Soy Protein Isolate-Stachyose Emulsion Gel for the Delivery of Vitamin D3: Effect on the Humoral Immune Response in Dairy Goats Under Heat Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Emulsion Gel
2.3. Fourier-Transform Infrared (FTIR)
2.4. X-Ray Diffraction
2.5. In-Vitro Dissolution Testing of the Emulsion Gel
2.6. Light Scattering In-Vitro Measurements of Oil Droplet Size
2.7. Zeta Potential In-Vitro Measurements of Oil Droplets
2.8. 17O NMR Measurements
2.9. 1H NMR Measurements
2.10. Determination of Embedding Rate
2.11. Differential Scanning Calorimetry (DSC)
2.12. Animals, Diets, Facilities, and Experimental Design
2.13. Sample Collection and Analysis of 25-(OH)-D3
2.14. Establishment of Humoral Response to a Nominal Antigen Chicken Egg Albumin (OVA)
2.15. Anti-OVA Specific IgG by ELISA
2.16. Statistical Analysis
3. Results and Discussion
3.1. Storage Stability
3.2. Thermal Stability
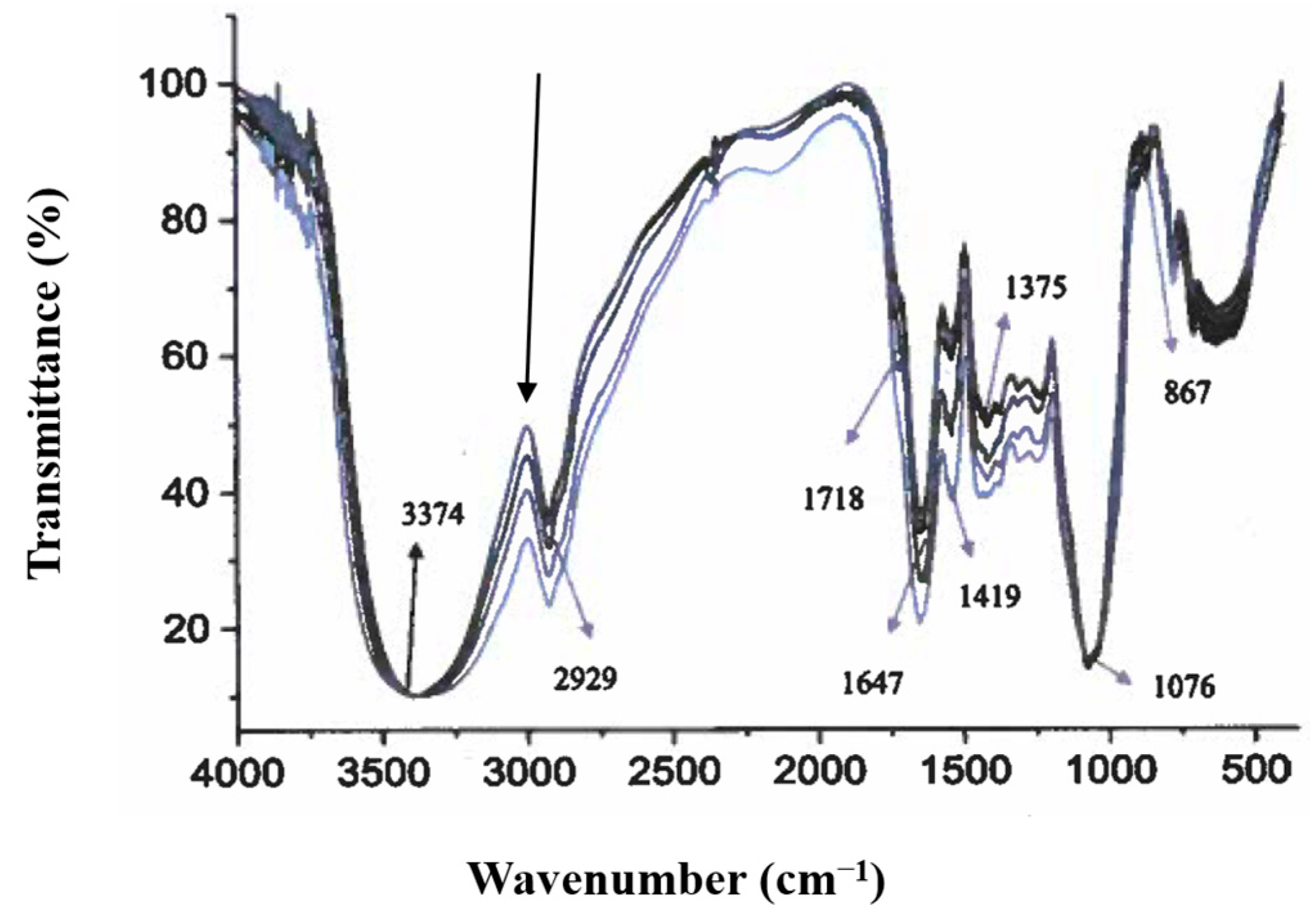
3.3. FTIR Spectroscopy
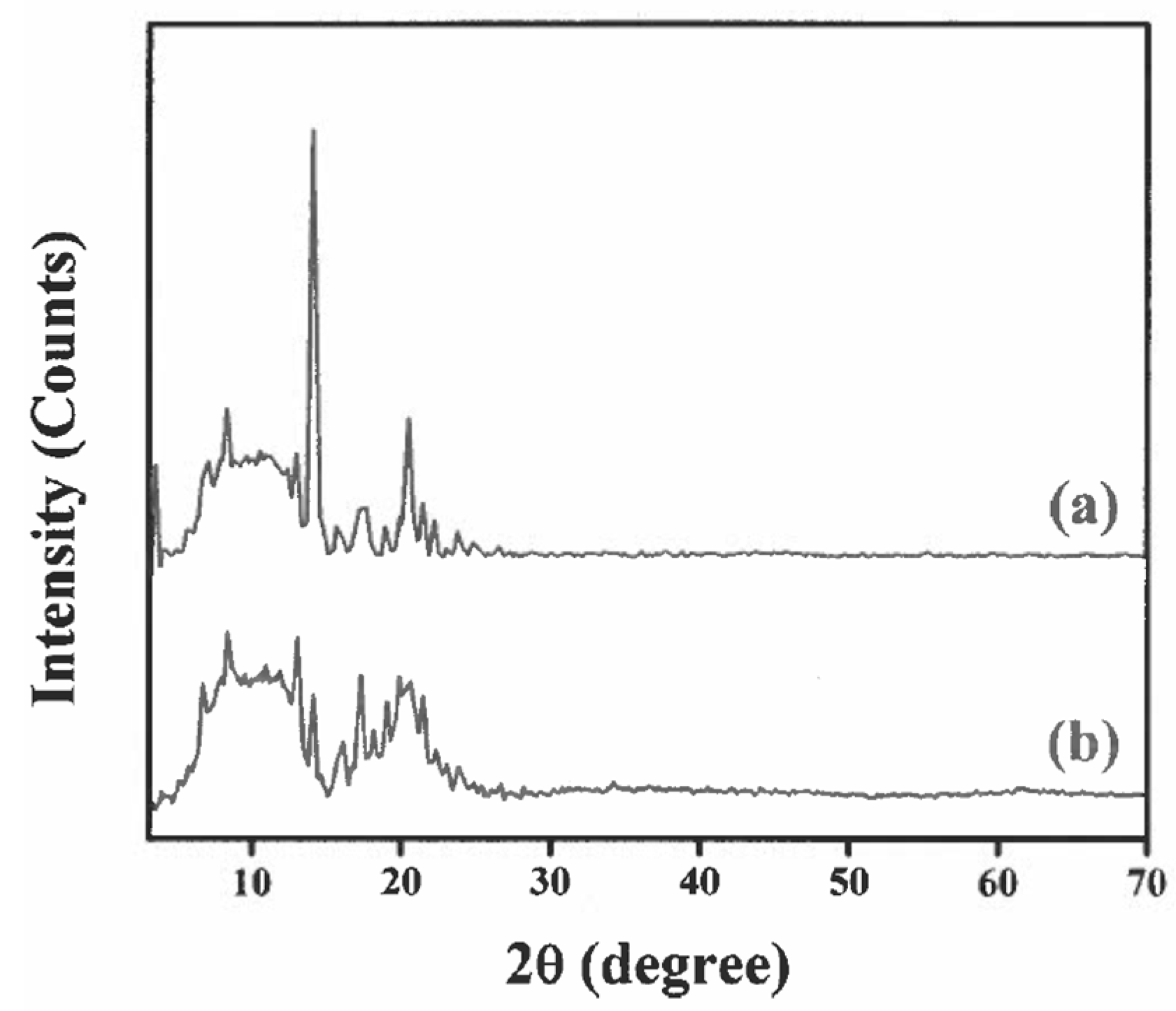
3.4. XRD Study
3.5. Physical Characterization
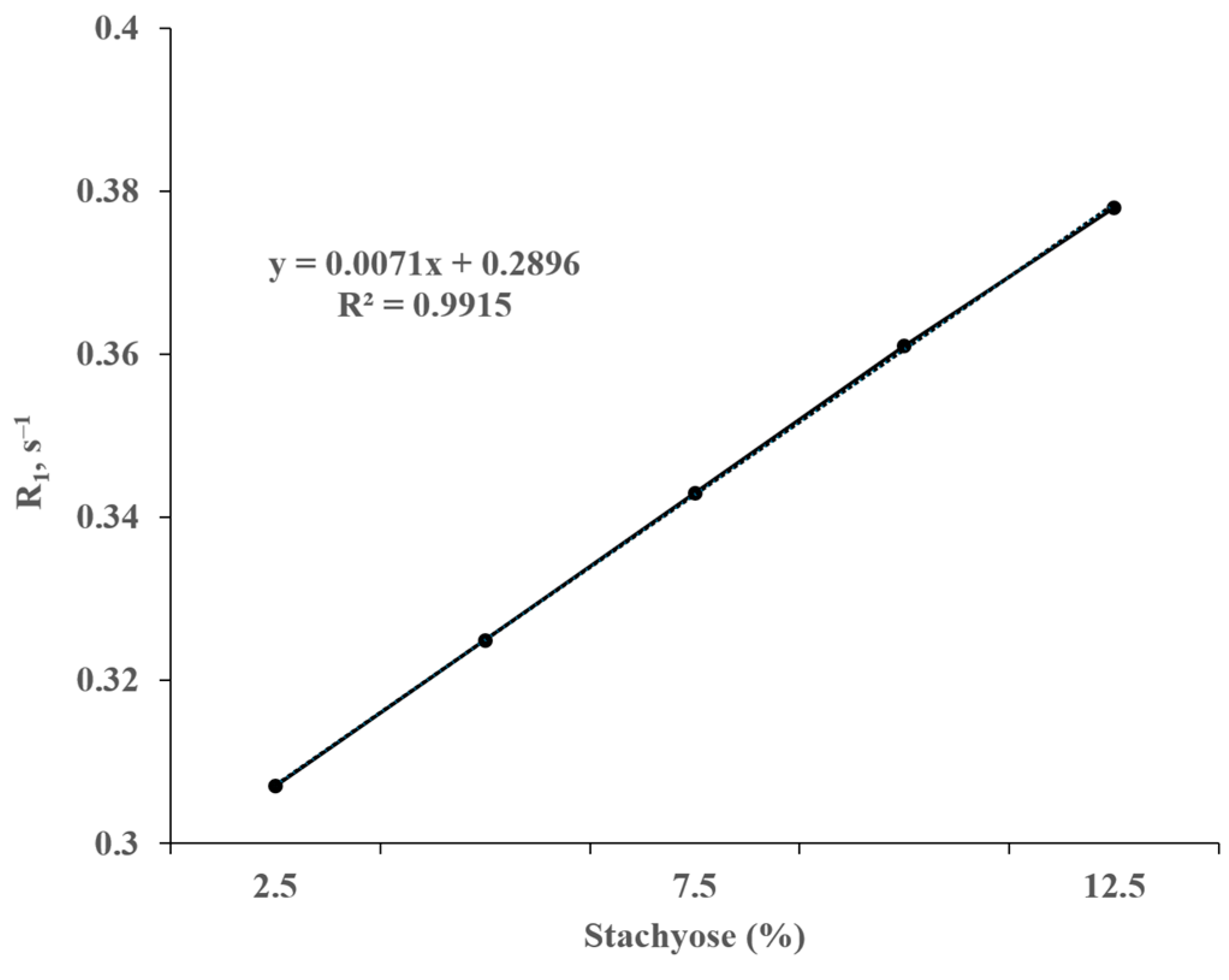
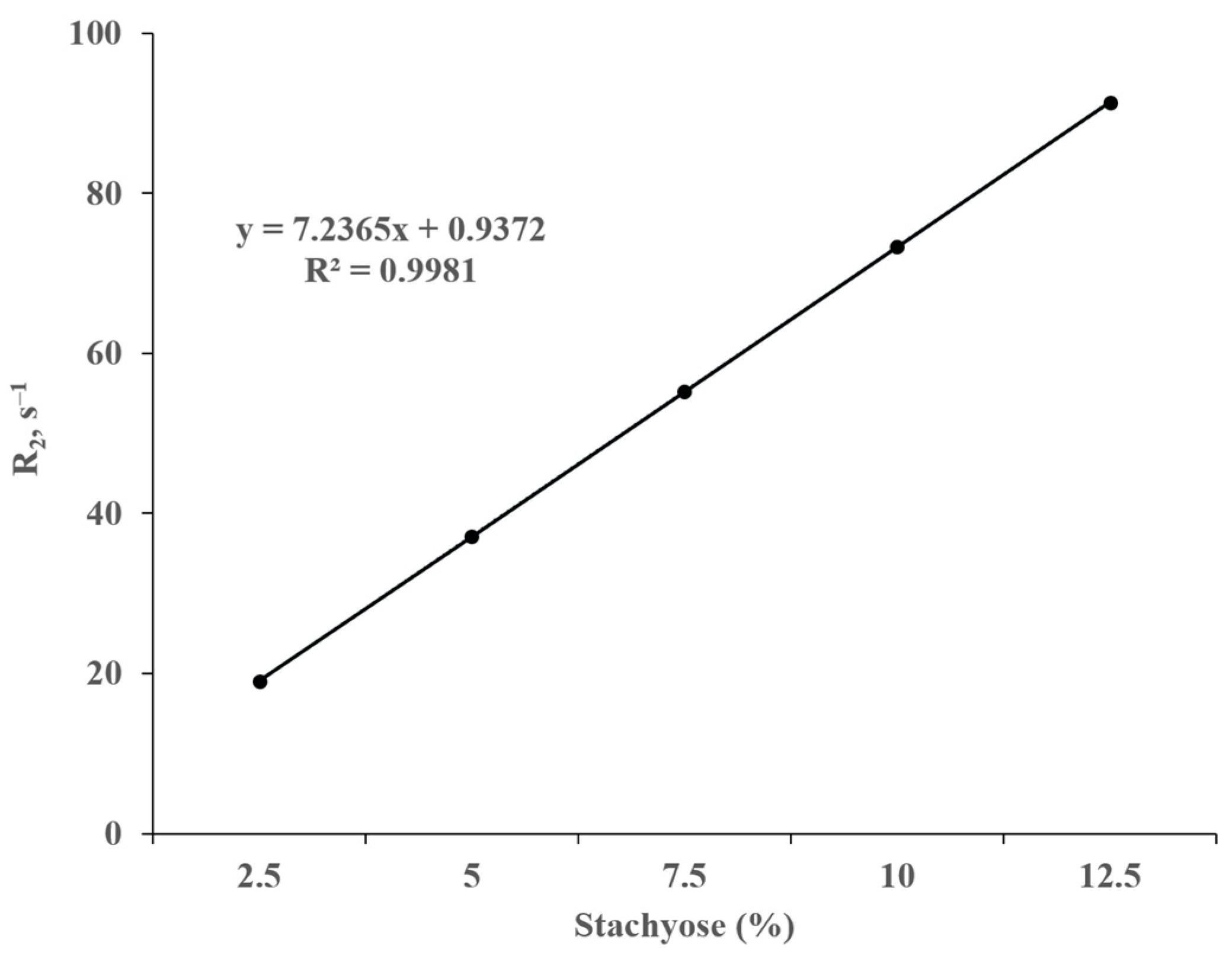
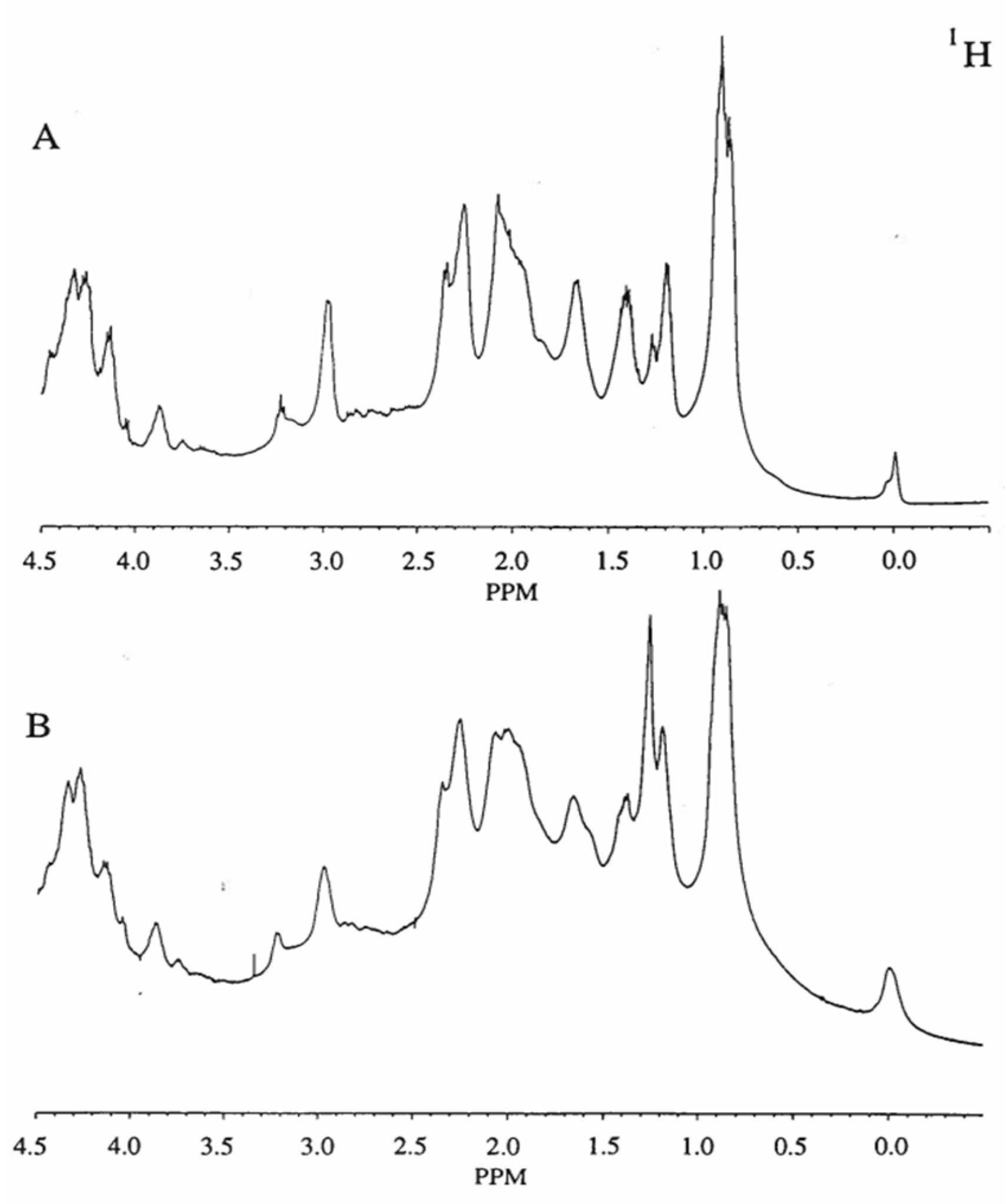
3.6. NMR Spectroscopy
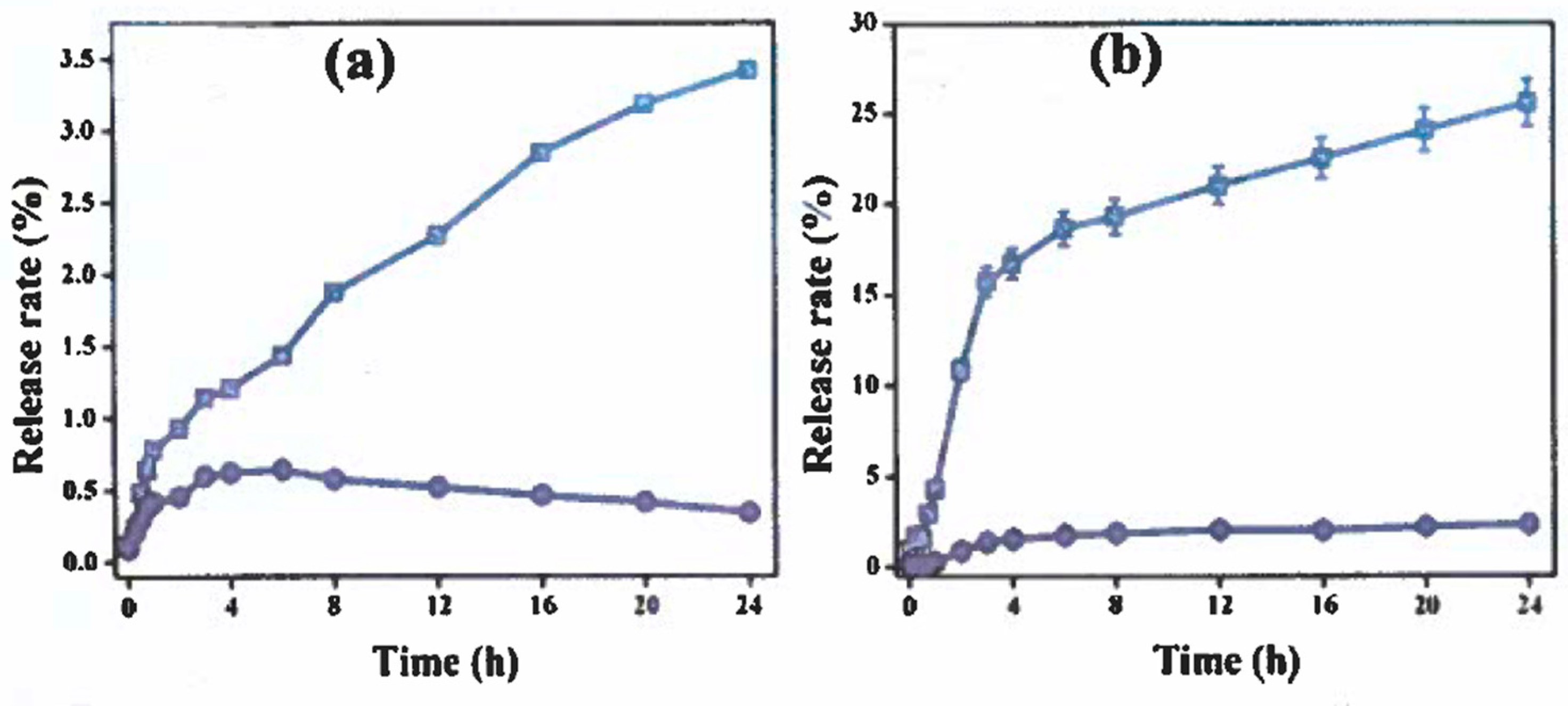
3.7. In-Vitro Study
3.8. In-Vivo Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ismailova, A.; White, J.H. Vitamin D, infections and immunity. Rev. Endocr. Metab. Disord. 2022, 23, 265–277. [Google Scholar] [CrossRef]
- Hodnik, J.J.; Ježek, J.; Starič, J. A review of vitamin D and its importance to the health of dairy cattle. J. Dairy Res. 2020, 87, 84–87. [Google Scholar] [CrossRef]
