Sexual Differences in Appendages of a Fossorial Narrow-Mouth Frog, Kaloula rugifera (Anura, Microhylidae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling
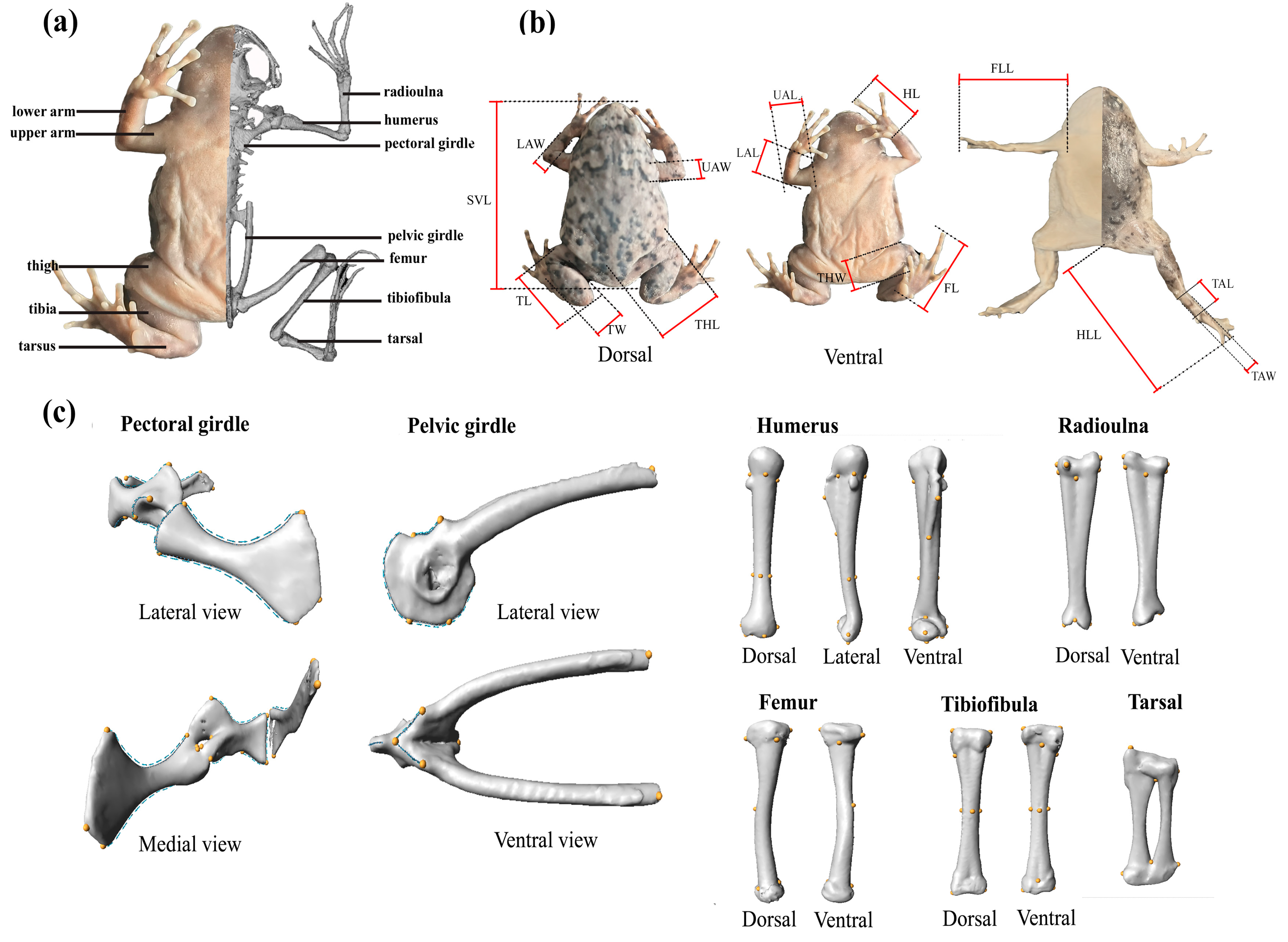
2.2. External Morphometrics Measurements
2.3. MicroCT Scanning
2.4. Geometric Morphometrics Landmarking
2.5. Statistical Analyses
3. Results
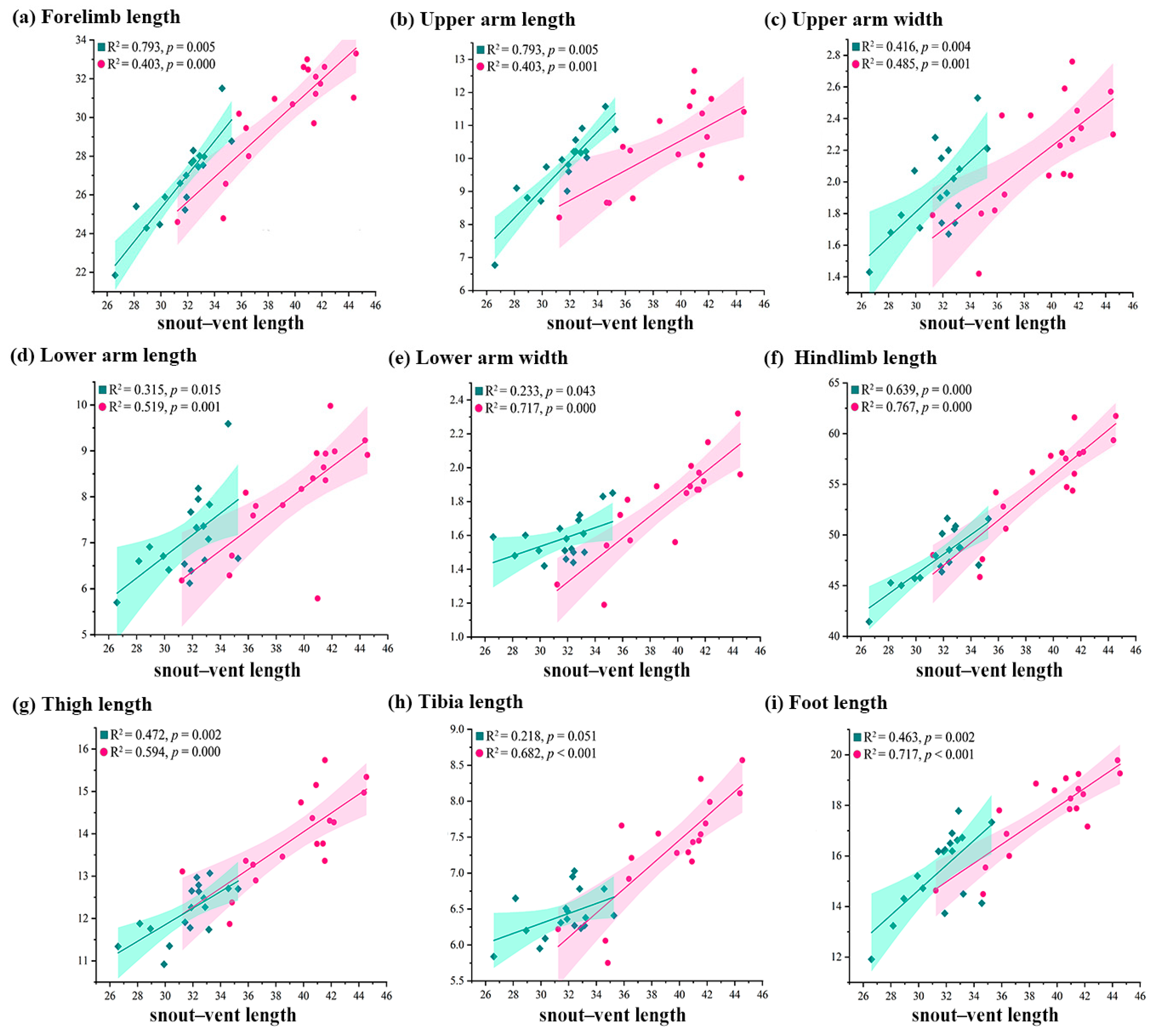
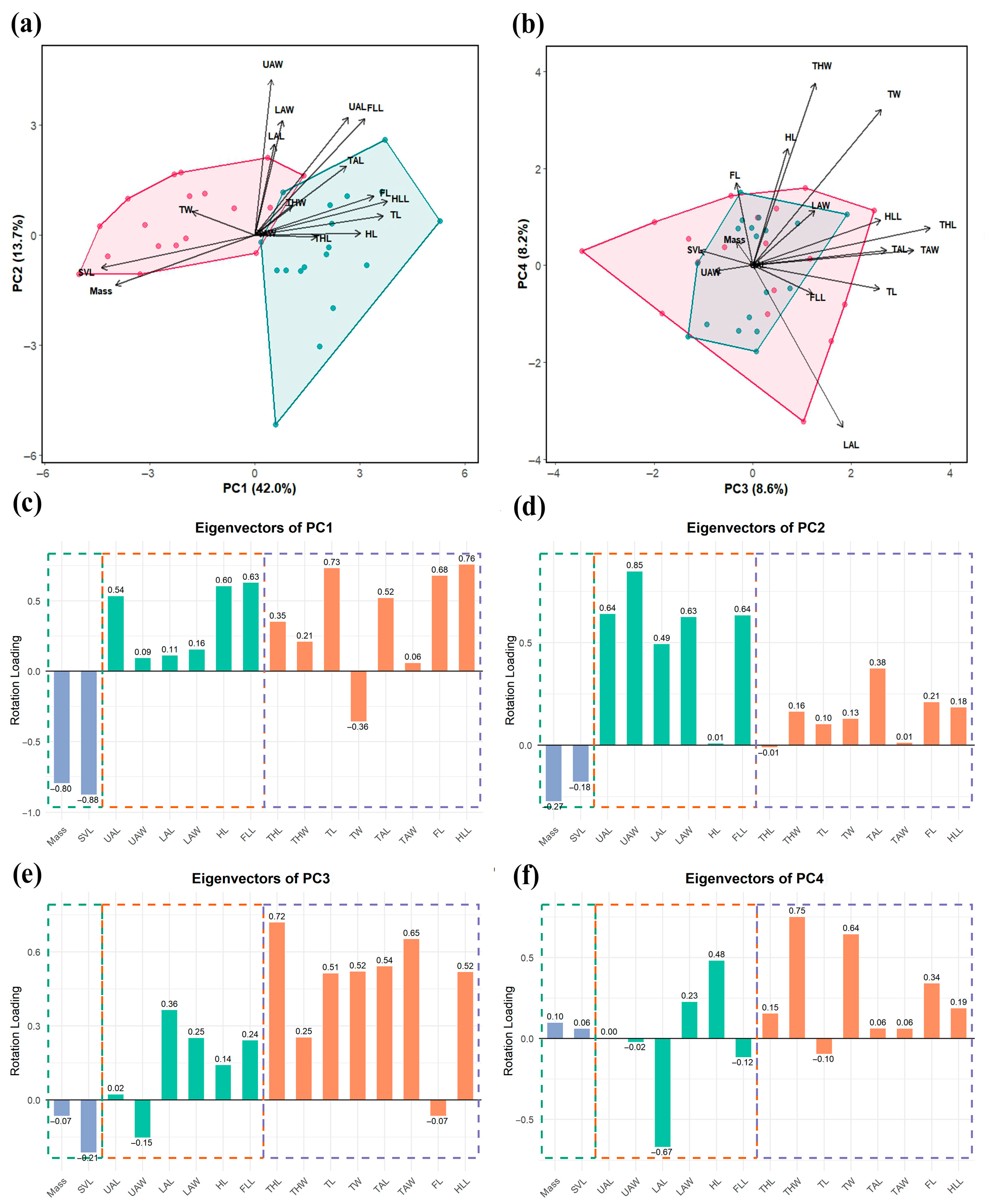
3.1. Comparisons of External Measurements
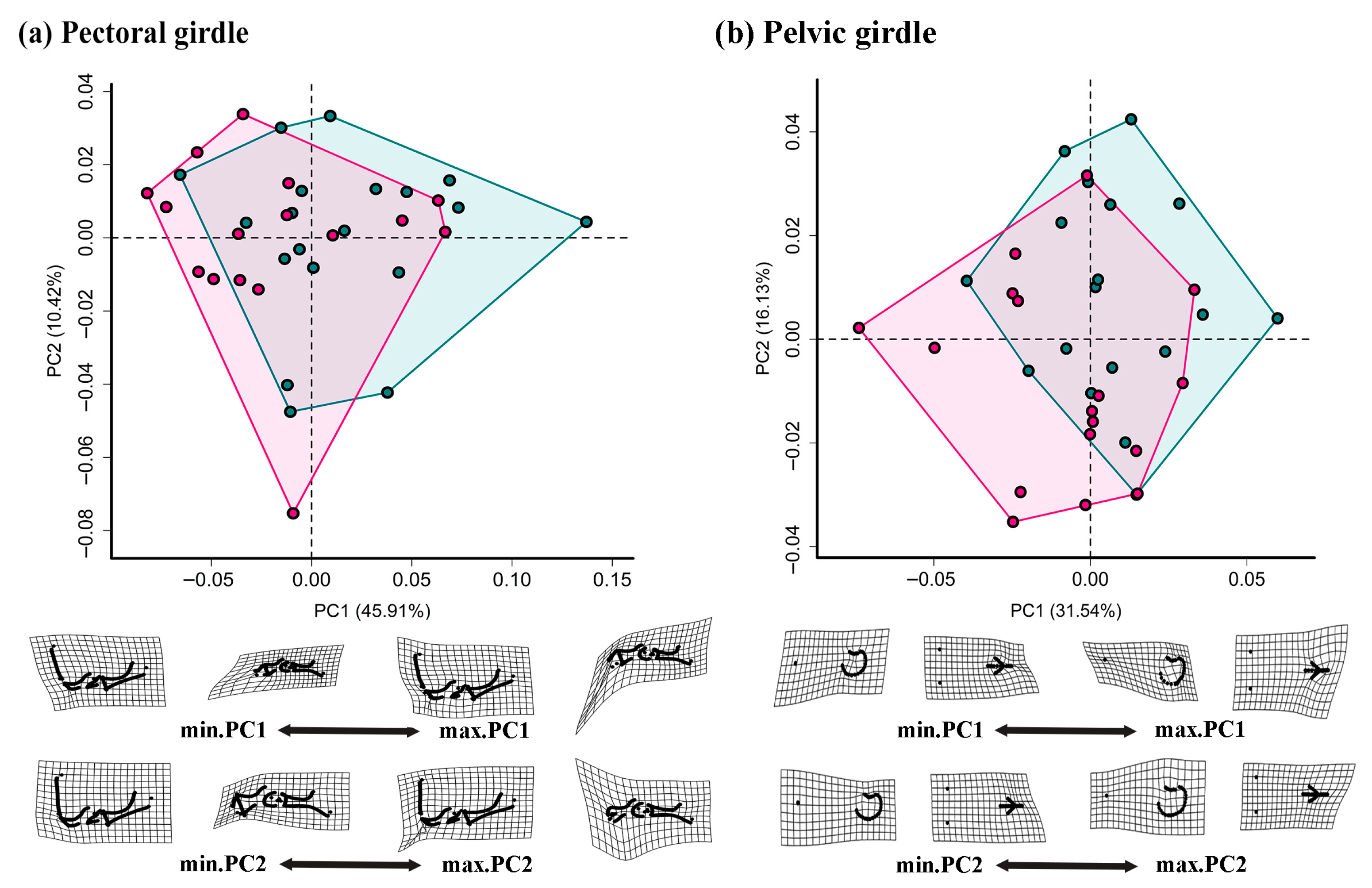
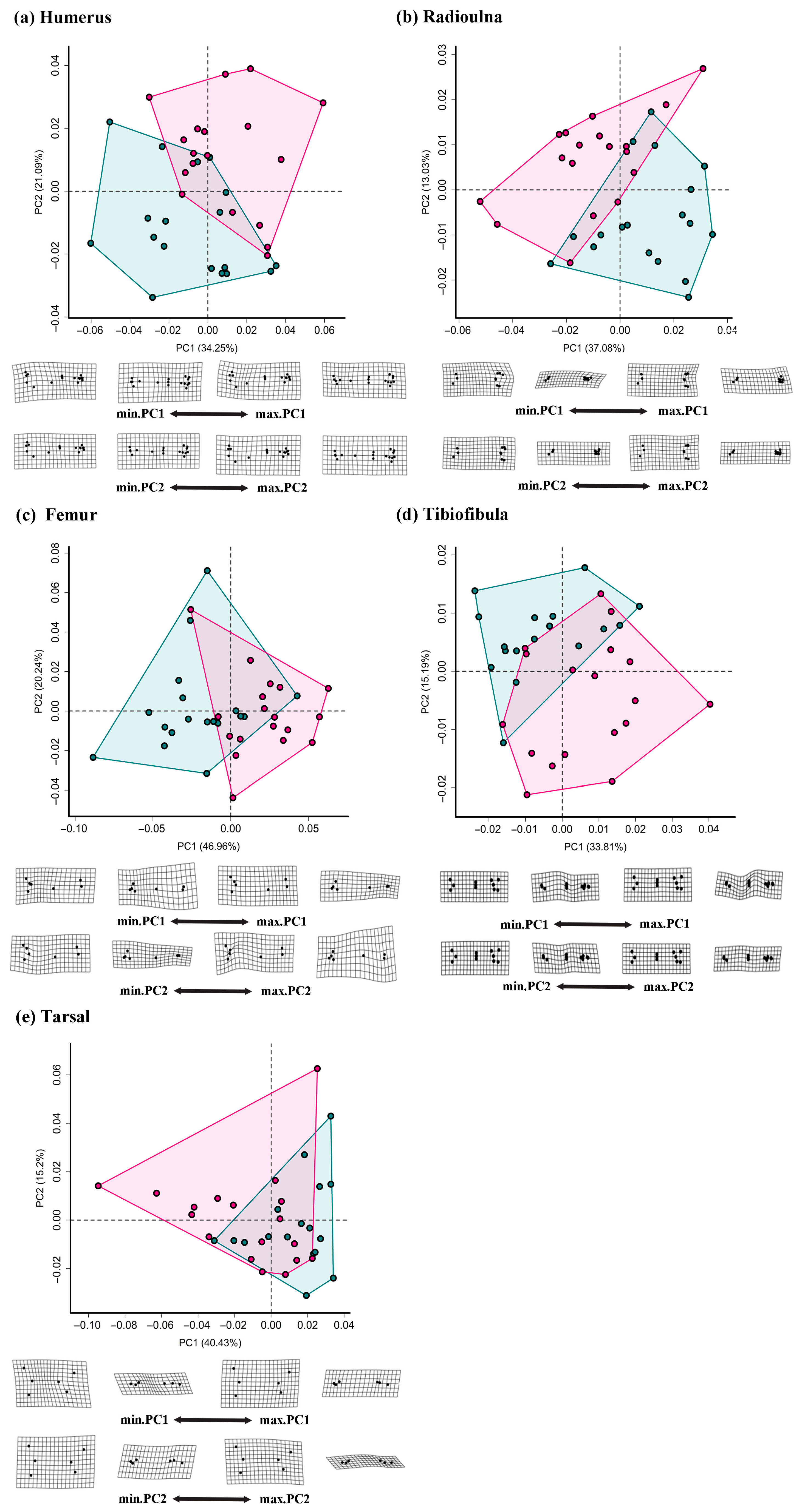
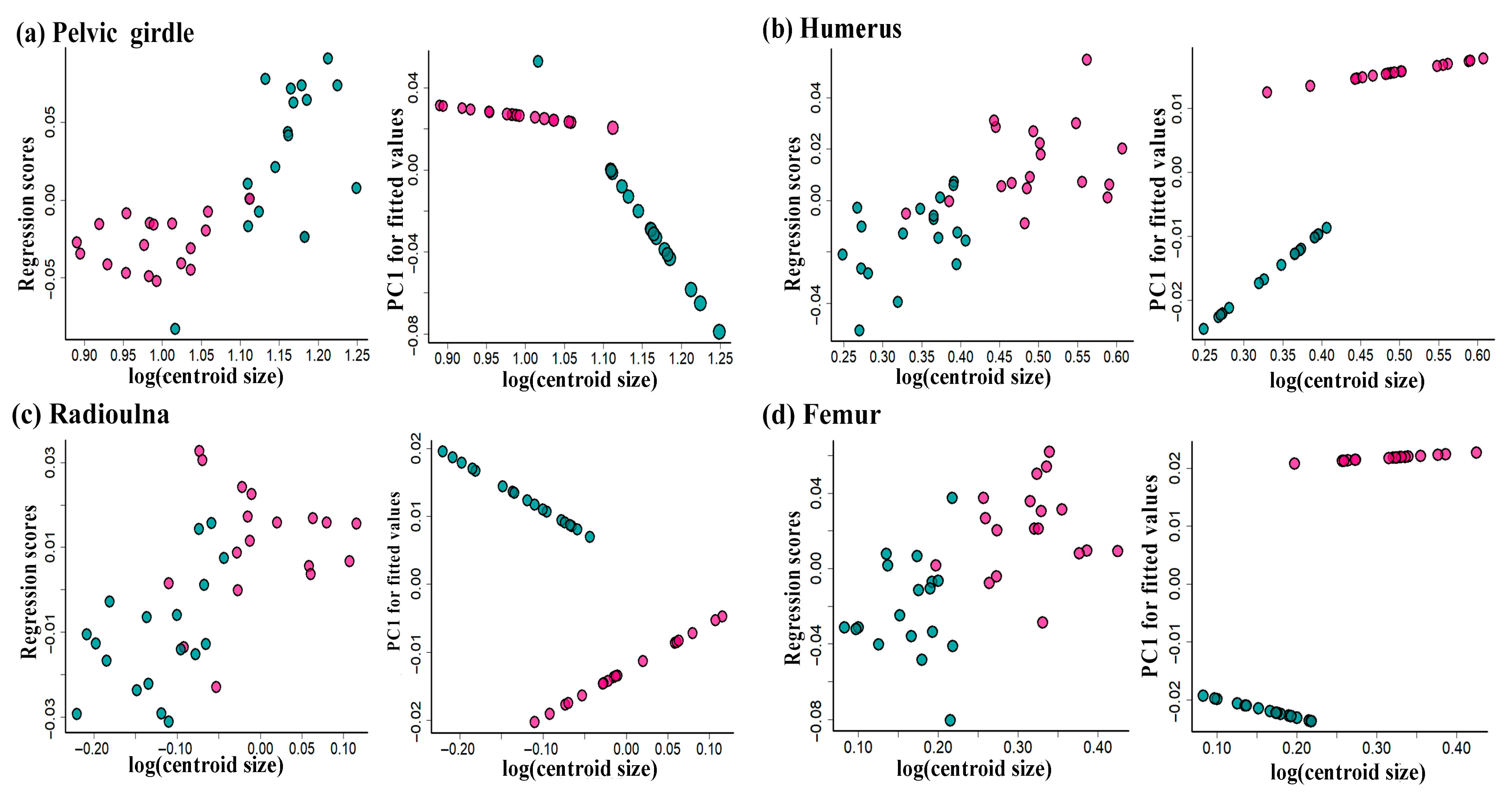
3.2. Comparisons of Girdle and Limb Shape
4. Discussion
4.1. Sexual Size Dimorphism in External Morphology
4.2. Sexual Shape Dimorphism in Appendicular Skeletons
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, M.; Li, Y.; Huang, X.; Gu, Y.; Li, S.; Yin, P.; Zhang, L.; Tang, P. Skeleton-vasculature chain reaction: A novel insight into the mystery of homeostasis. Bone Res. 2021, 9, 21. [Google Scholar] [CrossRef]
- Laurin, M.; Quilhac, A.; De Buffrénil, V. The vertebrate skeleton: A brief introduction. In Vertebrate Skeletal Histology and Paleohistology; Buffrénil, V., Ricqlès, A.J., Zylberberg, L., Padian, K., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 39–58. [Google Scholar]
- Zhang, M.; Zhu, W.; Wang, B.; Wang, S.; Chang, L.; Zhao, T.; Jiang, J. Osteological development of a small and fast metamorphic frog, Microhyla fissipes (Anura, Neobatrachia, Microhylidae). J. Anat. 2021, 239, 1318–1335. [Google Scholar] [CrossRef]
- Law, C.J.; Hlusko, L.J.; Tseng, Z.J. Uncovering the mosaic evolution of the carnivoran skeletal system. Biol. Lett. 2024, 20, 20230526. [Google Scholar] [CrossRef] [PubMed]
- Kongtueng, P.; Piboon, P.; Klinhom, S.; Aunsan, I.; Tongser, N.; Angkawanish, T.; Nganvongpanit, K.; Boonsri, B. Sexual Dimorphism in the Skeletal Morphology of Asian Elephants (Elephas maximus): A Preliminary Morphometric Study of Skull, Scapula, and Pelvis. Biology 2025, 14, 933. [Google Scholar] [CrossRef]
