Effect of L-Carnitine on Muscle Quality and Antioxidant Capacity of Hybrid Sheep at an Early Stage
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Trials and Ration
2.2. Collection and Documentation of Samples
2.3. Sample Laboratory Analysis
2.3.1. Quantification of L-Carnitine in Blood
2.3.2. Carcass Performance Assessment
2.3.3. Determination of Meat Quality in the Longissimus dorsi Muscle
2.3.4. Evaluation of Myoglobin Levels in Various Muscle Regions Through Coloration of the Longissimus dorsi Muscle
2.3.5. Measurement of the Longissimus dorsi Muscle Oxygenation Index
2.3.6. Measurement of Gene Expression Associated with Flesh Color and Oxidation in the Longissimus Dorsi Muscle
2.3.7. Statistical Analysis
3. Results
3.1. Effect of Dietary L-Carnitine Supplementation on the L-Carnitine Concentration in the Blood of Sheep
3.2. Effect of L-Carnitine in the Diet on the Growth of Sheep
3.3. Effect of L-Carnitine Supplementation in the Diet of Sheep on Carcass Traits and Meat Quality
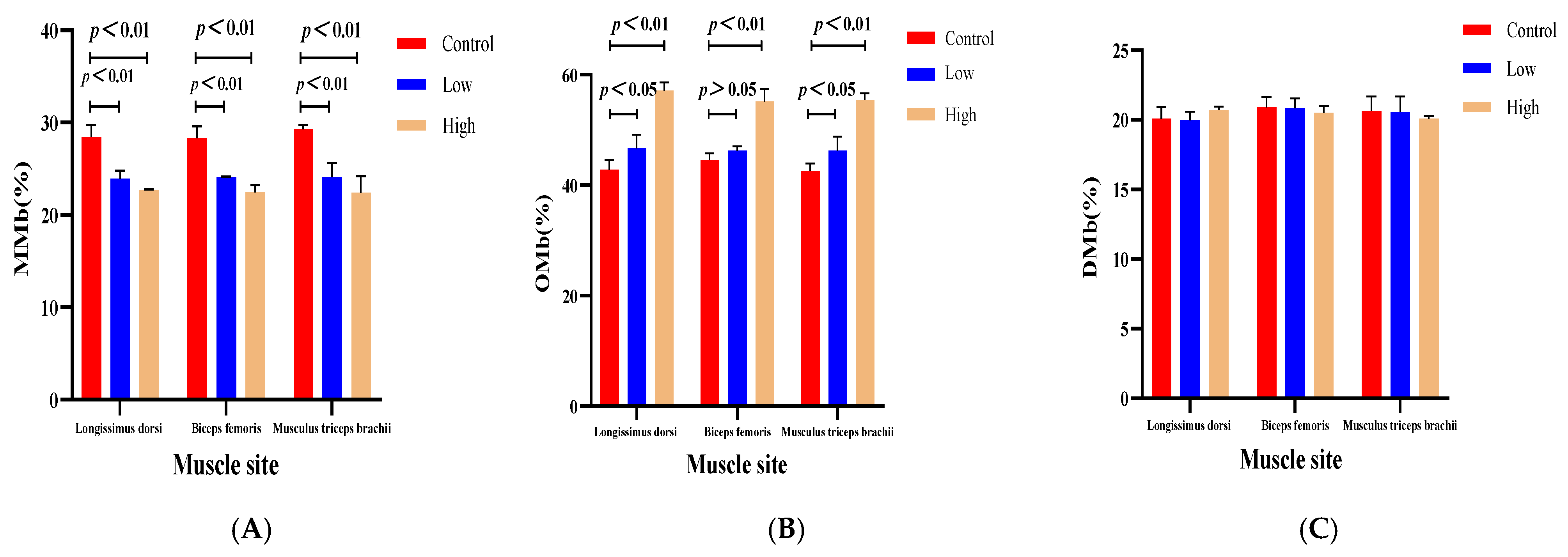
3.4. The Impact of Dietary L-Carnitine Supplementation on the Meat Pigmentation of Various Muscle Segments in Sheep
3.5. Effects of Dietary Inclusion of L-Carnitine on the Oxidation Reduction Status of the Longissimus Dorsi Muscle in Hybrid Sheep
3.6. The Relationship Between Concentrations of L-Carnitine (45 d) and Biochemical Indicators
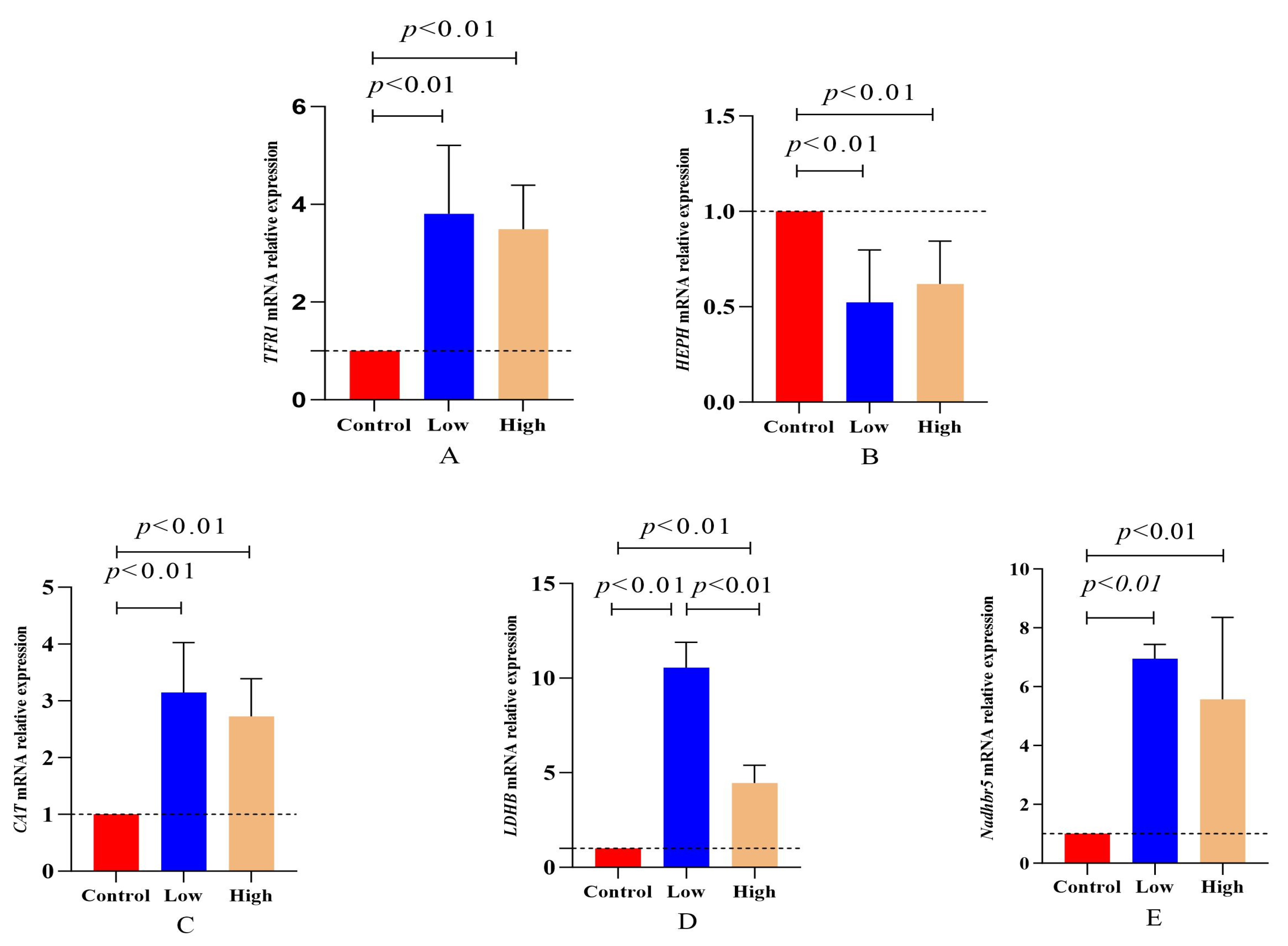
3.7. Assessment of mRNA Expression Levels of Genes Involved in Oxidation Reduction and Meat Color Formation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TMR | Total mixed ration |
| DM | Dry matter |
| DDGS | Distillers dried grains with solubles |
| ADFI | Average daily feed intake |
| ADG | Average daily gain |
| F/G | Feed-to-gain ratio |
| GR | Weight-adjusted fatness value |
| OMb | Oxymyoglobin |
| MMb | Metmyoglobin |
| DMb | Deoxymyoglobin |
| MRA | Metmyoglobin reductase activity |
| SOD | Superoxide dismutase |
| LDH | Lactate dehydrogenase |
| LDHB | Lactate dehydrogenase-B |
| NADHB5R | Nadh-cytochrome b5 reductase |
| H2O2 | Hydrogen peroxide |
| CAT | Catalase |
| ROS | Reactive oxygen species |
| TRF1 | Transferrin receptor 1 |
| HEPH | Hephaestin |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
References
- Fielding, R.; Riede, L.; Lugo, J.P.; Bellamine, A. l-Carnitine Supplementation in Recovery after Exercise. Nutrients 2018, 10, 349. [Google Scholar] [CrossRef]
- Ross, N.S.; Hoppel, C.L. Partial Muscle Carnitine Palmitoyltransferase-A Deficiency: Rhabdomyolysis Associated with Transiently Decreased Muscle Carnitine Content After Ibuprofen Therapy. JAMA 1987, 257, 62–65. [Google Scholar] [CrossRef]
