of genomic methods in bottom trawl surveys to improve stock assessments in Europe. Front. Mar. Sci. 2023, 10, 1095171. [Google Scholar] [CrossRef]
- Youn, B.I.; Choi, D.H.; Im, Y.J.; Kim, J.N.; Kim, M.J. A study on the characteristics of fish community in the coastal water of the Five West Sea Islands in Korea. J. Korean Soc. Fish. Ocean Technol. 2020, 56, 213–222. [Google Scholar] [CrossRef]
- Ye, D.Y. Focusing on jurisdictional issues. In Current Status and Tasks of Research on the Five West Sea Islands; Center for Korean Studies: Incheon, Republic of Korea, 2022; pp. 755–779. [Google Scholar]
- Taberlet, P.; Coissac, E.; Hajibabaei, M.; Rieseberg, L.H. Environmental DNA. Mol. Ecol. 2012, 21, 1789–1793. [Google Scholar] [CrossRef] [PubMed]
- Pascher, K.; Švara, V.; Jungmeier, M. Environmental DNA-based methods in biodiversity monitoring of protected areas: Application range, limitations, and needs. Diversity 2022, 14, 463. [Google Scholar] [CrossRef]
- Wang, X.; Wang, L.; Wang, A.; Zhao, L. New insights into fish diversity in the Yellow and Bohai Seas based on environmental DNA technology. Fishes 2024, 9, 435. [Google Scholar] [CrossRef]
- Thomsen, P.F.; Møller, P.R.; Sigsgaard, E.E.; Knudsen, S.W.; Jørgensen, O.A.; Willerslev, E. Environmental DNA from seawater samples correlate with trawl catches of subarctic, deepwater fishes. PLoS ONE 2016, 11, e0165252. [Google Scholar] [CrossRef]
- Lee, Y.D.; Lee, G.M.; Park, J.Y.; Gwak, W.S. Comparing environmental DNA metabarcoding and underwater visual census to monitor korean coastal fish community. Ocean Sci. J. 2022, 57, 592–606. [Google Scholar] [CrossRef]
- Lee, Y.D.; Lee, G.M.; Gwak, W.S. Assessment of fish diversity in the coastal waters off Nodaedo Island, Tongyeong, Korea, using an underwater visual census and environmental DNA metabarcoding. Mar. Biol. 2024, 171, 23. [Google Scholar] [CrossRef]
- NIFS. Briefing Book on the Impact and Adaptation of Climate Change on the Marine and Fisheries Sector 2025. Available online: https://www.nifs.go.kr/contents/actionContentsCons0149.do (accessed on 10 June 2025). (In Korean).
- Woo, H.J.; Bahk, J.J.; Lee, Y.G.; Je, J.G.; Choi, J.U. Characteristics of sediments in the Kanghwa tidal flat on the west coast of Korea. J. Wetl. Res. 2004, 6, 167–178. [Google Scholar]
- Elston, C.; Cowley, P.D.; von Brandis, R.G.; Lea, J. Stingray habitat use is dynamically influenced by temperature and tides. Front. Mar. Sci. 2022, 8, 754404. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.B.; Han, S.H.; Kim, M.J. Species composition and monthly variation of aquatic animals collected in the coastal waters off Yeonpyeong-do, Korea. J. Fish. Manag. Sci. Educ. 2022, 34, 471–483. [Google Scholar]
- Park, J.; Jeong, G.S.; Kim, J.N.; Im, Y.J.; Kim, M.J. Species composition and seasonal variation of aquatic organism caught by fish pots in the coastal waters off Baekryeong-do, Korea. J. Korean Soc. Fish. Ocean Technol. 2018, 54, 306–314. [Google Scholar] [CrossRef]
- NIFS (National Institute of Fisheries Science). Evaluation Report of Survey of Fishery Resources in the Baekryeong-do, Daecheong-do, Socheong-do, Taeyonpyong-do and Soyeonpyeong-do; NIFS: Busan, Republic of Korea, 2015; pp. 1–46. [Google Scholar]
- Walker, N.D.; Maxwell, D.L.; Quesne, W.J.F.L.; Jennings, S. Estimating efficiency of survey and commercial trawl gears from comparisons of catch-ratios. ICES J. Mar. Sci. 2017, 74, 1448–1457. [Google Scholar] [CrossRef]
- Kim, I.S.; Choi, Y.; Lee, C.L.; Lee, Y.J.; Kim, B.J.; Kim, J.H. Illustrated Book of Korean Fishes; Kyohak-Sa: Seoul, Republic of Korea, 2005; pp. 1–613. [Google Scholar]
- Uchii, K.; Doi, H.; Minamoto, T. A novel environmental DNA approach to quantify the cryptic invasion of non-native genotypes. Mol. Ecol. Resour. 2016, 16, 415–422. [Google Scholar] [CrossRef]
- Miya, M.; Sato, Y.; Fukunaga, T.; Sado, T.; Poulsen, J.Y.; Sato, K.; Minamoto, T.; Yamamoto, S.; Yamanaka, H.; Araki, H.; et al. MiFish, a set of universal PCR primers for metabarcoding environmental DNA from fishes: Detection of more than 230 subtropical marine species. R. Soc. Open Sci. 2015, 2, 150088. [Google Scholar] [CrossRef]
- FASTX-Toolkit. Available online: http://hannonlab.cshl.edu/fastx_toolkit/ (accessed on 16 August 2025).
