Effects of L-Carnitine on the Developmental Competence of Bovine Oocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Oocyte Collection and In Vitro Maturation of Bovine Oocytes
- (1)
- FBS (control): Oocytes matured in IVM medium supplemented with 10% FBS.
- (2)
- BSA: Oocytes matured in IVM medium without FBS and containing BSA (8 mg/mL).
- (3)
- FBS + 1.5 mM LC: Oocytes matured in IVM medium containing 10% FBS supplemented with 1.5 mM L-carnitine.
- (4)
2.3. Evaluation of Oocyte Maturation
2.4. Evaluation of the Lipid Content of In Vitro-Matured Oocytes
2.5. In Vitro Fertilization
2.6. In Vitro Embryo Culture
2.7. Cryopreservation of Embryos by Slow Freezing
2.8. Gene Expression Analysis
2.9. Statistical Analyses
3. Results
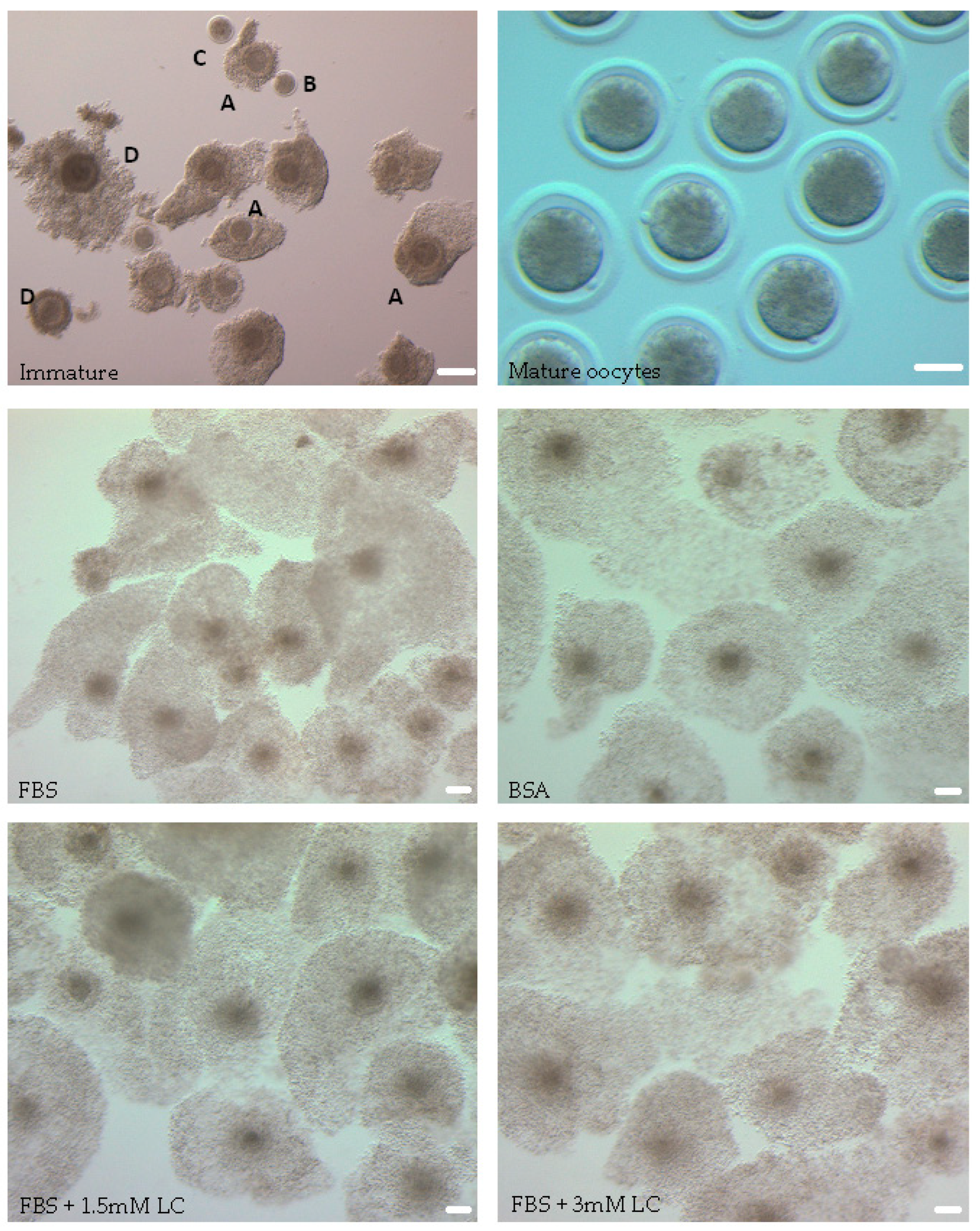
3.1. In Vitro Oocyte Maturation Status
3.1.1. Cumulus Expansion and First Polar Body Formation
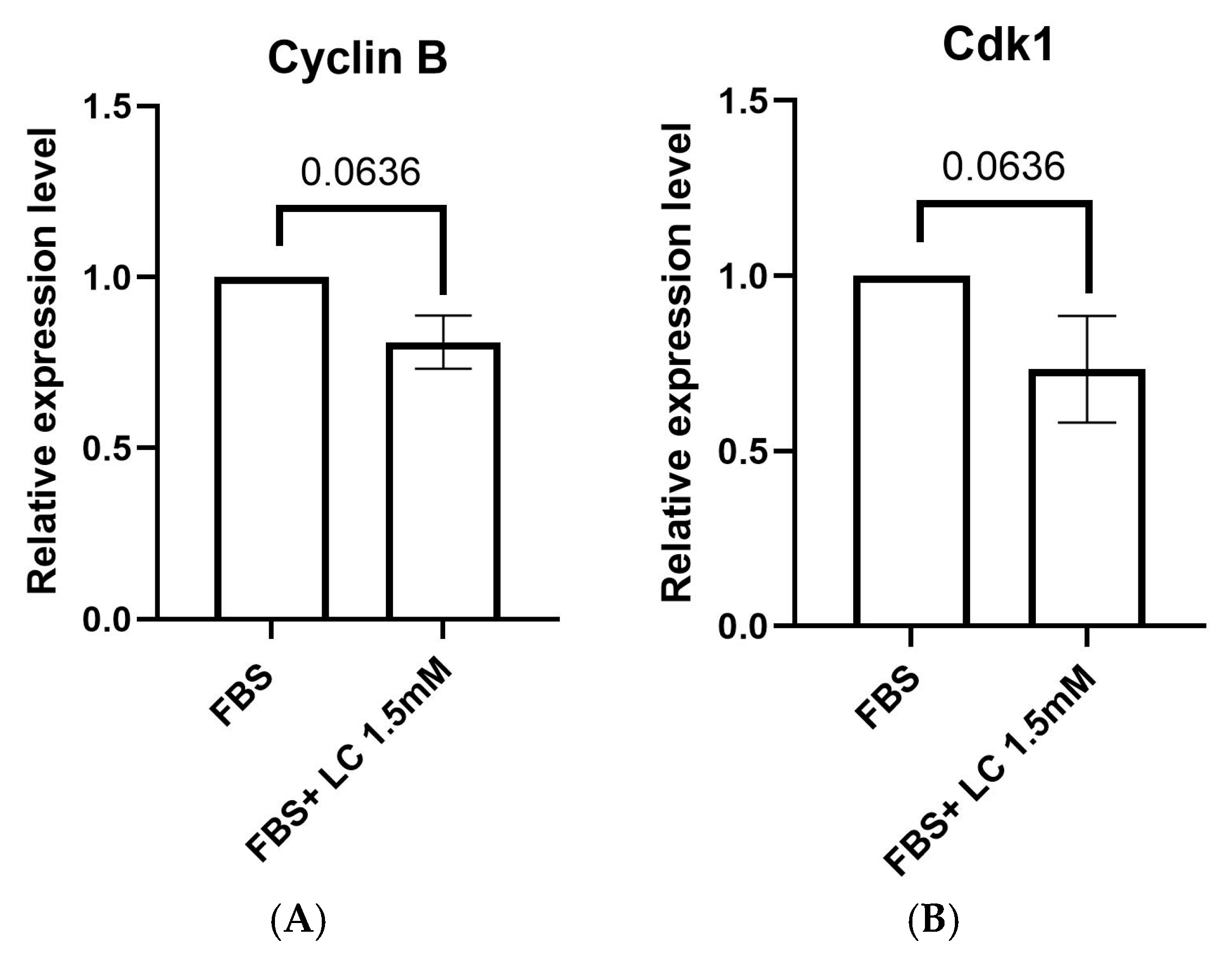
3.1.2. Expression of Oocyte Maturation Markers
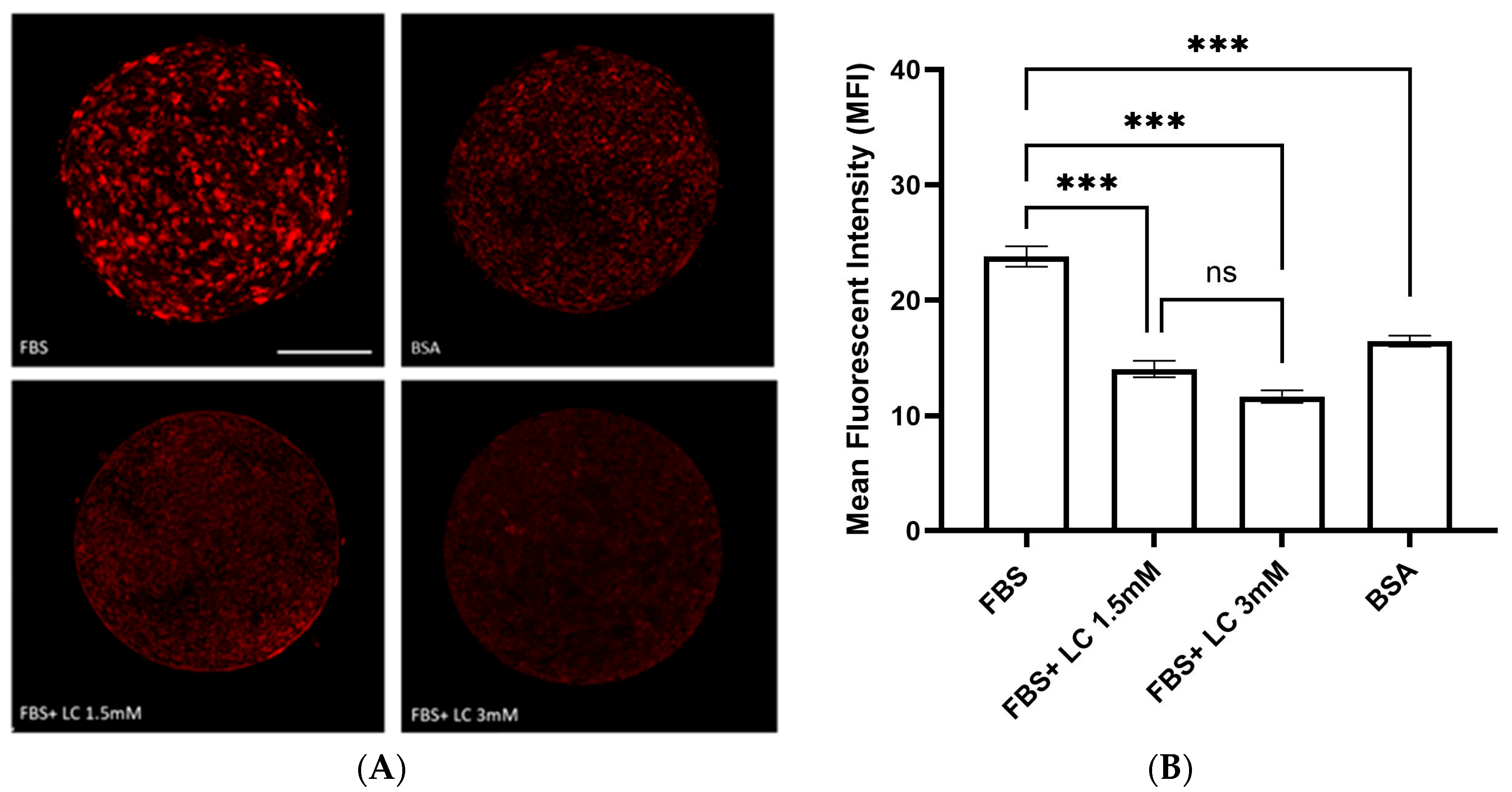
3.2. Lipid Content of Matured Oocytes
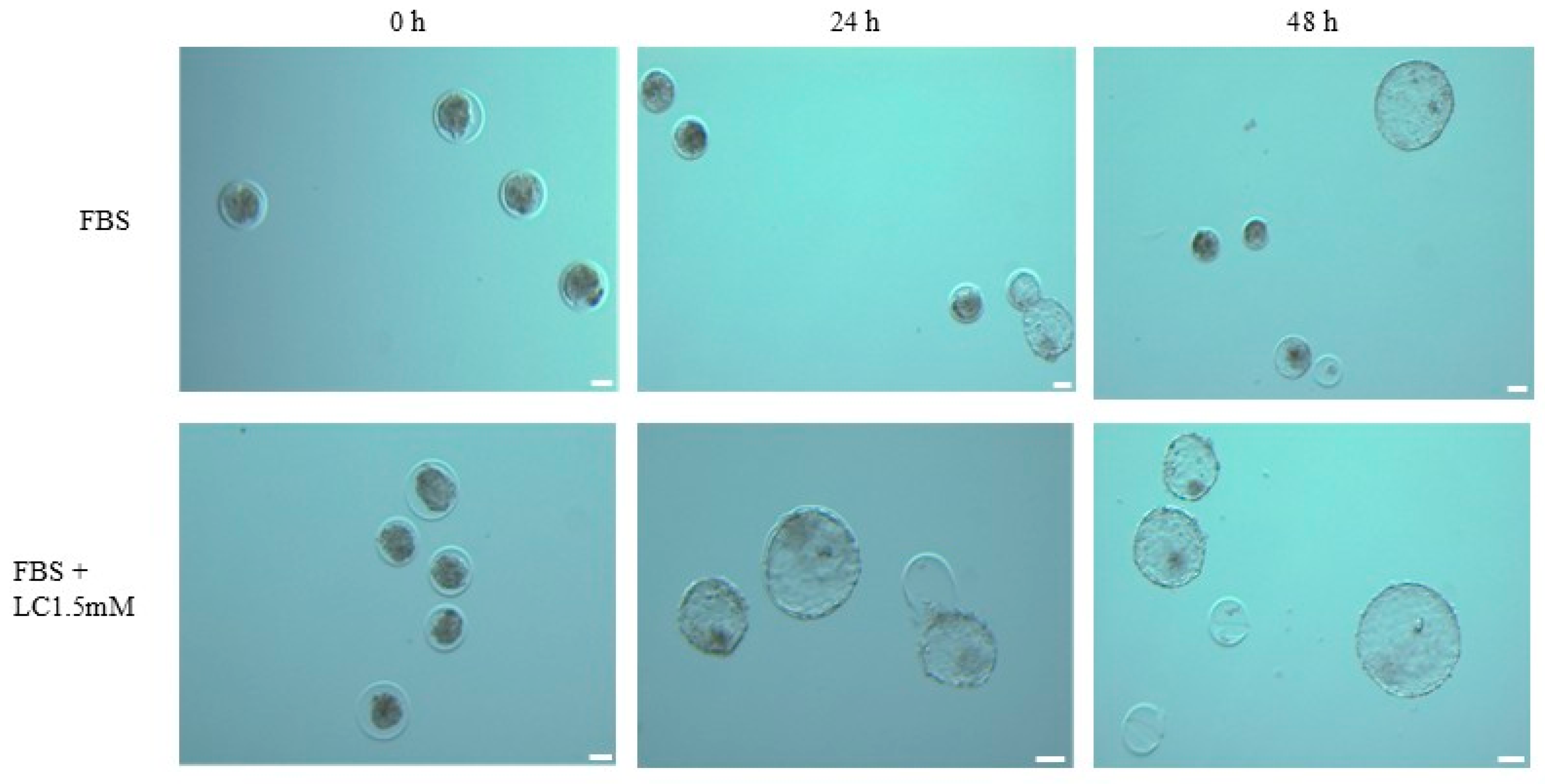
3.3. In Vitro Fertilization and Embryo Production
3.4. Post-Thaw Survival of Cryopreserved Embryos
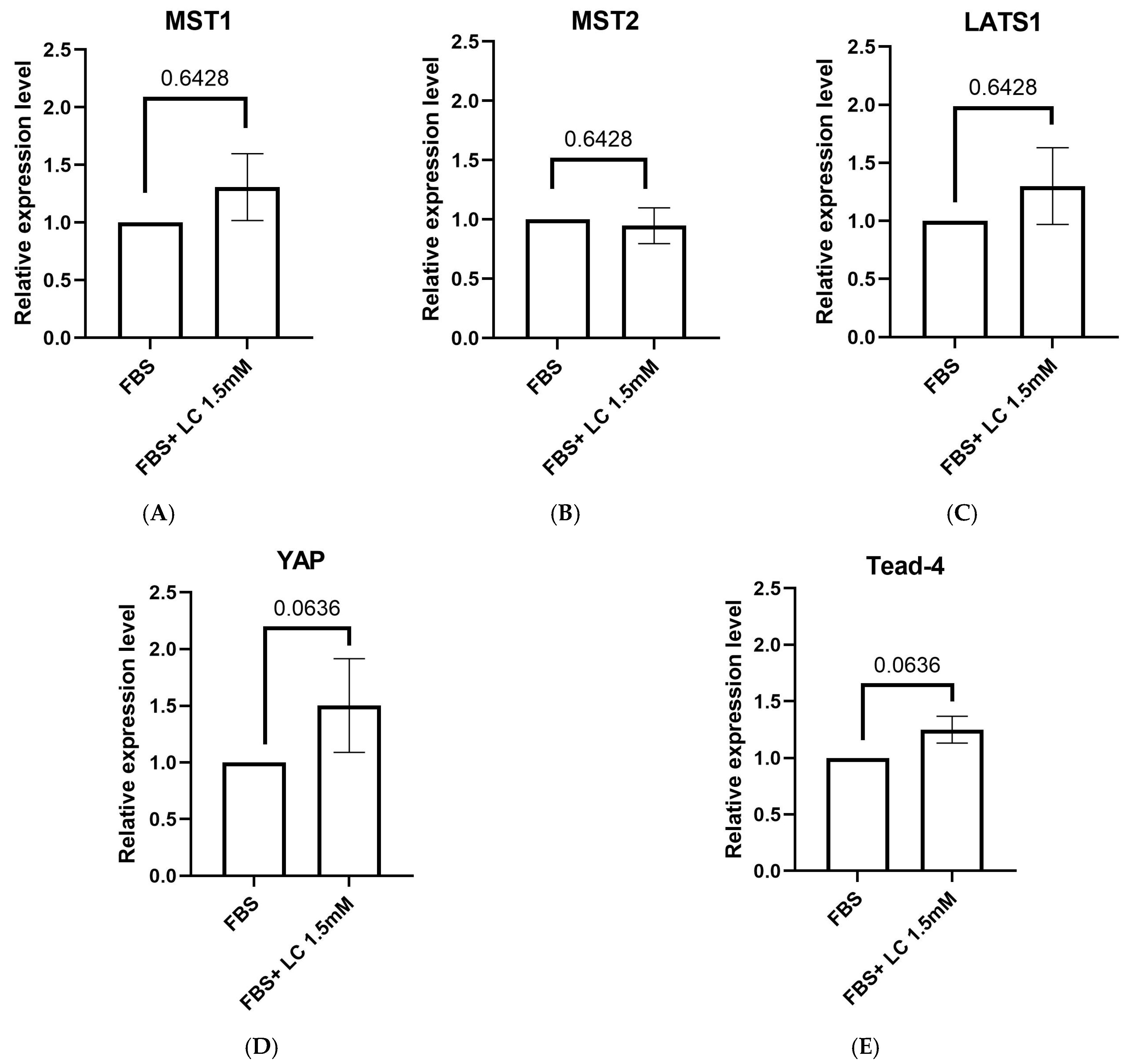
3.5. Expression of Hippo Signaling Pathway Component Genes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Daly, J.; Smith, H.; McGrice, H.A.; Kind, K.L.; van Wettere, W.H. Towards improving the outcomes of assisted reproductive technologies of cattle and sheep, with particular focus on recipient management. Animals 2020, 10, 293. [Google Scholar] [CrossRef]
- Rexroad, C.; Vallet, J.; Matukumalli, L.K.; Reecy, J.; Bickhart, D.; Blackburn, H.; Boggess, M.; Cheng, H.; Clutter, A.; Cockett, N. Genome to phenome: Improving animal health, production, and well-being–a new USDA blueprint for animal genome research 2018–2027. Front. Genet. 2019, 10, 327. [Google Scholar] [CrossRef] [PubMed]
- Salek, F.; Guest, A.; Johnson, C.; Kastelic, J.P.; Thundathil, J. Factors Affecting the Success of Ovum Pick-Up, In Vitro Production and Cryopreservation of Embryos in Cattle. Animals 2025, 15, 344. [Google Scholar] [CrossRef]
- Palasz, A.; Thundathil, J.; De La Fuente, J.; Mapletoft, R. Effect of reduced concentrations of glycerol and various macromolecules on the cryopreservation of mouse and cattle embryos. Cryobiology 2000, 41, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Inaba, Y.; Somfai, T.; Kaneda, M.; Geshi, M.; Nagai, T.; Manabe, N. Supplementation of culture medium with L-carnitine improves development and cryotolerance of bovine embryos produced in vitro. Reprod. Fertil. Dev. 2013, 25, 589–599. [Google Scholar] [CrossRef]