- Di Guida, N.S.; Cassini, G.H. Fractal Dimension and Suture Complexity During Postnatal Ontogeny in Neotropical Deer in Relation to Sexual Dimorphism and Other Biological Features. J. Exp. Zool. Part B 2025. [Google Scholar] [CrossRef] [PubMed]
- Rivera, J.A.; Fuentes-G, J.A.; Martins, E.P. Evolutionary Links Between Skull Shape and Body Size Suggest Allometric Forces and Selection at Work in a Generalist Group of Lizards. Ecol. Evol. 2024, 14, e70594. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, R.A.M.; Folly, M.; Carmo, L.F.; Martins, A. Growing towards disparity: Geometric morphometrics reveals sexual and allometric differences in Aparasphenodon brunoi (Anura: Hylidae: Lophyohylinae) head shape. Cuad. Herpetol. 2020, 34, 5–15. [Google Scholar] [CrossRef]
- Fischer, B.; Mitteroecker, P. Allometry and sexual dimorphism in the human pelvis. Anat. Rec. 2017, 300, 698–705. [Google Scholar] [CrossRef]
- Berns, C.M. The evolution of sexual dimorphism: Understanding mechanisms of sexual shape differences. In Sexual Dimorphism; Moriyama, H., Ed.; IntechOpen: London, UK, 2013; pp. 1–16. [Google Scholar]
- Tereshchenko, V.S. Sexual Dimorphism in the Postcranial Skeleton of Dinosaurs. Paleontol. J. 2020, 54, 1410–1433. [Google Scholar] [CrossRef]
- Soliz, M.C.; Ponssa, M.L.; Abdala, V. Comparative anatomy and development of pectoral and pelvic girdles in hylid anurans. J. Morphol. 2018, 279, 904–924. [Google Scholar] [CrossRef]
- Bi, X.P.; Zhang, G.J. Ancestral developmental potentials in early bony fish contributed to vertebrate water-to-land transition. Zool. Res. 2021, 42, 135. [Google Scholar] [CrossRef] [PubMed]
- Motani, R.; Huang, J.; Jiang, D.Y.; Tintori, A.; Rieppel, O.; You, H.; Hu, Y.C.; Zhang, R. Separating sexual dimorphism from other morphological variation in a specimen complex of fossil marine reptiles (Reptilia, Ichthyosauriformes, Chaohusaurus). Sci. Rep. 2018, 8, 14978. [Google Scholar] [CrossRef]
- Gómez, R.O.; Lires, A.I. High ecomorphological diversity among Early Cretaceous frogs from a large subtropical wetland of Iberia. CR. Palevol. 2019, 18, 711–723. [Google Scholar] [CrossRef]