- Joshi, N.A.; Fass, J.N. Sickle: A Sliding-Window, Adaptive, Quality-Based Trimming Tool for FastQ Files, Version 1.33; GitHub: San Francisco, CA, USA, 2014. Available online: https://github.com/najoshi/sickle (accessed on 10 June 2025).
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A versatile open source tool for metagenomics. PeerJ 2016, 4, e2584. [Google Scholar] [CrossRef]
- Iwasaki, W.; Fukunaga, T.; Isagozawa, R.; Yamada, K.; Maeda, Y.; Satoh, T.P.; Sado, T.; Mabuchi, K.; Takeshima, H.; Miya, M.; et al. MitoFish and MitoAnnotator: A mitochondrial genome database of fish with an accurate and automatic annotation pipeline. Mol. Biol. Evol. 2013, 30, 2531–2540. [Google Scholar] [CrossRef]
- Hwang, S.D.; McFarlane, G.A.; Choi, O.I.; Kim, J.S.; Hwang, H.J. Spatiotemporal distribution of pacific anchovy (Engraulis japonicus) eggs in the West Sea of Korea. J. Fish. Sci. Technol. 2007, 10, 74–85. [Google Scholar] [CrossRef]
- Lee, T.W.; Song, H.S. Distribution, and length and age composition of Johnius belengeri in the coastal waters of Korea. Korean J. Ichthyol. 1993, 5, 184–193. [Google Scholar]
- Seo, I.S.; Hong, J.S. Seasonal variation of fish assemblages on Jangbong tidal flat, Incheon, Korea. J. Korean Fish. Soc. 2010, 43, 510–520. [Google Scholar]
- Lee, B.W.; Chung, E.Y.; Lee, J.Y. Histological study on the reproductive cycle of Coilia nasus. J. Aquac. 2003, 16, 179–186. [Google Scholar]
- Ma, J.; Li, B.; Zhao, J.; Wang, X.; Hodgdon, C.T.; Tian, S. Environmental influences on the spatio-temporal distribution of Coilia nasus in the Yangtze River estuary. J. Appl. Ichthyol. 2020, 36, 315–325. [Google Scholar] [CrossRef]
- Youn, B.I.; Choi, D.H.; Roh, T.H.; Lee, S.H.; Han, K.H.; Kwon, D.H.; Kim, M.J. Age and growth characteristics of Okamejei kenojei in the West Sea of South Korea according to coronal vertebral microstructure. Fishes 2023, 8, 197. [Google Scholar] [CrossRef]
- Hayer, C.A.; Bayless, M.F.; George, A.E.; Thompson, N.; Richter, C.A.; Chapman, D. Use of environmental DNA to detect grass carp spawning events. Fishes 2020, 5, 27. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X.Q. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environ. Biol. Fish. 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Goldberg, C.S.; Turner, C.R.; Deiner, K.; Klymus, K.E.; Thomsen, P.F.; Murphy, M.A.; Spear, S.F.; McKee, A.; Oyler-McCance, S.J.; Cornman, R.S.; et al. Critical considerations for the application of environmental DNA methods to detect aquatic species. Methods. Ecol. Evol. 2016, 7, 9. [Google Scholar] [CrossRef]
- Yu, Z.; Ito, S.I.; Wong, M.K.S.; Yoshizawa, S.; Inoue, J.; Itoh, S.; Yukami, R.; Ishikawa, K.; Guo, C.; Ijichi, M.; et al. Comparison of species-specific qPCR and metabarcoding methods to detect small pelagic fish distribution from open ocean environmental DNA. PLoS ONE 2022, 17, e0273670. [Google Scholar] [CrossRef]