- Neumann-Schmidt, S.; Zierz, S. Carnitine acyltransferases in normal human skeletal muscle and in muscle of patients with carnitine palmitoyltransferase deficiency. Neuromuscul. Disord. 1991, 1, 253–260. [Google Scholar] [CrossRef]
- Zammit, V.A. Carnitine acyltransferases: Functional significance of subcellular distribution and membrane topology. Prog. Lipid Res. 1999, 38, 199–224. [Google Scholar] [CrossRef]
- Li, L.; Limbu, S.; Ma, Q.; Chen, L.; Zhang, M.; Du, Z. The metabolic regulation of dietary L-carnitine in aquaculture nutrition: Present status and future research strategies. Rev. Aquac. 2018, 11, 1228–1257. [Google Scholar] [CrossRef]
- Li, L.; Lu, D.; Jiang, Z.; Limbu, S.; Qiao, F.; Chen, L.; Zhang, M.; Du, Z. Dietary L-carnitine improves glycogen and protein accumulation in Nile tilapia via increasing lipid-sourced energy supply: An isotope-based metabolic tracking. Aquac. Rep. 2020, 17, 100302. [Google Scholar] [CrossRef]
- Yang, S.-D.; Wen, Y.-C.; Liou, C.-H.; Liu, F.-G. Influence of dietary l-carnitine on growth, biological traits and meat quality in Tilapia. Aquac. Res. 2009, 40, 1374–1382. [Google Scholar] [CrossRef]
- Chen, X.; Niu, J.; Wang, J.; Zhao, W. Effects of L-carnitine Supplementation in High-Fat Diet on Growth, Antioxidant Capacity and Lipid Metabolism of Golden Pompano (Trachinotus ovatus). Front. Mar. Sci. 2022, 9, 831153. [Google Scholar] [CrossRef]
- Victor, H.; Liu, Y.; Limbu, S.M.; Wang, Y. Dietary L-carnitine and nucleotides synergistically enhance growth performance, feed utilization, and antioxidant capacity of largemouth bass (Micropterus salmoides) juvenile fed a high-fat diet. Aquac. Int. 2024, 32, 4737–4756. [Google Scholar] [CrossRef]
- Victor, H.; Lei, M.; Liu, Y.; Limbu, S.M.; Wang, Y. Effect of L-carnitine supplementation on growth performance, feed utilization, body composition, and antioxidant capacity of large yellow croaker, Larimichthys crocea, fed at two dietary lipid levels. Aquac. Int. 2025, 33, 63. [Google Scholar] [CrossRef]
- Khatibjoo, A.; Nooreh, Z.; Fattahnia, F.; Akbari-Gharaei, M. Effects of L-Carnitine and Butyric Acid on Carcass Characteristics and Meat Quality of Broiler Chickens. Res. Anim. Prod. 2018, 8, 1–10. [Google Scholar] [CrossRef][Green Version]
- Akhoondzadeh, H.; Bouyeh, M.; Paz, E.; Seidavi, A.; Vlčková, R. The effect of dietary L-carnitine and fat on performance, carcass traits and blood components in broiler chickens. Anim. Sci. Pap. Rep. 2023, 41, 111–122. [Google Scholar] [CrossRef]
- Lösel, D.; Rehfeldt, C. Effects of l-carnitine supplementation to suckling piglets on carcass and meat quality at market age. Animal 2013, 7, 1191–1198. [Google Scholar] [CrossRef]
- Apple, J.; Sawyer, J.; Maxwell, C.; Yancey, J.; Frank, J.; Woodworth, J.; Musser, R. Effects of L-carnitine supplementation on quality characteristics of fresh pork bellies from pigs fed 3 levels of corn oil1. J. Anim. Sci. 2011, 89, 2878–2891. [Google Scholar] [CrossRef]