- Soliz, M.; Tulli, M.J.; Abdala, V. Osteological postcranial traits in hylid anurans indicate a morphological continuum between swimming and jumping locomotor modes. J. Morphol. 2017, 278, 403–417. [Google Scholar] [CrossRef]
- Engelkes, K.; Kath, L.; Kleinteich, T.; Hammel, J.U.; Beerlink, A.; Haas, A. Ecomorphology of the pectoral girdle in anurans (Amphibia, Anura): Shape diversity and biomechanical considerations. Ecol. Evol. 2020, 10, 11467–11487. [Google Scholar] [CrossRef] [PubMed]
- Buttimer, S.M.; Stepanova, N.; Womack, M. Evolution of the unique anuran pelvic and hind limb skeleton in relation to microhabitat, locomotor mode, and jump performance. Integr. Comp. Biol. 2020, 60, 1330–1345. [Google Scholar] [CrossRef]
- Soliz, M.C.; Abdala, V.; Tulli, M.J. The ecological drivers of variation in pectoral girdle anatomy in frogs. Acta. Zool. 2024, 106, 38–54. [Google Scholar] [CrossRef]
- Yan, C.; Ma, H.; Yang, Y.; Mi, Z. Sexual Dimorphism in the Limb Bones of Asiatic Toad (Bufo gargarizans) in Relation to Sexual Selection. Animals 2023, 13, 2638. [Google Scholar] [CrossRef]
- Petrović, T.G.; Vukov, T.D.; Kolarov, N.T. Sexual dimorphism in size and shape of traits related to locomotion in nine anuran species from Serbia and Montenegro. Folia. Zool. 2017, 66, 11–21. [Google Scholar] [CrossRef]
- Leavey, A.; Richards, C.T.; Porro, L.B. Comparative muscle anatomy of the anuran pelvis and hindlimb in relation to locomotor mode. J. Anat. 2024, 245, 751–774. [Google Scholar] [CrossRef]
- Juarez, B.H.; Adams, D.C. Evolutionary allometry of sexual dimorphism of jumping performance in anurans. Evol. Ecol. 2022, 36, 717–733. [Google Scholar] [CrossRef]
- Reilly, S.M.; Jorgensen, M.E. The evolution of jumping in frogs: Morphological evidence for the basal anuran locomotor condition and the radiation of locomotor systems in crown group anurans. J. Morphol. 2011, 272, 149–168. [Google Scholar] [CrossRef]