- Xu, H.J.; Jiang, X.; Zhang, C.R.; Ma, G.M.; Wang, L.H.; Zhang, Q.Y.; Zhang, Y.G. Effects of dietary 25-hydroxyvitamin D3 on the lactation performance, blood metabolites, antioxidant and immune function in dairy cows. Livest. Sci. 2021, 248, 104497. [Google Scholar] [CrossRef]
- Carsetti, R.; Quinti, I. Editorial: IgA and mucosal immunity in vaccinology and in protection from infection. Front. Cell. Infect. Microbiol. 2024, 14, 1409111. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Politis, A.P.; Mavrogianni, V.S.; Barbagianni, M.S.; Vasileiou, N.G.C.; Fthenakis, G.C.; Fragkou, I.A. Mammary defences and immunity against mastitis in sheep. Animal 2019, 9, 726. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Núñez de González, M.T.; Woldesenbet, S.; Attaie, R.; Jung, Y. Influence of deliverable form of dietary vitamin D3 on the immune response in late-lactating dairy goats. Dairy 2024, 5, 308–315. [Google Scholar] [CrossRef]
- Bhatia, J.; Greer, F. Use of soy protein-based formulas in infant feeding. Pediatrics 2008, 121, 1062–1068. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.W. Health effects of soy protein and isoflavones in humans. J. Nutr. 2008, 138, 12445–12495. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.; Ding, M.; Su, J.; Ye, J.; He, X.; Zhang, Y.; Shang, X. Stachyose in combination with L. rhamnosus GG ameliorates acute hypobaric hypoxia-induced intestinal barrier dysfunction through alleviating inflammatory response and oxidative stress. Free Radic. Biol. Med. 2024, 212, 505–519. [Google Scholar] [CrossRef] [PubMed]
- Bodera, P. Influence of Prebiotics on the Human Immune System (GALT). Recent Pat. Inflamm. Allergy Drug Discov. 2008, 2, 149–153. [Google Scholar] [CrossRef]