- Hur, S.B.; Yoo, J.M. Distribution of fish eggs and larvae in the Western Waters of Korea. J. Korean Fish. Soc. 1984, 17, 536–542. [Google Scholar]
- Yoo, J.M.; Kim, W.S.; Kim, S.; Lee, E.K. On the early life history of gunnel (Enedrias fangi). Korean J. Ichthyol. 1995, 7, 25–32. [Google Scholar]
- Hwang, S.D.; Lee, T.W. Reproduction and early Life history of gunnel, Pholis fangi in the Yellow Sea off Korea. Korean J. Ichthyol. 2001, 13, 6–18. [Google Scholar]
- Evans, N.T.; Olds, B.P.; Renshaw, M.A.; Turner, C.R.; Li, Y.; Jerde, C.L.; Mahon, A.R.; Pfrender, M.E.; Lamberti, G.A.; Lodge, D.M. Quantification of mesocosm fish and amphibian species diversity via environmental DNA metabarcoding. Mol. Ecol. Resour. 2016, 16, 29–41. [Google Scholar] [CrossRef]
- Kim, J.K.; Choi, O.I.; Kim, J.I.; Chang, D.S.; Park, K.D. Age and growth of the elongate ilisha Ilisha elongata. J. Fish. Sci. Technol. 2007, 10, 30–36. [Google Scholar] [CrossRef]
- Yamada, U.; Tagawa, M.; Kishida, S.; Honjjo, K. Fishes of the East China Sea and the Yellow Sea; Nihon Shinko: Nagasaki, Japan, 2005; pp. 1–501. [Google Scholar]
- Wang, X.; Yagi, Y.; Tojima, S.; Kinoshita, I.; Hirota, Y.; Fujita, S. Early life history of Ilisha elongata (Pristigasteridae, Clupeiformes, Pisces) in Ariake Sound, Shimabara Bay, Japan. Plankton Benthos Res. 2021, 16, 210–220. [Google Scholar] [CrossRef]
- Collins, R.A.; Wangensteen, O.S.; O’Gorman, E.J.; Mariani, S.; Sims, D.W.; Genner, M.J. Persistence of environmental DNA in marine systems. Commun. Biol. 2018, 1, 185. [Google Scholar] [CrossRef]
- FishBase. Thamnaconus modestus Summary Page. Available online: https://www.fishbase.se/summary/Thamnaconus-modestus (accessed on 12 June 2025).
- Jung, T.S. Inter-annual variation of tides on the Western Coasts of Korea. J. Korean Soc. Coastal Ocean Eng. 2016, 28, 81–91. [Google Scholar] [CrossRef]



| Month | Station | Average Vessel Speed (knot) | Towing Duration (m) |
|---|---|---|---|
| Mar. | 05 | 3.5 | 30 |
| 06 | 3.6 | 30 | |
| 08 | 4.2 | 30 | |
| 09 | 3.4 | 30 | |
| May | 05 | 4.5 | 30 |
| 06 | 3.6 | 30 | |
| 08 | 4.0 | 25 | |
| 09 | 3.9 | 13 | |
| Aug. | 01 | 4.0 | 10 |
| 05 | 4.6 | 30 | |
| 06 | 4.2 | 15 | |
| 08 | 3.7 | 30 | |
| 09 | 4.1 | 20 |
| Month | Sampling Station | Water Depth (m) | Surface | Bottom | ||
|---|---|---|---|---|---|---|
| Water Temperature (°C) | Salinity (psu) | Water Temperature (°C) | Salinity (psu) | |||
| Mar. | 01 | 7 | 5.4 | 31.1 | 5.3 | 31.1 |
| 02 | 10 | 5.4 | 30.9 | 5.3 | 30.9 | |
| 03 | 17 | 5.4 | 30.9 | 5.3 | 30.9 | |
| 04 | 16 | 5.4 | 31.3 | 5.3 | 31.3 | |
| 05 | 48 | 5.5 | 31.3 | 5.1 | 31.4 | |
| 06 | 52 | 5.6 | 31.5 | 5.3 | 31.5 | |
| 07 | 42 | 6.3 | 31.7 | 6.2 | 31.7 | |
| 08 | 21 | 6.1 | 31.6 | 6.1 | 31.6 | |
| 09 | 11 | 6.1 | 31.6 | 6.1 | 31.6 | |
| 10 | 37 | 5.6 | 31.4 | 5.7 | 31.5 | |
| May | 01 | 4 | 14.6 | 30.8 | 14.5 | 30.8 |