- Tharasanit, T.; Thuwanut, P. Oocyte cryopreservation in domestic animals and humans: Principles, techniques and updated outcomes. Animals 2021, 11, 2949. [Google Scholar] [CrossRef]
- Pero, M.E.; Zullo, G.; Esposito, L.; Iannuzzi, A.; Lombardi, P.; De Canditiis, C.; Neglia, G.; Gasparrini, B. Inhibition of apoptosis by caspase inhibitor Z-VAD-FMK improves cryotolerance of in vitro derived bovine embryos. Theriogenology 2018, 108, 127–135. [Google Scholar] [CrossRef]
- Dias, L.R.O.; Leme, L.O.; Sprícigo, J.F.W.; Pivato, I.; Dode, M.A.N. Effect of delipidant agents during in vitro culture on the development, lipid content, gene expression and cryotolerance of bovine embryos. Reprod. Domest. Anim. 2020, 55, 11–20. [Google Scholar] [CrossRef]
- Brown, D.A. Lipid droplets: Proteins floating on a pool of fat. Curr. Biol. 2001, 11, R446–R449. [Google Scholar] [CrossRef] [PubMed]
- Paczkowski, M.; Silva, E.; Schoolcraft, W.B.; Krisher, R.L. Comparative importance of fatty acid beta-oxidation to nuclear maturation, gene expression, and glucose metabolism in mouse, bovine, and porcine cumulus oocyte complexes. Biol. Reprod. 2013, 88, 111. [Google Scholar] [CrossRef]
- Sturmey, R.; Reis, A.; Leese, H.; McEvoy, T. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Reprod. Domest. Anim. 2009, 44, 50–58. [Google Scholar] [CrossRef]
- Del Collado, M.; Saraiva, N.Z.; Lopes, F.L.; Gaspar, R.C.; Padilha, L.C.; Costa, R.R.; Rossi, G.F.; Vantini, R.; Garcia, J.M. Influence of bovine serum albumin and fetal bovine serum supplementation during in vitro maturation on lipid and mitochondrial behaviour in oocytes and lipid accumulation in bovine embryos. Reprod. Fertil. Dev. 2016, 28, 1721–1732. [Google Scholar] [CrossRef]
- Reis, A.; Rooke, J.; McCallum, G.; Staines, M.; Ewen, M.; Lomax, M.; McEvoy, T. Consequences of exposure to serum, with or without vitamin E supplementation, in terms of the fatty acid content and viability of bovine blastocysts produced in vitro. Reprod. Fertil. Dev. 2003, 15, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Idrissi, S.J.; Le Bourhis, D.; Lefevre, A.; Emond, P.; Le Berre, L.; Desnoës, O.; Joly, T.; Buff, S.; Maillard, V.; Schibler, L. Lipid profile of bovine grade-1 blastocysts produced either in vivo or in vitro before and after slow freezing process. Sci. Rep. 2021, 11, 11618. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, R.; Ibayashi, M.; Tatsumi, T.; Yamamoto, A.; Kokubo, T.; Miyasaka, N.; Sato, K.; Ikeda, S.; Minami, N.; Tsukamoto, S. Synthesis and maintenance of lipid droplets are essential for mouse preimplantation embryonic development. Development 2019, 146, dev181925. [Google Scholar] [CrossRef]