- Cheong, K.-L.; Chen, S.; Teng, B.; Veeraperumal, S.; Zhong, S.; Tan, K. Oligosaccharides as potential regulators of gut microbiota and intestinal health in post-COVID-19 management. Pharmaceuticals 2023, 16, 860. [Google Scholar] [CrossRef]
- Kailasapathy, K.; Chin, J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol. Cell Biol. 2000, 78, 80–88. [Google Scholar] [CrossRef]
- Li, B.; Luan, H.; Qin, J.; Zong, A.; Liu, L.; Xu, Z.; Du, F.; Xu, T. Effect of soluble dietary fiber on soy protein isolate emulsion gel properties, stability and delivery of vitamin D3. Int. J. Biol. Macromol. 2024, 262, 129806. [Google Scholar] [CrossRef]
- Athanassiou, P.; Mavragani, C.; Athanassiou, L.; Kostoglou-Athanassiou, I.; Koutsilieris, M. Vitamin D deficiency in primary Sjögren’s Syndrome: Association with clinical manifestations and immune activation markers. Mediterr. J. Rheumatol. 2022, 33, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.; Jung, S.-C.; Kwak, K.; Kim, J.-S. The role of prebiotics in modulating gut microbiota: Implications for human health. Int. J. Mol. Sci. 2024, 25, 4834. [Google Scholar] [CrossRef]
- Gunn, K.M.; Holly, M.A.; Veith, T.L.; Buda, A.R.; Prasafd, R.; Rotz, C.A.; Soder, K.J.; Stoner, A.M.K. Projected heat stress challenges and abatement opportunities for U.S. milk production. PLoS ONE 2019, 14, e0214665. [Google Scholar] [CrossRef]