- Madsen, J.; Kreuzer, M.; Silacci, P.; Bee, G. Effect of sex and milk replacer with or without supplemental carnitine and arginine on growth characteristics, carcass, and meat quality of artificially reared low-birth weight pigs. J. Anim. Sci. 2024, 102, skae122. [Google Scholar] [CrossRef]
- Kunimura, K.; Nakamoto, M.; Ushijima, M. S-1-Propenylcysteine Enhances Endurance Capacity of Mice by Stimulating Fatty Acid Metabolism via Muscle Isoform of Carnitine Acyltransferase-1. J. Nutr. 2024, 154, 2707–2716. [Google Scholar] [CrossRef]
- Wafa, W.M.; El-Nagar, H.A. Reproductive efficiency, milk production, health status, antioxidant capacity, lipid profile, and metabolic hormones of lactating cows treated with coenzyme q10 and l-carnitine.h coenzyme q10 and l-carnitine. J. Anim. Health Prod. 2021, 9, 380–390. [Google Scholar] [CrossRef]
- Martín, A.; Giráldez, F.J.; Mateo, J.; Caro, I.; Andrés, S. Dietary administration of l-carnitine during the fattening period of early feed restricted lambs modifies lipid metabolism and meat quality. Meat Sci. 2023, 198, 109111. [Google Scholar] [CrossRef]
- Liu, G.; Ding, Y.; Chen, Y.; Yang, Y. Effect of energy intake and L-carnitine on fattening performance, carcass traits, meat quality, blood metabolites, and gene expression of lamb. Small Rumin. Res. 2020, 183, 106025. [Google Scholar] [CrossRef]
- Delgado, J.; Ansorena, D.; Van Hecke, T.; Astiasarán, I.; De Smet, S.; Estévez, M. Meat lipids, NaCl and carnitine: Do they unveil the conundrum of the association between red and processed meat intake and cardiovascular diseases?—Invited Review. Meat Sci. 2021, 171, 108278. [Google Scholar] [CrossRef]
- Holloway, G.; Bézaire, V.; Heigenhauser, G.; Tandon, N.; Glatz, J.; Luiken, J.; Bonen, A.; Spriet, L. Mitochondrial long chain fatty acid oxidation, fatty acid translocase/CD36 content and carnitine palmitoyltransferase I activity in human skeletal muscle during aerobic exercise. J. Physiol. 2006, 571, 201–210. [Google Scholar] [CrossRef]
- Dobbins, R.; Szczepaniak, L.; Bentley, B.; Esser, V.; Myhill, J.; McGarry, J. Prolonged Inhibition of Muscle Carnitine Palmitoyltransferase-1 Promotes Intramyocellular Lipid Accumulation and Insulin Resistance in Rats. Diabetes 2001, 50, 123–130. [Google Scholar] [CrossRef]
- Lheureux, P.; Hantson, P. Carnitine in the treatment of valproic acid-induced toxicity. Clin. Toxicol. 2009, 47, 101–111. [Google Scholar] [CrossRef]
- Yano, J.; Kaida, Y.; Nakayama, Y.; Ito, S.; Kurokawa, Y.; Nakamura, N.; Hazama, T.; Maeda, T.; Hashida, R.; Tashiro, K.; et al. Carnitine deficiency is associated with decreased exercise activity in hemodialysis patients. Ren. Replace. Ther. 2019, 5, 2. [Google Scholar] [CrossRef]