| 02 | 9 | 16.4 | 30.3 | 16.4 | 30.2 | |
| 03 | 19 | 16.4 | 30.3 | 16.3 | 30.2 | |
| 04 | 15 | 15.3 | 30.7 | 14.7 | 30.7 | |
| 05 | 24 | 14.6 | 30.9 | 12.9 | 31.2 | |
| 06 | 45 | 15.5 | 31.1 | 10.4 | 31.3 | |
| 07 | 46 | 15.1 | 31.1 | 10.4 | 31.3 | |
| 08 | 14 | 13.8 | 31.3 | 11.1 | 31.5 | |
| 09 | 16 | 11.1 | 31.5 | 11.1 | 31.5 | |
| 10 | 34 | 11.5 | 31.4 | 11.4 | 31.4 | |
| Aug. | 01 | 6 | 26.1 | 28.1 | 26.1 | 27.9 |
| 02 | 13 | 27.2 | 27.3 | 27.0 | 27.1 | |
| 03 | 20 | 27.0 | 27.1 | 27.0 | 27.0 | |
| 04 | 16 | 26.3 | 27.5 | 25.8 | 28.1 | |
| 05 | 18 | 26.2 | 28.8 | 25.1 | 27.1 | |
| 06 | 54 | 28.0 | 30.3 | 13.4 | 31.6 | |
| 07 | 43 | 27.6 | 30.2 | 15.2 | 31.4 | |
| 08 | 26 | 21.1 | 30.4 | 15.6 | 31.3 | |
| 09 | 23 | 19.4 | 30.8 | 19.4 | 30.8 | |
| 10 | 36 | 20.7 | 30.5 | 18.7 | 30.9 | |
| Class | Order | Family | Species | eDNA Metabarcoding | Bottom Trawl Surveys |
|---|---|---|---|---|---|
| Elasmobranchii | Rajiformes | Rajidae | Beringraja pulchra | O | O |
| Rajiformes | Rajidae | Okamejei kenojei | O | O | |
| Teleostei | Clupeiformes | Alosidae | Sardinops sagax | O | |
| Clupeiformes | Engraulidae | Coilia nasus | O | ||
| Clupeiformes | Engraulidae | Engraulis japonicus | O | O | |
| Clupeiformes | Engraulidae | Setipinna tenuifilis | O | O | |
| Clupeiformes | Engraulidae | Thryssa kammalensis | O | O | |
| Clupeiformes | Pristigasteridae | Ilisha elongata | O | ||
| Carangaria incertae sedis | Sphyraenidae | Sphyraena pinguis | O | ||
| Eupercaria incertae sedis | Sciaenidae | Collichthys lucidus | O | ||
| Eupercaria incertae sedis | Sciaenidae | Collichthys niveatus | O | ||
| Eupercaria incertae sedis | Sciaenidae | Johnius grypotus | O | O | |
| Eupercaria incertae sedis | Sciaenidae | Larimichthys polyactis | O | ||
| Eupercaria incertae sedis | Sciaenidae | Pennahia argentata | O | ||
| Eupercaria incertae sedis | Sparidae | Pagrus major | O | O | |
| Gobiiformes | Gobiidae | Chaeturichthys stigmatias | O | ||
| Gobiiformes | Gobiidae | Pterogobius zonoleucus | O | ||
| Gobiiformes | Gobiidae | Tridentiger barbatus | O | ||
| Lophiiformes | Lophiidae | Lophius litulon | O | O | |
| Perciformes | Ammodytidae | Ammodytes personatus | O | O | |
| Perciformes | Hemitripteridae | Hemitripterus villosus | O | ||
| Perciformes | Hexagrammidae | Hexagrammos otakii | O | ||
| Perciformes | Liparidae | Liparis tanakae | O | ||
| Perciformes | Pholidae | Pholis fangi | O | O | |
| Perciformes | Platycephalidae | Cociella crocodilus | O | ||
| Perciformes | Platycephalidae | Platycephalus indicus | O | ||
| Perciformes | Sebastidae | Sebastes schlegelii | O | ||
| Perciformes | Zoarcidae | Zoarces gillii | O | O | |
| Pleuronectiformes | Paralichthyidae | Paralichthys olivaceus | O | O | |
| Pleuronectiformes | Cynoglossidae | Cynoglossus abbreviatus | O | O | |
| Pleuronectiformes | Cynoglossidae | Cynoglossus joyneri | O | ||