- Caroprese, M.; Marzano, A.; Marino, R.; Gliatta, G.; Muscio, A.; Sevi, A. Immune response of cows fed polyunsaturated fatty acids under high ambient temperatures. J. Dairy Sci. 2009, 92, 2796–2803. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, J.; Ye, G.; Harnid, M.; Lia, S.; Huang, K. Protective effects of zymosan on heat stress-induced immunosuppression and apoptosis in dairy cows and peripheral blood mononuclear cells. Cell Stress Chaperones 2018, 23, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Zmrhal, V.; Svodarova, A.; Venusova, E.; Slama, P. The influence of heat stress on chicken immune system and mitigation of negative impacts by baicalin and baicalein. Animals 2023, 13, 2564. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, A.; Joy, A.; Dunshea, F.R.; Chauhan, S.S. The impact of heat stress on immune status of dairy cattle and strategies to ameliorate the negative effects. Animals 2022, 13, 107. [Google Scholar] [CrossRef]
- Haug, I.J.; Sagmo, L.B.; Zeiss, D.; Olsen, I.C.; Draget, K.I.; Seternes, T. Bioavailability of EPA and DHA delivered by gelled emulsions and soft gel capsules. Eur. J. Lipid Sci. Technol. 2011, 113, 137–145. [Google Scholar] [CrossRef]
- Temova, Ž.; Roškar, R. Stability-indicating HPLC-UV method for vitamin D3 determination in solutions, nutritional supplements and pharmaceuticals. J. Chromatogr. Sci. 2016, 54, 1180–1186. [Google Scholar] [CrossRef] [PubMed]
- Mora-Gutierrez, A.; Farrell, H.M.; Kumosinski, T.F. Comparison of hydration behavior of bovine and caprine caseins as determined by oxygen-17 nuclear magnetic resonance: pH/pD dependence. J. Agric. Food Chem. 1996, 44, 796–803. [Google Scholar] [CrossRef]
- Covington, A.K.; Paabo, M.; Robinson, R.A.; Bates, R.G. Use of the glass electrode in deuterium oxide and the relation between the standardized pD (pa0) scale and the operational pH in heavy water. Anal. Chem. 1968, 40, 700–706. [Google Scholar] [CrossRef]