- Heinonen, O.J. 971 Effect of muscle carnitine concentration on fatty acid oxidation and exercise capacity in rats. Med. Sci. Sports Exerc. 1994, 26, S173. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Scientific Opinion on the substantiation of health claims related to L-carnitine and faster recovery from muscle fatigue after exercise (ID 738, 1492, 1493), skeletal muscle tissue repair (ID 738, 1492, 1493), increase in endurance capacity (ID 4305, 4684), maintenance of normal blood LDL-cholesterol concentrations (ID 1494, 4684), contribution to normal spermatogenesis (ID 1822), “energy metabolism” (ID 1821), and increasing L-carnitine concentrations and/or decreasing free fatty acids in blood during pregnancy (ID 1495) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011, 9, 2212. [Google Scholar] [CrossRef][Green Version]
- Li, J.-L.; Wang, Q.-Y.; Luan, H.-Y.; Kang, Z.-C.; Wang, C.-B. Effects of L-carnitine against oxidative stress in human hepatocytes: Involvement of peroxisome proliferator-activated receptor alpha. J. Biomed. Sci. 2012, 19, 32. [Google Scholar] [CrossRef]
- Keshani, M.; Alikiaii, B.; Babaei, Z.; Askari, G.; Heidari, Z.; Sharma, M.; Bagherniya, M. The effects of L-carnitine supplementation on inflammation, oxidative stress, and clinical outcomes in critically Ill patients with sepsis: A randomized, double-blind, controlled trial. Nutr. J. 2024, 23, 31. [Google Scholar] [CrossRef]
- Golzar Adabi, S.; Cooper, R.G.; Ceylan, N.; Corduk, M. L-carnitine and its functional effects in poultry nutrition. World’s Poult. Sci. J. 2011, 67, 277–296. [Google Scholar] [CrossRef]
- Owen, K.Q.; Nelssen, J.L.; Goodband, R.D.; Weeden, T.L.; Blum, S.A. Effect of L-carnitine and soybean oil on growth performance and body composition of early-weaned pigs2. J. Anim. Sci. 1996, 74, 1612–1619. [Google Scholar] [CrossRef]
- Ahmadipour, B.; Hasani, F.F.; Hassanpour, H.; Khajali, F. Effect of l-carnitine and emulsifier on growth performance, lipid profile, lipogenesis, and oxidative stress of broiler chickens raised at high altitude. Poult. Sci. 2025, 104, 105157. [Google Scholar] [CrossRef]
- Song, B.; Zheng, C.; Zheng, J.; Zhang, S.; Zhong, Y.; Guo, Q.; Li, F.; Long, C.; Xu, K.; Duan, Y.; et al. Comparisons of carcass traits, meat quality, and serum metabolome between Shaziling and Yorkshire pigs. Anim. Nutr. 2022, 8, 125–134. [Google Scholar] [CrossRef]
- Zhu, Y.; Jameson, E.; Crosatti, M.; Schäfer, H.; Rajakumar, K.; Bugg, T.D.H.; Chen, Y. Carnitine metabolism to trimethylamine by an unusual Rieske-type oxygenase from human microbiota. Proc. Natl. Acad. Sci. USA 2014, 111, 4268–4273. [Google Scholar] [CrossRef]
- NY/T 816-2004; Chinese Meat Sheep Feeding Standards. Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004.