- Prates, E.; Nunes, J.; Pereira, R. A role of lipid metabolism during cumulus-oocyte complex maturation: Impact of lipid modulators to improve embryo production. Mediat. Inflamm. 2014, 2014, 692067. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Sengupta, P.; Durairajanayagam, D. Role of L-carnitine in female infertility. Reprod. Biol. Endocrinol. 2018, 16, 5. [Google Scholar] [CrossRef]
- Montjean, D.; Entezami, F.; Lichtblau, I.; Belloc, S.; Gurgan, T.; Menezo, Y. Carnitine content in the follicular fluid and expression of the enzymes involved in beta oxidation in oocytes and cumulus cells. J. Assist. Reprod. Genet. 2012, 29, 1221–1225. [Google Scholar] [CrossRef]
- Jiang, W.; Li, Y.; Zhao, Y.; Gao, Q.; Jin, Q.; Yan, C.; Xu, Y. L-carnitine supplementation during in vitro culture regulates oxidative stress in embryos from bovine aged oocytes. Theriogenology 2020, 143, 64–73. [Google Scholar] [CrossRef]
- Placidi, M.; Di Emidio, G.; Virmani, A.; D’Alfonso, A.; Artini, P.G.; D’Alessandro, A.M.; Tatone, C. Carnitines as mitochondrial modulators of oocyte and embryo bioenergetics. Antioxidants 2022, 11, 745. [Google Scholar] [CrossRef]
- Knitlova, D.; Hulinska, P.; Jeseta, M.; Hanzalova, K.; Kempisty, B.; Machatkova, M. Supplementation of l-carnitine during in vitro maturation improves embryo development from less competent bovine oocytes. Theriogenology 2017, 102, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Catandi, G.D.; Cheng, M.-H.; Chicco, A.J.; Chen, T.; Carnevale, E.M. L-carnitine enhances developmental potential of bovine oocytes matured under high lipid concentrations in vitro. Anim. Reprod. Sci. 2023, 252, 107249. [Google Scholar] [CrossRef]
- Liang, Y.; Yoisungnern, T.; Huang, Y.; Parnpai, R. Effects of L-carnitine on embryo development of vitrified swamp buffalo oocytes following in vitro fertilization. Livest. Sci. 2020, 232, 103933. [Google Scholar] [CrossRef]
- Chankitisakul, V.; Somfai, T.; Inaba, Y.; Techakumphu, M.; Nagai, T. Supplementation of maturation medium with L-carnitine improves cryo-tolerance of bovine in vitro matured oocytes. Theriogenology 2013, 79, 590–598. [Google Scholar] [CrossRef]
- Dunning, K.R.; Akison, L.K.; Russell, D.L.; Norman, R.J.; Robker, R.L. Increased beta-oxidation and improved oocyte developmental competence in response to l-carnitine during ovarian in vitro follicle development in mice. Biol. Reprod. 2011, 85, 548–555. [Google Scholar] [CrossRef]