- Choi, I.; Shim, J.H.; Ricklefs, R.E. Morphometric relationships of take-off speed in anuran amphibians. J. Exp. Biol. A 2003, 299, 99–102. [Google Scholar] [CrossRef]
- Scherz, M.D.; Rakotoarison, A.; Hawlitschek, O.; Vences, M.; Glaw, F. Leaping towards a saltatorial lifestyle? An unusually long-legged new species of Rhombophryne (Anura, Microhylidae) from the Sorata massif in northern Madagascar. Zoosyst. Evol. 2015, 91, 105–114. [Google Scholar] [CrossRef][Green Version]
- Jorgensen, M.; Reilly, S. Phylogenetic patterns of skeletal morphometrics and pelvic traits in relation to locomotor mode in frogs. J. Evolution. Biol. 2013, 26, 929–943. [Google Scholar] [CrossRef]
- Citadini, J.M.; Brandt, R.; Williams, C.R.; Gomes, F.R. Evolution of morphology and locomotor performance in anurans: Relationships with microhabitat diversification. J. Evolution. Biol. 2018, 31, 371–381. [Google Scholar] [CrossRef]
- Vidal-García, M.; Byrne, P.; Roberts, J.; Keogh, J.S. The role of phylogeny and ecology in shaping morphology in 21 genera and 127 species of Australo-Papuan myobatrachid frogs. J. Evolution. Biol. 2014, 27, 181–192. [Google Scholar] [CrossRef]
- Enriquez-Urzelai, U.; Montori, A.; Llorente, G.A.; Kaliontzopoulou, A. Locomotor mode and the evolution of the hindlimb in western Mediterranean anurans. Evol. Biol. 2015, 42, 199–209. [Google Scholar] [CrossRef]
- Keeffe, R.; Blackburn, D.C. Diversity and function of the fused anuran radioulna. J. Anat. 2022, 241, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, R.; Blackburn, D.C. Comparative morphology of the humerus in forward-burrowing frogs. Biol. J. Linn. Soc. 2020, 131, 291–303. [Google Scholar] [CrossRef]
- Moen, D.S. What determines the distinct morphology of species with a particular ecology? The roles of many-to-one mapping and trade-offs in the evolution of frog ecomorphology and performance. Am. Nat. 2019, 194, E81–E95. [Google Scholar] [CrossRef] [PubMed]