- Meng, L.; Jin, X.; Qi, Z.; Mi, L. Effects of dietary minerals deficiency and supplementation on different parts of muscle minerals content in grazing Mongolian sheep. Front. Vet. Sci. 2024, 11, 1301852. [Google Scholar] [CrossRef]
- Bourguignon, L.; Chu, A.; Jin, H.; Brandt, N. Ryanodine Receptor-Ankyrin Interaction Regulates Internal Ca2+ Release in Mouse T-lymphoma Cells. J. Biol. Chem. 1995, 270, 17917–17922. [Google Scholar] [CrossRef]
- Destefanis, G.; Brugiapaglia, A.; Barge, M.T.; Dal Molin, E. Relationship between beef consumer tenderness perception and Warner–Bratzler shear force. Meat Sci. 2008, 78, 153–156. [Google Scholar] [CrossRef]
- Byrne, C.E.; Troy, D.J.; Buckley, D.J. Postmortem changes in muscle electrical properties of bovine M. longissimus dorsi and their relationship to meat quality attributes and pH fall. Meat Sci. 2000, 54, 23–34. [Google Scholar] [CrossRef]
- Alarcón-Rojo, A.; Lucero, V.; Carrillo-López, L.; Janacua, H. Use of apple pomace in animal feed as an antioxidant of meat. S. Afr. J. Anim. Sci. 2019, 49, 131. [Google Scholar] [CrossRef]
- Atkin, A.J.; Lynam, J.M.; Moulton, B.E.; Sawle, P.; Motterlini, R.; Boyle, N.M.; Pryce, M.T.; Fairlamb, I.J.S. Modification of the deoxy-myoglobin/carbonmonoxy-myoglobin UV-vis assay for reliable determination of CO-release rates from organometallic carbonyl complexes. Dalton Trans. 2011, 40, 5755–5761. [Google Scholar] [CrossRef]
- Tang, J.; Faustman, C.; Hoagland, T.A. Krzywicki Revisited: Equations for Spectrophotometric Determination of Myoglobin Redox Forms in Aqueous Meat Extracts. J. Food Sci. 2004, 69, C717–C720. [Google Scholar] [CrossRef]
- Barber, R.; Harmer, D.; Coleman, R.; Clark, B. GAPDH as a housekeeping gene: Analysis of GAPDH mRNA expression in a panel of 72 human tissues. Physiol. Genom. 2005, 21, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Li, Y.; Tian, Y.; Zhou, F.; Ma, J.; Xia, S.; Yang, T.; Ma, L.; Zeng, Q.; Liu, G.; et al. Obese Ningxiang pig-derived microbiota rewires carnitine metabolism to promote muscle fatty acid deposition in lean DLY pigs. Innovation 2023, 4, 100486. [Google Scholar] [CrossRef] [PubMed]
- Gnoni, A.; Longo, S.; Gnoni, G.V.; Giudetti, A.M. Carnitine in Human Muscle Bioenergetics: Can Carnitine Supplementation Improve Physical Exercise? Molecules 2020, 25, 182. [Google Scholar] [CrossRef]
- Posridee, K.; Kupittayanant, S.; Rachnavy, P.; Oonsivilai, A.; Oonsivilai, R. Role of Ginseng and L-Carnitine in Modulating Exercise Endurance and Oxidative Stress in Rats. Nutrients 2025, 17, 568. [Google Scholar] [CrossRef] [PubMed]
- Koves, T.R.; Li, P.; An, J.; Akimoto, T.; Slentz, D.; Ilkayeva, O.; Dohm, G.L.; Yan, Z.; Newgard, C.B.; Muoio, D.M. Peroxisome proliferator-activated receptor-gamma co-activator 1alpha-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J. Biol. Chem. 2005, 280, 33588–33598. [Google Scholar] [CrossRef]
- Dunning, K.R.; Russell, D.L.; Robker, R.L. Lipids and oocyte developmental competence: The role of fatty acids and β-oxidation. Reproduction 2014, 148, R15–R27. [Google Scholar] [CrossRef]
- Walker, D.K.; Lambert, B.D.; Woodworth, J.C. Effects of dietary ruminally protected L-carnitine on plasma metabolites in sheep following a sub-lethal ammonia challenge. J. Anim. Physiol. Anim. Nutr. 2005, 89, 413–418. [Google Scholar] [CrossRef]
- Chapa, A.M.; Fernandez, J.M.; White, T.W.; Bunting, L.D.; Gentry, L.R.; Ward, T.L.; Blum, S.A. Influence of intravenous L-carnitine administration in sheep preceding an oral urea drench. J. Anim. Sci. 1998, 76, 2930–2937. [Google Scholar] [CrossRef]