- Clark, K.L.; George, J.W.; Przygrodzka, E.; Plewes, M.R.; Hua, G.; Wang, C.; Davis, J.S. Hippo signaling in the ovary: Emerging roles in development, fertility, and disease. Endocr. Rev. 2022, 43, 1074–1096. [Google Scholar] [CrossRef]
- Gerri, C.; McCarthy, A.; Scott, G.M.; Regin, M.; Brumm, S.; Simon, C.S.; Lee, J.; Montesinos, C.; Hassitt, C.; Hockenhull, S. A conserved role of Hippo signaling in initiation of the first lineage specification event across mammals. bioRxiv 2022. [Google Scholar] [CrossRef]
- Sharma, J.; Antenos, M.; Madan, P. A comparative analysis of hippo signaling pathway components during murine and bovine early mammalian embryogenesis. Genes 2021, 12, 281. [Google Scholar] [CrossRef]
- Sonnen, K.F.; Janda, C.Y. Signalling dynamics in embryonic development. Biochem. J. 2021, 478, 4045–4070. [Google Scholar] [CrossRef] [PubMed]
- Rausch, V.; Hansen, C.G. The Hippo pathway, YAP/TAZ, and the plasma membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Y.; Chen, B.; Chan, W.N.; Mui, C.W.; Cheung, A.H.; Zhang, J.; Wong, K.Y.; Yu, J.; Kang, W. Targeting the hippo pathway in gastric cancer and other malignancies in the digestive system: From bench to bedside. Biomedicines 2022, 10, 2512. [Google Scholar] [CrossRef] [PubMed]
- Negrón-Pérez, V.M.; Zhang, Y.; Hansen, P.J. Single-cell gene expression of the bovine blastocyst. Reproduction 2017, 154, 627–644. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef]
- Negrón-Pérez, V.M.; Hansen, P.J. Role of yes-associated protein 1, angiomotin, and mitogen-activated kinase kinase 1/2 in development of the bovine blastocyst. Biol. Reprod. 2018, 98, 170–183. [Google Scholar] [CrossRef]
- Sharma, J.; Madan, P. Characterisation of the Hippo signalling pathway during bovine preimplantation embryo development. Reprod. Fertil. Dev. 2020, 32, 392–401. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.; Madan, P. Differential regulation of Hippo signaling pathway components between 8-cell and blastocyst stages of bovine preimplantation embryogenesis. Mol. Reprod. Dev. 2022, 89, 146–161. [Google Scholar] [CrossRef]
- Zolini, A.M.; Carrascal-Triana, E.; de King, A.R.; Hansen, P.J.; Torres, C.A.A.; Block, J. Effect of addition of L-carnitine to media for oocyte maturation and embryo culture on development and cryotolerance of bovine embryos produced in vitro. Theriogenology 2019, 133, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Romek, M.; Gajda, B.; Krzysztofowicz, E.; Kepczynski, M.; Smorag, Z. New technique to quantify the lipid composition of lipid droplets in porcine oocytes and pre-implantation embryos using Nile Red fluorescent probe. Theriogenology 2011, 75, 42–54. [Google Scholar] [CrossRef]