- Nauwelaerts, S.; Aerts, P. Take-off and landing forces in jumping frogs. J. Exp. Biol. 2006, 209, 66–77. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Chen, X. Osteology of Quasipaa robertingeri (Anura: Dicroglossidae). Asian Herpetol. Res. 2016, 7, 242–250. [Google Scholar] [CrossRef]
- Blázquez-Orta, R.; Rodríguez, L.; González, M.M.; Estaca-Gómez, V.; De Gaspar, I.; Feranec, R.S.; Carretero, J.M.; Arsuaga, J.L.; García, N. Dogs from the past: Exploring morphology in mandibles from Iberian archaeological sites using 3D geometric morphometrics. J. Archaeol. Sci.-Rep. 2024, 57, 104660. [Google Scholar] [CrossRef]
- Ilić, M.; Jojić, V.; Stamenković, G.; Marković, V.; Simić, V.; Paunović, M.; Crnobrnja-Isailović, J. Geometric vs. traditional morphometric methods for exploring morphological variation of tadpoles at early developmental stages. Amphib.-Reptil. 2019, 40, 499–509. [Google Scholar] [CrossRef]
- Kleisner, K.; Trnka, J.; Tureček, P. FACEDIG automated tool for placing landmarks on facial portraits for geometric morphometrics users. Sci. Rep. 2025, 15, 24330. [Google Scholar] [CrossRef]
- Mitteroecker, P.; Schaefer, K. Thirty years of geometric morphometrics: Achievements, challenges, and the ongoing quest for biological meaningfulness. Am. J. Biol. Anthropol. 2022, 178, 181–210. [Google Scholar] [CrossRef]
- Pogoda, P.; Kupfer, A. Sexual shape dimorphism in the cranium and pelvic girdle of Northern spectacled salamanders, Salamandrina perspicillata, investigated via 3D geometric morphometrics. Salamandra 2020, 56, 113–122. [Google Scholar]
- Nascimento, F.A.C.D.; Vilela, B.; Matias Dubeux, M.J.; Galdino, J.Y.A.; de Araújo-Neto, J.V.; Leal, F.; de Sá, R. Reproductive biology and sexual dimorphism of the poorly known frog Chiasmocleis alagoana (Microhylidae, Gastrophryninae), with an updated diagnosis for the species. Stud. Neotrop. Fauna E. 2022, 57, 96–110. [Google Scholar] [CrossRef]
- Frost, D.R. Amphibian Species of the World: An Online Reference. Version 6.2. Electronic Database; American Museum of Natural History: New York, NY, USA, 2025; Available online: https://amphibiansoftheworld.amnh.org/index.php (accessed on 23 March 2025).