| Pleuronectiformes | Cynoglossidae | Cynoglossus robustus | O | ||
| Pleuronectiformes | Cynoglossidae | Cynoglossus semilaevis | O | ||
| Pleuronectiformes | Pleuronectidae | Kareius bicoloratus | O | ||
| Pleuronectiformes | Pleuronectidae | Pseudopleuronectes yokohamae | O | ||
| Scombriformes | Trichiurudae | Trichiurus japonicus | O | ||
| Scombriformes | Scombridae | Scomber japonicus | O | ||
| Scombriformes | Scombridae | Scomberomorus niphonius | O | O | |
| Scombriformes | Stromateidae | Pampus argenteus | O | ||
| Scombriformes | Stromateidae | Pampus echinogaster | O | ||
| Tetraodontiformes | Monacanthidae | Rudarius ercodes | O | ||
| Tetraodontiformes | Monacanthidae | Thamnaconus modestus | O | ||
| Tetraodontiformes | Tetraodontidae | Takifugu chinensis | O | ||
| Tetraodontiformes | Tetraodontidae | Takifugu niphobles | O | ||
| Tetraodontiformes | Tetraodontidae | Takifugu rubripes | O |
| Species | Mar. | May | Aug. | Total | ||||
|---|---|---|---|---|---|---|---|---|
| N | W | N | W | N | W | N | W | |
| Ammodytes personatus | 420 | 19.5 | 4367 | 234 | 4787 | 253.5 | ||
| Chaeturichthys stigmatias | 40 | 5 | 694 | 8 | 734 | 13 | ||
| Cociella crocodilus | 87 | 131 | 87 | 131 | ||||
| Coilia nasus | 1860 | 613.5 | 39 | 76 | 1899 | 689.5 | ||
| Collichthys lucidus | 157 | 96 | 157 | 96 | ||||
| Collichthys niveatus | 380 | 151.5 | 380 | 151.5 | ||||
| Cynoglossus abbreviatus | 50 | 124.5 | 50 | 124.5 | ||||
| Cynoglossus joyneri | 104 | 68 | 772 | 258 | 876 | 326 | ||
| Cynoglossus robustus | 145 | 199 | 145 | 199 | ||||
| Engraulis japonicus | 455,221 | 81,180 | 455,221 | 81,180 | ||||
| Hemitriperus villosus | 269 | 1224.5 | 269 | 1224.5 | ||||
| Hexagrammos otakii | 42 | 1 | 54 | 3 | 96 | 4 | ||
| Johnius grypotus | 240 | 133.5 | 366,518 | 16,024 | 366,758 | 16,157.5 | ||
| Kareius bicoloratus | 101 | 103 | 108 | 901 | 209 | 1004 | ||
| Larimichthys polyactis | 51 | 16.5 | 40 | 48.5 | 8270 | 2262 | 8361 | 2327 |
| Liparis tanakae | 103 | 9 | 528 | 36 | 631 | 45 | ||
| Lophius litulon | 108 | 1206 | 108 | 1206 | ||||
| Okamejei kenojei | 666 | 3962 | 5778 | 42,463.5 | 858 | 7193.5 | 7302 | 53,619 |
| Pagrus major | 54 | 1896 | 54 | 1896 | ||||
| Pampus echinogaster | 50 | 56.5 | 330 | 638 | 380 | 694.5 | ||
| Paralichthys olivaceus | 103 | 4277.5 | 54 | 1414 | 241 | 17,190 | 398 | 22,881.5 |
| Pennahia argentata | 520 | 366 | 520 | 366 | ||||
| Pholis fangi | 360 | 25 | 360 | 25 | ||||
| Beringraja pulchra | 48 | 1487 | 48 | 1487 | ||||
| Sardinops sagax | 113 | 48 | 113 | 48 | ||||
| Scomber japonicus | 193 | 96 | 193 | 96 | ||||
| Scomberomorus niphonius | 117 | 147 | 117 | 147 | ||||
| Sebastes schlegelii | 42 | 0.5 | 42 | 0.5 | ||||
| Setipinna tenuifilis | 2674 | 1695.5 | 2398 | 1534 | 5072 | 3229.5 | ||
| Sphyraena pinguis | 313 | 12 | 313 | 12 | ||||
| Takifugu chinensis | 113 | 668 | 113 | 668 | ||||