- Williams, P. Nutritional composition of red meat. Nutr. Diet. 2007, 64, S113–S119. [Google Scholar] [CrossRef]
- Qiao, M.; Fletcher, D.; Smith, D.; Northcutt, J. The Effect of Broiler Breast Meat Color on pH, Moisture, Water-Holding Capacity, and Emulsification Capacity. Poult. Sci. 2001, 80, 676–680. [Google Scholar] [CrossRef]
- Gault, N. The relationship between water-holding capacity and cooked meat tenderness in some beef muscles as influenced by acidic conditions below the ultimate pH. Meat Sci. 1985, 15, 15–30. [Google Scholar] [CrossRef]
- Roca, M.; Incze, K. Fermented sausages. Food Rev. Int. 1990, 6, 91–118. [Google Scholar] [CrossRef]
- Samir, H.; Swelum, A.A.; Abdelnaby, E.A.; El-Sherbiny, H.R. Incorporation of L-Carnitine in the OvSynch protocol enhances the morphometrical and hemodynamic parameters of the ovarian structures and uterus in ewes under summer climatic conditions. BMC Vet. Res. 2023, 19, 246. [Google Scholar] [CrossRef]
- Yousefi, J.; Taherpour, K.; Ghasemi, H.A.; Akbari Gharaei, M.; Mohammadi, Y.; Rostami, F. Effects of emulsifier, betaine, and L-carnitine on growth performance, immune response, gut morphology, and nutrient digestibility in broiler chickens exposed to cyclic heat stress. Br. Poult. Sci. 2023, 64, 384–397. [Google Scholar] [CrossRef] [PubMed]
- Pujol, A.; Ospina-E, J.C.; Alvarez, H.; Muñoz, D.A. Myoglobin content and oxidative status to understand meat products’ color: Phenomenological based model. J. Food Eng. 2023, 348, 111439. [Google Scholar] [CrossRef]
- Tamai, I.; Ohashi, R.; Nezu, J.-i.; Yabuuchi, H.; Oku, A.; Shimane, M.; Sai, Y.; Tsuji, A. Molecular and Functional Identification of Sodium Ion-dependent, High Affinity Human Carnitine Transporter OCTN2. J. Biol. Chem. 1998, 273, 20378–20382. [Google Scholar] [CrossRef]
- Ma, D.; Kim, Y.; Cooper, B.; Oh, J.; Chun, H.; Choe, J.; Schoonmaker, J.; Ajuwon, K.; Min, B. Metabolomics Profiling to Determine the Effect of Postmortem Aging on Color and Lipid Oxidative Stabilities of Different Bovine Muscles. J. Agric. Food Chem. 2017, 65, 6708–6716. [Google Scholar] [CrossRef]
- Bulgaru, V.; Popescu, L. Dry-Aged Beef: Color Parameters and Sensory Characteristics. J. Eng. Sci. 2023, 30, 155–163. [Google Scholar] [CrossRef]
- Zhou, X.; Huang, G.; Wang, L.; Zhao, Y.; Li, J.; Chen, D.; Wei, L.; Chen, Z.; Yang, B. L-carnitine promotes liver regeneration after hepatectomy by enhancing lipid metabolism. J. Transl. Med. 2023, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Wakil, S.; Abu-Elheiga, L. Fatty acid metabolism: Target for metabolic syndrome. J. Lipid Res. 2008, 50, S138–S143. [Google Scholar] [CrossRef]
- Shalaby, M.; Ramadan, A.; El-Rahman, S.; Fayed, H. Hepatoprotective Effect of L-Carnitine is Achieved via Activating Nrf2 and Targeting TLR4 Signaling Pathways in Thioacetamide—Induced Liver Fibrosis in Rats. EAS J. Vet. Med. Sci. 2024, 6, 74–85. [Google Scholar] [CrossRef]
- Placidi, M.; Vergara, T.; Casoli, G.; Flati, I.; Capece, D.; Artini, P.G.; Virmani, A.; Zanatta, S.; D’Alessandro, A.M.; Tatone, C.; et al. Acyl-Carnitines Exert Positive Effects on Mitochondrial Activity under Oxidative Stress in Mouse Oocytes: A Potential Mechanism Underlying Carnitine Efficacy on PCOS. Biomedicines 2023, 11, 2474. [Google Scholar] [CrossRef]