- Asare-Addo, K.; Conway, B.R.; Larhrib, H.; Levina, M.; Rajabi-Siahboomi, A.R.; Tetteh, J.; Boateng, J.; Nokhodchi, A. The effect of pH and ionic strength of dissolution media on in vitro release of two model drugs of different solubilities from HPMC matrices. Colloids Surf. B Biointerfaces 2013, 111, 384–391. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Baianu, I.C. 1H NMR relaxation and viscosity measurements in solutions and suspensions of carbohydrates and starch from corn: The investigation of carbohydrate hydration and stereochemical and aggregation effects in relation to 17O and 13C NMR data for carbohydrate solutions. J. Agric. Food Chem. 1989, 37, 1459–1465. [Google Scholar] [CrossRef]
- Núñez de González, M.; Attaie, R.; Woldesenbet, S.; Mora-Gutierrez, A.; Kirven, J.; Jung, Y.; Myers, D. Effect of feeding a low level of encapsulated fish oil to dairy goats on milk yield, composition, and fatty acid profile. J. Am. Oil Chem. Soc. 2020, 97, 281–288. [Google Scholar] [CrossRef]
- Kiely, M.E.; Zhang, J.Y.; Kinsella, M.; Khashan, A.S.; Kenny, L.C. Vitamin D status is associated with uteroplacental dysfunction indicated by pre-eclampsia and small-for-gestational-age birth in a large prospective pregnancy cohort in Ireland with low vitamin D status. Am. J. Clin. Nutr. 2016, 104, 354–361. [Google Scholar] [CrossRef]