- Gorin, V.A.; Scherz, M.D.; Korost, D.V.; Poyarkov, N.A. Consequences of parallel miniaturisation in Microhylinae (Anura, Microhylidae), with the description of a new genus of diminutive South East Asian frogs. Zoosyst. Evol. 2021, 97, 21–54. [Google Scholar] [CrossRef]
- Emerson, S.B. Functional analysis of frog pectoral girdles. The epicoracoid cartilages. J. Zool. 1983, 201, 293–308. [Google Scholar] [CrossRef]
- De Sá, R.O.; Tonini, J.F.R.; van Huss, H.; Long, A.; Cuddy, T.; Forlani, M.C.; Peloso, P.L.; Zaher, H.; Haddad, C.F. Multiple connections between Amazonia and Atlantic Forest shaped the phylogenetic and morphological diversity of Chiasmocleis Mehely, 1904 (Anura: Microhylidae: Gastrophryninae). Mol. Phylogenet. Evol. 2019, 130, 198–210. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Hu, C.; Ye, C.Y.; Huang, Y.Z. Fauna Sinica, Amphibia, Vol. 2, Anura; Science Press: Beijing, China, 2009. [Google Scholar]
- Chen, W.; Ren, L.; He, D.J.; Wang, Y.; Pike, D. Reproductive ecology of Sichuan digging frogs (Microhylidae: Kaloula rugifera). Acta Herpetol. 2015, 10, 17–21. [Google Scholar] [CrossRef]
- Fei, L.; Ye, C.Y. Observation on the Reproductive Habits of Kaloula rugifera. Chin. J. Zool. 1983, 18, 4–8. [Google Scholar] [CrossRef]
- Zeng, X.M.; Wu, G.F. The Karyotype of Kaloula Rugifera; China Forestry Publishing House: Beijing, China, 1990. [Google Scholar]
- Li, B.; Wang, J.S.; Yuan, S.Q.; Liu, N.; Rao, D.Q.; Liang, H.; Wang, X.R. Study on the Phylogeny of Kaloula (Microhylidae, Amphibia) Based on the Mitochondrial Gene. J. W. China Forest. Sci. 2012, 41, 51–55. [Google Scholar] [CrossRef]
- Chen, W.; Yang, P.; Fu, Z.; Wen, L.; Guan, T.; Jia, X.; Liu, H.; You, Z. Advertisement Call Analysis of Kaloula rugifera. Mianyang Norm. Univ. 2017, 36, 23–26. [Google Scholar] [CrossRef]
- Torreilles, S.L.; McClure, D.E.; Green, S.L. Evaluation and refinement of euthanasia methods for Xenopus laevis. J. Am. Assoc. Lab. Anim. 2009, 48, 512–516. [Google Scholar] [CrossRef]
- Underwood, W.; Anthony, R. AVMA Guidelines for the Euthanasia of Animals: 2020 Edition. 2020. Available online: https://www.avma.org/sites/default/files/2020-02/Guidelines-on-Euthanasia-2020.pdf (accessed on 27 August 2025).
- Watters, J.L.; Cummings, S.T.; Flanagan, R.L.; Siler, C.D. Review of morphometric measurements used in anuran species descriptions and recommendations for a standardized approach. Zootaxa 2016, 4072, 477–495. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, X.; Ye, C.; Fei, L.; Li, P.; Jiang, J.; Wang, B. Osteology of the Asian narrow-mouth toad Kaloula borealis (Amphibia, Anura, Microhylidae) with comments on its osteological adaptation to fossorial life. Acta Zool. 2020, 101, 366–383. [Google Scholar] [CrossRef]
- Stepanova, N.; Womack, M.C. Anuran limbs reflect microhabitat and distal, later-developing bones are more evolutionarily labile. Evolution 2020, 74, 2005–2019. [Google Scholar] [CrossRef]
- Adams, D.; Collyer, M.; Kaliontzopoulou, A. Geometric morphometric Analyses of 2D/3D Landmark Data. R Package Version 4.0.10. 2020. Available online: https://cran.r-project.org/package=geomorph (accessed on 27 August 2025).