- Dogan, S.; Aydın, T.; Köroğlu, N.; Yılmazer, Y.; Albayrak, N.; Cetin, F.; Moshfeghi, E.; Çelik, Ö. Assessing the efficacy of a novel sperm-washing medium enriched with serotonin, L-carnitine, and coenzyme Q10: An observational cohort study. Asian J. Androl. 2024, 26, 635–639. [Google Scholar] [CrossRef]
- Grossini, E.; Marchi, F.; Venkatesan, S.; Mele, A.; Ferrante, D.; Mazzini, L. Effects of Acetyl-L-Carnitine on Oxidative Stress in Amyotrophic Lateral Sclerosis Patients: Evaluation on Plasma Markers and Members of the Neurovascular Unit. Antioxidants 2023, 12, 1887. [Google Scholar] [CrossRef]
- Nanke, K.; Sebranek, J.; Olson, D. Color Characteristics of Irradiated Vacuum-Packaged Pork, Beef, and Turkey. J. Food Sci. 1998, 63, 1001–1006. [Google Scholar] [CrossRef]
- Nakanishi, K.; Tajima, F.; Nakamura, A.; Yagura, S.; Ookawara, T.; Yamashita, H.; Suzuki, K.; Taniguchi, N.; Ohno, H. Effects of hypobaric hypoxia on antioxidant enzymes in rats. J. Physiol. 1995, 489, 869–876. [Google Scholar] [CrossRef]
- Han, D.; Antunes, F.; Canali, R.; Rettori, D.; Cadenas, E. Voltage-dependent Anion Channels Control the Release of the Superoxide Anion from Mitochondria to Cytosol. J. Biol. Chem. 2003, 278, 5557–5563. [Google Scholar] [CrossRef]
- Shan, J.; Jin, X.; Zhang, C.; Huang, M.; Xing, J.; Li, Q.; Cui, Y.; Niu, Q.; Chen, X.; Wang, X. Metal natural product complex Ru-procyanidins with quadruple enzymatic activity combat infections from drug-resistant bacteria. Acta Pharm. Sin. B 2024, 14, 2298–2316. [Google Scholar] [CrossRef]
- Ligtenberg, M.; Mougiakakos, D.; Mukhopadhyay, M.; Witt, K.; Lladser, Á.; Chmielewski, M.; Riët, T.; Abken, H.; Kiessling, R. Coexpressed Catalase Protects Chimeric Antigen Receptor–Redirected T Cells as well as Bystander Cells from Oxidative Stress–Induced Loss of Antitumor Activity. J. Immunol. 2015, 196, 759–766. [Google Scholar] [CrossRef]
- Kellner, D.; Hung, S.; Weiss, K.; Sligar, S. Kinetic Characterization of Compound I Formation in the Thermostable Cytochrome P450 CYP119. J. Biol. Chem. 2002, 277, 9641–9644. [Google Scholar] [CrossRef]
- Qiao, B.; Zhang, Q.; Liu, D.; Wang, H.; Yin, J.; Wang, R.; He, M.; Cui, M.; Shang, Z.; Wang, D.; et al. A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J. Exp. Bot. 2015, 66, 5853–5866. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Faustman, C.; Mancini, R.; Seyfert, M.; Hunt, M. Mitochondrial Reduction of Metmyoglobin: Dependence on the Electron Transport Chain. J. Agric. Food Chem. 2005, 53, 5449–5455. [Google Scholar] [CrossRef]
- Pigeon, C.; Ilyin, G.; Courselaud, B.; Leroyer, P.; Turlin, B.; Brissot, P.; Loréal, O. A New Mouse Liver-specific Gene, Encoding a Protein Homologous to Human Antimicrobial Peptide Hepcidin, Is Overexpressed during Iron Overload. J. Biol. Chem. 2001, 276, 7811–7819. [Google Scholar] [CrossRef]
- Mackenzie, E.; Iwasaki, K.; Tsuji, Y. Intracellular Iron Transport and Storage: From Molecular Mechanisms to Health Implications. Antioxid. Redox Signal. 2008, 10, 997–1030. [Google Scholar] [CrossRef] [PubMed]
- Qatato, M.; Bonadonna, M.; Palais, G.; Ertl, A.; Schmidt, G.; Polycarpou-Schwarz, M.; Karim, Z.; Galy, B. IRE-dependent Regulation of Intestinal Dmt1 Prevails During Chronic Dietary Iron Deficiency but is Dispensable in Conditions of Acute Erythropoietic Stress. HemaSphere 2022, 6, e693. [Google Scholar] [CrossRef]
- Volek, J.; Kraemer, W.; Rubin, M.; Gómez, A.; Ratamess, N.; Gaynor, P. l-Carnitinel-tartrate supplementation favorably affects markers of recovery from exercise stress. AJP Endocrinol. Metab. 2002, 282, E474–E482. [Google Scholar] [CrossRef]