- Caroprese, M.; Ciliberti, M.G.; Santillo, A.; Marino, R.; Sevi, A.; Albenzio, M. Immune response, productivity and quality of milk from grazing goats as affected by dietary polyunsaturated fatty acid supplementation. Res. Vet. Sci. 2016, 105, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, K.; Huang, Q.; Geng, F. Microwave pretreatment enhanced the properties of ovalbumin-inulin-oil emulsion gels and improved the storage stability of pomegranate seed oil. Food Hydrocoll. 2021, 113, 106548. [Google Scholar] [CrossRef]
- Mora-Gutierrez, A.; Kumosinski, T.F.; Farrell, H.M., Jr. κ-carrageenan interaction with bovine and caprine caseins as shown by sedimentation and NMR spectroscopy techniques. J. Agric. Food Chem. 1998, 46, 4987–4996. [Google Scholar] [CrossRef]
- Zhang, G.; Sun, C.; Song, J.; Jin, W.; Tang, Y.; Zhou, D.; Song, L. Glycation of whey protein isolate and stachyose modulates their in vitro digestibility: Promising prebiotics as functional ingredients. Food Biosci. 2023, 52, 102379. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, F.; Yang, X.; Mao, Y.; Qi, Q.; Zheng, M.; Xu, X.; Cao, Y.; Wu, Y.; Liu, J. Synergistic influence of ultrasound and dietary fiber addition on transglutaminase-induced peanut protein gel and its application for encapsulation of lutein. Food Hydrocoll. 2023, 137, 108374. [Google Scholar] [CrossRef]
- Joy, A.; Dunshea, F.R.; Leury, B.J.; Clarke, I.J.; DiGiacomo, K.; Chauhan, S.S. Resilience of small ruminants to climate change and increased environmental temperature: A review. Animals 2020, 10, 867. [Google Scholar] [CrossRef]
- Ruiz-González, A.; Suissi, W.; Baumgard, L.H.; Martel-Kennes, Y.; Chouinard, P.Y.; Gervais, R.; Rico, D.E. Increased dietary vitamin D3 and calcium partially alleviate heat stress symptoms and inflammation in lactating Holstein cows independent of dietary concentrations of vitamin A and selenium. J. Dairy Sci. 2023, 106, 3984–4001. [Google Scholar] [CrossRef]