- Stringfellow, D.A.; Givens, M.D. Manual of the International Embryo Transfer Society: A Procedural Guide and General Information for the Use of Embryo Transfer Technology Emphasizing Sanitary Procedures; International Embryo Transfer Society: Champaign, IL, USA, 2010. [Google Scholar]
- Youngs, C.R. Preimplantasyon Sığır Embriyolar, Koyun ve Keçi, Kriyoprezervasyon. J. Vis. Exp. (JoVE) 2011, e2764. [Google Scholar] [CrossRef]
- Mun, S.; Kim, Y.-J.; Markkandan, K.; Shin, W.; Oh, S.; Woo, J.; Yoo, J.; An, H.; Han, K. The whole-genome and transcriptome of the Manila clam (Ruditapes philippinarum). Genome Biol. Evol. 2017, 9, 1487–1498. [Google Scholar] [CrossRef]
- Calder, M.D.; Caveney, A.N.; Sirard, M.-A.; Watson, A.J. Effect of serum and cumulus cell expansion on marker gene transcripts in bovine cumulus-oocyte complexes during maturation in vitro. Fertil. Steril. 2005, 83, 1077–1085. [Google Scholar] [CrossRef]
- Sutton-McDowall, M.L.; Gilchrist, R.B.; Thompson, J.G. Cumulus expansion and glucose utilisation by bovine cumulus–oocyte complexes during in vitro maturation: The influence of glucosamine and follicle-stimulating hormone. Reproduction 2004, 128, 313–319. [Google Scholar] [CrossRef]
- Brunner, D.; Frank, J.; Appl, H.; Schöffl, H.; Pfaller, W.; Gstraunthaler, G. The serum-free media interactive online database. ALTEX-Altern. Anim. Exp. 2010, 27, 53–62. [Google Scholar]
- Chelladurai, K.S.; Christyraj, J.D.S.; Rajagopalan, K.; Yesudhason, B.V.; Venkatachalam, S.; Mohan, M.; Vasantha, N.C.; Christyraj, J.R.S.S. Alternative to FBS in animal cell culture-An overview and future perspective. Heliyon 2021, 7, e07686. [Google Scholar] [CrossRef]
- Leibfried-Rutledge, M.; Critser, E.; First, N. Effects of fetal calf serum and bovine serum albumin on in vitro maturation and fertilization of bovine and hamster cumulus-oocyte complexes. Biol. Reprod. 1986, 35, 850–857. [Google Scholar] [CrossRef]
- Leisinger, C.A.; Coffman, E.; da Silva, M.C.; Forshey, B.; Pinto, C. Factors affecting in vitro maturation of alpaca (Lama paco) oocytes. Anim. Reprod. Sci. 2014, 150, 70–75. [Google Scholar] [CrossRef][Green Version]
- Pilgrim, C.R.; McCahill, K.A.; Rops, J.G.; Dufour, J.M.; Russell, K.A.; Koch, T.G. A review of fetal bovine serum in the culture of mesenchymal stromal cells and potential alternatives for veterinary medicine. Front. Vet. Sci. 2022, 9, 859025. [Google Scholar] [CrossRef]