- Dursun, C.; Gül, S.; Özdemir, N.J.A.H. Age estimation and body size of the Parsley Frog, Pelodytes caucasicus Boulenger, 1896 from Lake Borçka Karagöl, Turkey. Acta Herpetol. 2022, 18, 11–22. [Google Scholar] [CrossRef]
- Başkale, E.; Ulubeli, S.A.; Kaska, Y. Age structures and growth parameters of the Levantine frog, Pelophylax bedriagae, at different localities in Denizli, Turkey. Acta Herpetol. 2018, 13, 147–154. [Google Scholar] [CrossRef]
- Pincheira-Donoso, D.; Harvey, L.P.; Grattarola, F.; Jara, M.; Cotter, S.C.; Tregenza, T.; Hodgson, D.J. The multiple origins of sexual size dimorphism in global amphibians. Glob. Ecol. Biogeogr. 2021, 30, 443–458. [Google Scholar] [CrossRef]
- Nali, R.C.; Zamudio, K.R.; Haddad, C.F.; Prado, C.P. Size-dependent selective mechanisms on males and females and the evolution of sexual size dimorphism in frogs. Am. Nat. 2014, 184, 727–740. [Google Scholar] [CrossRef]
- Li, H.; Chen, S.; Jiang, J.; He, B.; Zhang, M. Exploring sexual differences in external morphology and limb muscles of Hylarana guentheri (Anura: Ranidae) during non-breeding season. Acta Zool. 2023, 104, 647–656. [Google Scholar] [CrossRef]
- Gillooly, J.F.; Brown, J.H.; West, G.B.; Savage, V.M.; Charnov, E.L. Effects of size and temperature on metabolic rate. Science 2001, 293, 2248–2251. [Google Scholar] [CrossRef] [PubMed]
- Wells, K.D. The Ecology and Behavior of Amphibians; University of Chicago Press: Chicago, IL, USA, 2019. [Google Scholar]
- Bowcock, H.; Brown, G.P.; Shine, R. Sexual selection in cane toads Rhinella marina: A male’s body size affects his success and his tactics. Curr. Zool. 2013, 59, 747–753. [Google Scholar] [CrossRef]
- Emerson, S.B. A biomechanical perspective on the use of forelimb length as a measure of sexual selection in frogs. J. Evol. Biol. 1991, 4, 671–678. [Google Scholar] [CrossRef]
- Dursun, C.; Gül, S.; Özdemir, N. Sexual size and shape dimorphism in Turkish common toads (Bufo bufo Linnaeus 1758). Anat. Rec. 2022, 305, 1548–1558. [Google Scholar] [CrossRef]
- Herrel, A.; Gonwouo, L.; Fokam, E.; Ngundu, W.; Bonneaud, C. Intersexual differences in body shape and locomotor performance in the aquatic frog, Xenopus tropicalis. J. Zool. 2012, 287, 311–316. [Google Scholar] [CrossRef]
- Clarke, G.S.; Shine, R.; Phillips, B.L. May the (selective) force be with you: Spatial sorting and natural selection exert opposing forces on limb length in an invasive amphibian. J. Evol. Biol. 2019, 32, 994–1001. [Google Scholar] [CrossRef]
- Haddad, C.F.; Bastos, R.P. Predation on the toad Bufo crucifer during reproduction (Anura: Bufonidae). Amphib.-Reptil. 1997, 18, 295–298. [Google Scholar] [CrossRef]
- Juarez, B.H.; Moen, D.S.; Adams, D.C. Ecology, sexual dimorphism, and jumping evolution in anurans. J. Evol. Biol. 2023, 36, 829–841. [Google Scholar] [CrossRef]
- Klank, J.; Protti-Sánchez, F.; Mora-Rojas, P.; Rowland, H.M.; Stynoski, J.L. How to move and when to escape: Quantifying intraspecific exploratory and anti-predator behavior in an aposematic poison frog. Evol. Ecol. 2024, 38, 175–192. [Google Scholar] [CrossRef]
- Beer, R.D.; Chiel, H.J. Locomotion, invertebrate. In The Hand Book of Brain Theory and Neural Networks; MIT Press: Cambridge, MA, USA, 2003; pp. 553–556. [Google Scholar]
- Collings, A.J.; Richards, C.T. Digital dissection of the pelvis and hindlimb of the red-legged running frog, Phlyctimantis maculatus, using diffusible iodine contrast enhanced computed microtomography (DICE μCT). PeerJ 2019, 7, e7003. [Google Scholar] [CrossRef] [PubMed]
- Sherman, M.A.; Seth, A.; Delp, S.L. What is a moment arm? Calculating muscle effectiveness in biomechanical models using generalized coordinates. Proc. Asme Des. Eng. Tech. Conf. 2013, 2013, V07BT10A052. [Google Scholar] [CrossRef]
- Griep, S.; Schilling, N.; Marshall, P.; Amling, M.; Hahne, L.M.; Haas, A. Pectoral girdle movements and the role of the glenohumeral joint during landing in the toad, Rhinella marina (Linnaeus, 1758). Zoomorphology 2013, 132, 325–338. [Google Scholar] [CrossRef]
- Přikryl, T.; Aerts, P.; Havelková, P.; Herrel, A.; Roček, Z. Pelvic and thigh musculature in frogs (Anura) and origin of anuran jumping locomotion. J. Anat. 2009, 214, 100–139. [Google Scholar] [CrossRef]
- Gaitonde, N.; Giri, V.; Kunte, K. ‘On the rocks’: Reproductive biology of the endemic toad Xanthophryne (Anura: Bufonidae) from the Western Ghats, India. J. Nat. Hist. 2016, 50, 2557–2572. [Google Scholar] [CrossRef]
- Gosnell, W.C.; Butcher, M.T.; Maie, T.; Blob, R.W. Femoral loading mechanics in the Virginia opossum, Didelphis virginiana: Torsion and mediolateral bending in mammalian locomotion. J. Exp. Biol. 2011, 214, 3455–3466. [Google Scholar] [CrossRef][Green Version]
- Wilson, M.P.; Espinoza, N.R.; Shah, S.R.; Blob, R.W. Mechanical properties of the hindlimb bones of bullfrogs and cane toads in bending and torsion. Anat. Rec. 2009, 292, 935–944. [Google Scholar] [CrossRef]
- Butcher, M.T.; Blob, R.W. Mechanics of limb bone loading during terrestrial locomotion in river cooter turtles (Pseudemys concinna). J. Exp. Biol. 2008, 211, 1187–1202. [Google Scholar] [CrossRef]
- Vera, M.C.; Ferretti, J.L.; Abdala, V.; Cointry, G.R. Biomechanical properties of anuran long bones: Correlations with locomotor modes and habitat use. J. Anat. 2020, 236, 1112–1125. [Google Scholar] [CrossRef] [PubMed]