- El-Abbadi, N.H.; Dao, M.C.; Meydani, S.N. Yogurt: Role in healthy and active aging. Am. J. Clin. Nutr. 2014, 99, 1263S–1270S. [Google Scholar] [CrossRef] [PubMed]
- Bäumler, A.J.; Sperandio, V. Interactions between the microbiota and pathogenic bacteria in the gut. Nature 2016, 535, 85–93. [Google Scholar] [CrossRef]

| Time (h) | Rectal Temperature (°C) | ||
|---|---|---|---|
| Control | Diet 1 | Diet 2 | |
| 0800 | 39.37 | 39.93 | 39.98 |
| 1200 | 39.82 | 39.67 | 39.89 |
| 1700 | 39.67 | 39.78 | 39.38 |
| Average ± SD | 39.62 ± 0.23 | 39.79 ± 0.13 | 39.75 ± 0.32 |
| Item | Quantity |
|---|---|
| Dry matter (g/kg) | 891 |
| Crude protein (%) | 18.1 |
| Crude fat (%) | 4.2 |
| Fiber (%) | 8.7 |
| Total calcium (%) | 0.9 |
| Phosphate | 0.6 |
| Magnesium (%) | 0.29 |
| Manganese (mg/kg) | 141 |
| Vitamin A (IU/kg) | 20,296 |
| Vitamin D (IU/kg) | 4428 |
| Vitamin E (IU/kg) | 68 |
| Selenium (mg/kg) | 0.8 |
| NDF (%) | 24.1 |
| ADF (%) | 11.1 |
| NEL (Mcal kg−1) | 1.68 |
| Stachyose (%) | pH/pD 1 | ||
|---|---|---|---|
| 2.4 | 6.5 | 7.6 | |
| 0 | 0.00458 ± 0.000349 c | 0.00335 ± 0.000306 c | 0.00687 ± 0.000090 b |
| 0.1 | 0.00691 ± 0.000037 b | 0.00349 ± 0.000112 c | 0.00730 ± 0.000642 ab |
| 0.2 | 0.00721 ± 0.000408 b | 0.00382 ± 0.000066 c | 0.00746 ± 0.000277 ab |
| 0.3 | 0.00829 ± 0.000197 b | 0.00405 ± 0.000160 c | 0.00861 ± 0.000160 a |
| Diet 2 | Baseline (0 d) 2 | Feeding Period (56 d) | p-Value |
|---|---|---|---|
| Control 1 | 21.71 ± 0.644 | 23.71 ± 0.647 | 0.229 |
| Diet 1 | 21.41 ± 1.470 | 27.24 ± 0.813 | 0.001 |
| Diet 2 | 21.75 ± 0.462 | 34.29 ± 1.550 | <0.0001 |