- Şen, U.; Şirin, E.; Önder, H.; Özyürek, S.; Kolenda, M.; Sitkowska, B. Macromolecules influence cellular competence and expression level of IGFs genes in bovine oocytes in vitro. Animals 2022, 12, 2604. [Google Scholar] [CrossRef] [PubMed]
- Arias, M.E.; Vargas, T.; Gallardo, V.; Aguila, L.; Felmer, R. Simple and efficient chemically defined in vitro maturation and embryo culture system for bovine embryos. Animals 2022, 12, 3057. [Google Scholar] [CrossRef] [PubMed]
- Zare, Z.; Abouhamzeh, B.; Farahani, R.M.; Salehi, M.; Mohammadi, M. Supplementation of L-carnitine during in vitro maturation of mouse oocytes affects expression of genes involved in oocyte and embryo competence: An experimental study. Int. J. Reprod. Biomed. 2017, 15, 779. [Google Scholar] [CrossRef]
- Carrillo-González, D.F.; Rodríguez-Osorio, N.; Long, C.R.; Vásquez-Araque, N.A.; Maldonado-Estrada, J.G. L-carnitine supplementation during in vitro maturation and in vitro culture does not affect the survival rates after vitrification and warming but alters Inf-T and ptgs2 gene expression. Int. J. Mol. Sci. 2020, 21, 5601. [Google Scholar] [CrossRef]
- Zhao, B.; Li, H.; Zhang, H.; Ren, S.; Li, Y.; Wang, X.; Lan, X.; Qiao, H.; Ma, H.; Zhang, Y. The effect of L-carnitine supplementation during in vitro maturation on oocyte maturation and somatic cloned embryo development. Reprod. Biol. 2024, 24, 100853. [Google Scholar] [CrossRef]
- Shirazi, A.; Ardali, M.A.; Ahmadi, E.; Nazari, H.; Mamuee, M.; Heidari, B. The effect of macromolecule source and type of media during in vitro maturation of sheep oocytes on subsequent embryo development. J. Reprod. Infertil. 2012, 13, 13. [Google Scholar] [PubMed]
- Abe, H.; Yamashita, S.; Satoh, T.; Hoshi, H. Accumulation of cytoplasmic lipid droplets in bovine embryos and cryotolerance of embryos developed in different culture systems using serum-free or serum-containing media. Mol. Reprod. Dev. 2002, 61, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Sanches, B.V.; Zangirolamo, A.F.; da Silva, N.C.; Morotti, F.; Seneda, M.M. Cryopreservation of in vitro-produced embryos: Challenges for commercial implementation. Anim. Reprod. (AR) 2018, 14, 521–527. [Google Scholar] [CrossRef]
- Ghanem, N.; Fakruzzaman, M.; Batawi, A.H.; Kong, I.-K. Post-thaw viability, developmental and molecular deviations in in vitro produced bovine embryos cultured with l-carnitine at different levels of fetal calf serum. Theriogenology 2022, 191, 54–66. [Google Scholar] [CrossRef]
- Han, H.; Qi, R.; Zhou, J.J.; Ta, A.P.; Yang, B.; Nakaoka, H.J.; Seo, G.; Guan, K.-L.; Luo, R.; Wang, W. Regulation of the Hippo pathway by phosphatidic acid-mediated lipid-protein interaction. Mol. Cell 2018, 72, 328–340.e328. [Google Scholar] [CrossRef]