| Takifugu niphobles | 117 | 80 | 117 | 80 | ||||
| Takifugu rubripes | 120 | 1548 | 120 | 1548 | ||||
| Thryssa kammalensis | 514 | 79 | 520 | 66 | 1034 | 145 | ||
| Trichiurus japonicus | 313 | 192 | 313 | 192 | ||||
| Zoarces gillii | 54 | 72 | 54 | 72 | ||||
| Total | 3388 | 9002.5 | 15,946 | 53,385 | 838,097 | 129,952 | 857,431 | 192,339 |
| No. of species | 9 | 21 | 23 | 36 | ||||
| Species | Mar. | May | Aug. | Total |
|---|---|---|---|---|
| Read | Read | Read | Read | |
| Ammodytes personatus | 9763 | 0 | 0 | 9763 |
| Cynoglossus abbreviatus | 0 | 8413 | 0 | 8413 |
| Cynoglossus semilaevis | 0 | 0 | 13 | 13 |
| Engraulis japonicus | 0 | 61,335 | 57,691 | 119,026 |
| Ilisha elongata | 4775 | 0 | 0 | 4775 |
| Johnius grypotus | 0 | 0 | 238 | 238 |
| Lophius litulon | 6712 | 0 | 0 | 6712 |
| Okamejei kenojei | 118 | 0 | 0 | 118 |
| Pagrus major | 0 | 20,740 | 0 | 20,740 |
| Pampus argenteus | 0 | 0 | 6850 | 6850 |
| Paralichthys olivaceus | 0 | 0 | 82 | 82 |
| Pholis fangi | 23,606 | 14,977 | 0 | 38,583 |
| Platycephalus indicus | 0 | 3778 | 0 | 3778 |
| Pleuronectes yokohamae | 0 | 0 | 65 | 65 |
| Pterogobius zonoleucus | 2960 | 0 | 0 | 2960 |
| Beringraja pulchra | 0 | 0 | 13 | 13 |
| Rudarius ercodes | 4179 | 0 | 0 | 4179 |
| Scomberomorus niphonius | 0 | 0 | 106 | 106 |
| Setipinna tenuifilis | 0 | 18,520 | 0 | 18,520 |
| Thamnaconus modestus | 2919 | 0 | 0 | 2919 |
| Thryssa kammalensis | 0 | 10,969 | 0 | 10,969 |
| Tridentiger barbatus | 25,394 | 0 | 0 | 25,394 |
| Zoarces gillii | 0 | 0 | 42 | 42 |
| Total | 80,426 | 138,732 | 65,100 | 284,258 |
| No. of species | 9 | 7 | 9 | 23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoo, Y.-J.; An, S.-Y.; Lee, S.-H.; Lee, S.-J.; Gwak, W.-S. Spatiotemporal Patterns of Fish Diversity in the Waters Around the Five West Sea Islands of South Korea: Integrating Bottom Trawl and Environmental DNA (eDNA) Methods. Animals 2025, 15, 2613. https://doi.org/10.3390/ani15172613
Yoo Y-J, An S-Y, Lee S-H, Lee S-J, Gwak W-S. Spatiotemporal Patterns of Fish Diversity in the Waters Around the Five West Sea Islands of South Korea: Integrating Bottom Trawl and Environmental DNA (eDNA) Methods. Animals. 2025; 15(17):2613. https://doi.org/10.3390/ani15172613
Chicago/Turabian StyleYoo, Young-Ji, So-Yeon An, Seung-Hwan Lee, Soo-Jeong Lee, and Woo-Seok Gwak. 2025. "Spatiotemporal Patterns of Fish Diversity in the Waters Around the Five West Sea Islands of South Korea: Integrating Bottom Trawl and Environmental DNA (eDNA) Methods" Animals 15, no. 17: 2613. https://doi.org/10.3390/ani15172613
APA StyleYoo, Y.-J., An, S.-Y., Lee, S.-H., Lee, S.-J., & Gwak, W.-S. (2025). Spatiotemporal Patterns of Fish Diversity in the Waters Around the Five West Sea Islands of South Korea: Integrating Bottom Trawl and Environmental DNA (eDNA) Methods. Animals, 15(17), 2613. https://doi.org/10.3390/ani15172613