| Sampling Time (Days) | Experimental Diets 2 | ||
|---|---|---|---|
| Control (n = 6) | Diet 1 (n = 6) | Diet 2 (n = 6) | |
| 0 1 | 1.90 ± 0.213 c C | 1.87 ± 0.184 c C | 1.93 ± 0.080 c D |
| 14 | 1.93 ± 0.180 c C | 1.90 ± 0.103 c C | 1.98 ± 0.217 c D |
| 28 | 6.20 ± 1.168 c A | 11.67 ± 2.106 b A | 17.57 ± 2.635 a C |
| 42 | 3.22 ± 0.796 c B | 13.83 ± 1.376 b A | 23.83 ± 1.487 a B |
| 56 | 2.57 ± 0.403 c C | 8.37 ± 2.345 b B | 30.08 ± 1.101 a A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora-Gutierrez, A.; Núñez de González, M.T.; Attaie, R.; Jung, Y. Soy Protein Isolate-Stachyose Emulsion Gel for the Delivery of Vitamin D3: Effect on the Humoral Immune Response in Dairy Goats Under Heat Stress. Animals 2025, 15, 2588. https://doi.org/10.3390/ani15172588
Mora-Gutierrez A, Núñez de González MT, Attaie R, Jung Y. Soy Protein Isolate-Stachyose Emulsion Gel for the Delivery of Vitamin D3: Effect on the Humoral Immune Response in Dairy Goats Under Heat Stress. Animals. 2025; 15(17):2588. https://doi.org/10.3390/ani15172588
Chicago/Turabian StyleMora-Gutierrez, Adela, Maryuri T. Núñez de González, Rahmat Attaie, and Yoonsung Jung. 2025. "Soy Protein Isolate-Stachyose Emulsion Gel for the Delivery of Vitamin D3: Effect on the Humoral Immune Response in Dairy Goats Under Heat Stress" Animals 15, no. 17: 2588. https://doi.org/10.3390/ani15172588
APA StyleMora-Gutierrez, A., Núñez de González, M. T., Attaie, R., & Jung, Y. (2025). Soy Protein Isolate-Stachyose Emulsion Gel for the Delivery of Vitamin D3: Effect on the Humoral Immune Response in Dairy Goats Under Heat Stress. Animals, 15(17), 2588. https://doi.org/10.3390/ani15172588