- Kashiwagi, A.; Kanno, T.; Arita, K.; Ishisaka, R.; Utsumi, T.; Utsumi, K. Suppression of T3-and fatty acid-induced membrane permeability transition by L-carnitine. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 130, 411–418. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Taouk, G.M. A potential role of YAP/TAZ in the interplay between metastasis and metabolic alterations. Front. Oncol. 2020, 10, 928. [Google Scholar] [CrossRef] [PubMed]
- Fan, R.; Kim, N.-G.; Gumbiner, B.M. Regulation of Hippo pathway by mitogenic growth factors via phosphoinositide 3-kinase and phosphoinositide-dependent kinase-1. Proc. Natl. Acad. Sci. USA 2013, 110, 2569–2574. [Google Scholar] [CrossRef]
- Miller, E.; Yang, J.; DeRan, M.; Wu, C.; Su, A.I.; Bonamy, G.M.; Liu, J.; Peters, E.C.; Wu, X. Identification of serum-derived sphingosine-1-phosphate as a small molecule regulator of YAP. Chem. Biol. 2012, 19, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Straßburger, K.; Tiebe, M.; Pinna, F.; Breuhahn, K.; Teleman, A.A. Insulin/IGF signaling drives cell proliferation in part via Yorkie/YAP. Dev. Biol. 2012, 367, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Nita, A.; Moroishi, T. Hippo pathway in cell–cell communication: Emerging roles in development and regeneration. Inflamm. Regen. 2024, 44, 18. [Google Scholar] [CrossRef]
- Deng, Y.; Lu, J.; Li, W.; Wu, A.; Zhang, X.; Tong, W.; Ho, K.K.; Qin, L.; Song, H.; Mak, K.K. Reciprocal inhibition of YAP/TAZ and NF-κB regulates osteoarthritic cartilage degradation. Nat. Commun. 2018, 9, 4564. [Google Scholar] [CrossRef]
- Holmes, B.; Benavides-Serrato, A.; Saunders, J.T.; Kumar, S.; Nishimura, R.N.; Gera, J. mTORC2-mediated direct phosphorylation regulates YAP activity promoting glioblastoma growth and invasive characteristics. Neoplasia 2021, 23, 951–965. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wu, H.; Jiang, K.; Wang, Y.; Zhang, W.; Chu, Q.; Li, J.; Huang, H.; Cai, T.; Ji, H. MAPK-mediated YAP activation controls mechanical-tension-induced pulmonary alveolar regeneration. Cell Rep. 2016, 16, 1810–1819. [Google Scholar] [CrossRef]
- Zhu, J.; Wu, T.; Lin, Q. Non-hippo kinases: Indispensable roles in YAP/TAZ signaling and implications in cancer therapy. Mol. Biol. Rep. 2023, 50, 4565–4578. [Google Scholar] [CrossRef]
- Santinon, G.; Pocaterra, A.; Dupont, S. Control of YAP/TAZ activity by metabolic and nutrient-sensing pathways. Trends Cell Biol. 2016, 26, 289–299. [Google Scholar] [CrossRef]