- Bonneau, N.; Simonis, C.; Seringe, R.; Tardieu, C. Study of femoral torsion during prenatal growth: Interpretations associated with the effects of intrauterine pressure. Am. J. Phys. Anthropol. 2011, 145, 438–445. [Google Scholar] [CrossRef]
- Young, L.A.; Munro, E.; Somanchi, P.; Bemis, A.; Smith, S.M.; Shefelbine, S.J. Analysis of bone structure in PEROMYSCUS: Effects of burrowing behavior. Anat. Rec. 2024, 307, 3506–3518. [Google Scholar] [CrossRef]
- Durão, A.F.; Muñoz-Muñoz, F.; Ventura, J. Three-dimensional geometric morphometric analysis of the humerus: Comparative postweaning ontogeny between fossorial and semiaquatic water voles (Arvicola). J. Morphol. 2020, 281, 1679–1692. [Google Scholar] [CrossRef] [PubMed]
- Ponssa, M.L.; Medina, R.G. Comparative morphometrics in leptodactyline frogs (Anura, Leptodactylidae, Leptodactylinae): Does burrowing behavior relate to sexual dimorphism? J. Herpetol. 2016, 50, 604–615. [Google Scholar] [CrossRef]
- Nomura, F.; Rossa-Feres, D.C.; Langeani, F. Burrowing behavior of Dermatonotus muelleri (Anura, Microhylidae) with reference to the origin of the burrowing behavior of Anura. J. Ethol. 2009, 27, 195–201. [Google Scholar] [CrossRef]
- Mendoza, E.; Azizi, E.; Moen, D.S. What explains vast differences in jumping power within a clade? Diversity, ecology and evolution of anuran jumping power. Funct. Ecol. 2020, 34, 1053–1063. [Google Scholar] [CrossRef]
- Lewton, K.L.; Brankovic, R.; Byrd, W.A.; Cruz, D.; Morales, J.; Shin, S. The effects of phylogeny, body size, and locomotor behavior on the three-dimensional shape of the pelvis in extant carnivorans. PeerJ 2020, 8, e8574. [Google Scholar] [CrossRef]
| Measurements | Levene’s Test | Mean ± Standard Deviation | t | p | ||
|---|---|---|---|---|---|---|
| F | p | Male | Female | |||
| Snout–vent length | 6.495 | 0.016 | 31.661 ± 2.181 | 39.315 ± 3.644 | 7.647 | <0.001 |
| Body mass | 17.362 | <0.001 | 3.207 ± 0.684 | 6.801 ± 1.994 | 7.232 | <0.001 |
| External Morphology of Limbs (standardized to SVL) | ||||||
| Upper arm length | 3.092 | 0.088 | 0.309 ± 0.019 | 0.264 ± 0.025 | −6.041 | <0.001 |
| Upper arm width | 0.025 | 0.876 | 0.061 ± 0.007 | 0.055 ± 0.007 | −2.706 | 0.011 |
| Lower arm length | 1.240 | 0.273 | 0.224 ± 0.023 | 0.204 ± 0.020 | −2.724 | 0.010 |
| Lower arm width | 0.018 | 0.894 | 0.050 ± 0.004 | 0.046 ± 0.004 | −3.188 | 0.003 |
| Hand length | 4.787 | 0.036 | 0.312 ± 0.016 | 0.302 ± 0.022 | −1.533 | 0.135 |
| Forelimb length | 0.768 | 0.387 | 0.845 ± 0.030 | 0.771 ± 0.037 | −6.590 | 0.000 |
| Thigh length | 0.344 | 0.561 | 0.434 ± 0.036 | 0.413 ± 0.026 | −1.938 | 0.061 |
| Thigh width | 0.628 | 0.434 | 0.101 ± 0.008 | 0.098 ± 0.009 | −0.972 | 0.338 |
| Tibia length | 0.009 | 0.924 | 0.386 ± 0.020 | 0.355 ± 0.023 | −4.330 | <0.001 |
| Tibia width | 0.004 | 0.948 | 0.075 ± 0.009 | 0.079 ± 0.009 | 1.063 | 0.295 |
| Tarsus length | 0.126 | 0.725 | 0.203 ± 0.013 | 0.187 ± 0.012 | −3.936 | <0.001 |
| Tarsus width | 0.641 | 0.429 | 0.063 ± 0.008 | 0.060 ± 0.004 | −1.456 | 0.155 |
| Foot length | 2.596 | 0.116 | 0.488 ± 0.035 | 0.451 ± 0.023 | −3.787 | 0.001 |
| Hindlimb length | 0.251 | 0.620 | 1.511 ± 0.061 | 1.405 ± 0.066 | −4.968 | <0.001 |
| Skeletons | Parameters | R2 | F | p |
|---|---|---|---|---|
| Pectoral girdle | sex | 0.100 | 1.787 | 0.084 |
| size | 0.050 | 1.440 | 0.179 | |
| sex: size | 0.023 | 0.825 | 0.468 | |
| Pelvic girdle | sex | 0.122 | 4.729 | 0.001 |
| size | 0.053 | 2.181 | 0.023 | |
| sex: size | 0.051 | 2.098 | 0.024 | |
| Humerus | sex | 0.134 | 5.689 | <0.001 |
| size | 0.030 | 2.344 | 0.034 | |
| sex: size | 0.055 | 2.352 | 0.036 | |
| Radioulna | sex | 0.058 | 2.432 | 0.023 |
| size | 0.045 | 1.919 | 0.064 | |
| sex: size | 0.140 | 5.531 | 0.001 | |
| Femur | sex | 0.205 | 8.757 | <0.001 |
| size | 0.037 | 1.688 | 0.118 | |
| sex: size | 0.060 | 2.726 | 0.023 | |
| Tibiofibula | sex | 0.088 | 3.284 | <0.001 |
| size | 0.031 | 1.184 | 0.278 | |
| sex: size | 0.047 | 1.813 | 0.051 | |
| Tarsal | sex | 0.124 | 4.822 | 0.001 |
| size | 0.091 | 3.791 | 0.002 | |
| sex: size | 0.014 | 0.594 | 0.743 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Wang, X.; Huang, J.; Wang, X.; Wang, B.; Jiang, J.; Dong, B.; Zhang, M. Sexual Differences in Appendages of a Fossorial Narrow-Mouth Frog, Kaloula rugifera (Anura, Microhylidae). Animals 2025, 15, 2566. https://doi.org/10.3390/ani15172566
Zhang W, Wang X, Huang J, Wang X, Wang B, Jiang J, Dong B, Zhang M. Sexual Differences in Appendages of a Fossorial Narrow-Mouth Frog, Kaloula rugifera (Anura, Microhylidae). Animals. 2025; 15(17):2566. https://doi.org/10.3390/ani15172566
Chicago/Turabian StyleZhang, Wenyi, Xianzheng Wang, Jin Huang, Xiuping Wang, Bin Wang, Jianping Jiang, Bingjun Dong, and Meihua Zhang. 2025. "Sexual Differences in Appendages of a Fossorial Narrow-Mouth Frog, Kaloula rugifera (Anura, Microhylidae)" Animals 15, no. 17: 2566. https://doi.org/10.3390/ani15172566
APA StyleZhang, W., Wang, X., Huang, J., Wang, X., Wang, B., Jiang, J., Dong, B., & Zhang, M. (2025). Sexual Differences in Appendages of a Fossorial Narrow-Mouth Frog, Kaloula rugifera (Anura, Microhylidae). Animals, 15(17), 2566. https://doi.org/10.3390/ani15172566