- Yu, B.; van Tol, H.T.; Oei, C.H.; Stout, T.A.; Roelen, B.A. Lysophosphatidic acid accelerates bovine in vitro-produced blastocyst formation through the hippo/YAP pathway. Int. J. Mol. Sci. 2021, 22, 5915. [Google Scholar] [CrossRef]
| Target | Accession Number | Primer Sequence | Primer Efficiency (%) |
|---|---|---|---|
| PPIA | NM_178320.2 | TCTTGTCCATGGCAAATGCTG | 99.29 |
| TTTCACCTTGCCAAAGTACCAC | |||
| MST1 | NM_001075677.2 | CCCACTTGCCTGCTTTACTC | 104.07 |
| GTCGGACAGAAAGCTTCAGG | |||
| MST2 | NM_001079607.2 | AACGGATACAATGGCGAAAC | 102.87 |
| GTTGGTGGTGGGTTTGTAGG | |||
| LATS1 | NM_001192866.1 | CAGCAGCTGCCAGACCTATTA | 103.83 |
| TCCAGCTCTGTTTGCGGTTA | |||
| YAP | XM_015466436.1 | ACGGTGCTTTGACCTAATCG | 104.48 |
| ATGCCACCCAATACAACCAG | |||
| TEAD-4 | XM_059887105.1 | TCCACAGTAAAATGACCCTCACC | 101.131 |
| GTGTAGGTTTGCTGGGAGAAAG | |||
| Cyclin B | NM_001045872.1 | CATGGAAACATCTGGCTGTG | 98.96 |
| TGACTGCTTGCTCTTCCTCA | |||
| CDK1 | L26547.1 | TGTGCTTATGCAGGATTCCA | 99.37 |
| GGCCAAAATCTGCCAACTTA |
| Group | Total n | Oocytes with Polar Body n (%) |
|---|---|---|
| FBS | 305 | 254 (83.2) a |
| FBS + LC 1.5 mM | 312 | 256 (82.0) a |
| FBS + LC 3 mM | 310 | 248 (80.0) a |
| BSA | 286 | 202 (70.6) b |
| Group | Total n | Cleaved Embryos n (%) | Blastocysts n (%) |
|---|---|---|---|
| FBS | 217 | 146 (67.3) a | 69 (31.8) a |
| FBS + LC 1.5 mM | 225 | 156 (69.3) a | 71 (31.6) a |
| BSA | 232 | 133 (57.3) a | 52 (22.4) b |
| 24 h | 48 h | ||||
|---|---|---|---|---|---|
| Group | Total | Re-Expansion n (%) | Hatching n (%) | Re-Expansion n (%) | Hatching n (%) |
| FBS | 52 | 30 (57.7) | 10 (33.3) | 33 (63.5) | 12 (36.4) |
| FBS + LC 1.5 mM | 52 | 41 (78.8) | 15 (36.6) | 43 (82.7) | 17 (39.5) |
| BSA | 50 | 37 (74.0) | 12 (32.4) | 38 (76.0) | 13 (34.2) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salek, F.; Hashem, M.F.; Thundathil, J.C. Effects of L-Carnitine on the Developmental Competence of Bovine Oocytes. Animals 2025, 15, 2576. https://doi.org/10.3390/ani15172576
Salek F, Hashem MF, Thundathil JC. Effects of L-Carnitine on the Developmental Competence of Bovine Oocytes. Animals. 2025; 15(17):2576. https://doi.org/10.3390/ani15172576
Chicago/Turabian StyleSalek, Farzaneh, Mohamed F. Hashem, and Jacob C. Thundathil. 2025. "Effects of L-Carnitine on the Developmental Competence of Bovine Oocytes" Animals 15, no. 17: 2576. https://doi.org/10.3390/ani15172576
APA StyleSalek, F., Hashem, M. F., & Thundathil, J. C. (2025). Effects of L-Carnitine on the Developmental Competence of Bovine Oocytes. Animals, 15(17), 2576. https://doi.org/10.3390/ani15172576