- Hau, J.; Kaltwasser, S.; Muras, V.; Casutt, M.; Vohl, G.; Claußen, B.; Steffen, W.; Leitner, A.; Bill, E.; Cutsail, G.; et al. Conformational coupling of redox-driven Na+-translocation in Vibrio cholerae NADH:quinone oxidoreductase. Nat. Struct. Mol. Biol. 2023, 30, 1686–1694. [Google Scholar] [CrossRef]
- Kerschbaumer, B.; Totaro, M.; Frieß, M.; Breinbauer, R.; Bijelic, A.; Macheroux, P. Loop 6 and the β-hairpin flap are structural hotspots that determine cofactor specificity in the FMN-dependent family of ene-reductases. FEBS J. 2024, 291, 1560–1574. [Google Scholar] [CrossRef]
- Higuchi, M.; Yamamoto, Y.; Poole, L.; Shimada, M.; Sato, Y.; Takahashi, N.; Kamio, Y. Functions of Two Types of NADH Oxidases in Energy Metabolism and Oxidative Stress of Streptococcus mutans. J. Bacteriol. 1999, 181, 5940–5947. [Google Scholar] [CrossRef]
- Riganti, C.; Gazzano, E.; Polimeni, M.; Costamagna, C.; Bosìa, A.; Ghigo, D. Diphenyleneiodonium Inhibits the Cell Redox Metabolism and Induces Oxidative Stress. J. Biol. Chem. 2004, 279, 47726–47731. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Keeton, J.; Hunt, M.; Savell, J. Effects of L- or D-lactate-enhancement on the internal cooked colour development and biochemical characteristics of beef steaks in high-oxygen modified atmosphere. Food Chem. 2010, 119, 918–922. [Google Scholar] [CrossRef]
- Gao, X.; Wang, Z.; Miao, J.; Xie, L.; Dai, Y.; Li, X.; Chen, Y.; Luo, H.; Dai, R. Influence of different production strategies on the stability of color, oxygen consumption and metmyoglobin reducing activity of meat from Ningxia Tan sheep. Meat Sci. 2014, 96, 769–774. [Google Scholar] [CrossRef]
- Lacour, T.; Achstetter, T.; Dumas, B. Characterization of Recombinant Adrenodoxin Reductase Homologue (Arh1p) from Yeast: Implication in in vitro cytochrome p45011β monooxygenase system. J. Biol. Chem. 1998, 273, 23984–23992. [Google Scholar] [CrossRef][Green Version]
- Syed, K.; Kattamuri, C.; Thompson, T.B.; Yadav, J.S. Cytochrome b5 reductase–cytochrome b5 as an active P450 redox enzyme system in Phanerochaete chrysosporium: Atypical properties and in vivo evidence of electron transfer capability to CYP63A2. Arch. Biochem. Biophys. 2011, 509, 26–32. [Google Scholar] [CrossRef]
- Dewilde, S.; Kiger, L.; Burmester, T.; Hankeln, T.; Baudin-Creuza, V.; Aerts, T.; Marden, M.C.; Caubergs, R.; Moens, L. Biochemical Characterization and Ligand Binding Properties of Neuroglobin, a Novel Member of the Globin Family. J. Biol. Chem. 2001, 276, 38949–38955. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.-J.; Jana, C.K.; Das, N. Role of Catalase in Oxidative Stress- and Age-Associated Degenerative Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, C.; Xu, J.; Wang, W.; Zhang, C.; Tian, J.; Li, C.; Ma, Q. Dietary Phaffia rhodozyma-Synthesized 3S, 3′S-Astaxanthin Promotes Body Coloration and Muscle Quality in Pacific White Shrimp Litopenaeus vannamei. Aquac. Nutr. 2025, 2025, 9993234. [Google Scholar] [CrossRef]
- Martín, A.; Giráldez, F.J.; Cremonesi, P.; Castiglioni, B.; Biscarini, F.; Ceciliani, F.; Santos, N.; Andrés, S. Dietary Administration of L-Carnitine During the Fattening Period of Early Feed Restricted Lambs Modifies Ruminal Fermentation but Does Not Improve Feed Efficiency. Front. Physiol. 2022, 13, 840065. [Google Scholar] [CrossRef]
| Ingredients | Composition (%) | Nutritive Index | Nutrient Levels |
|---|---|---|---|
| Corn | 40.0 | Dry matter, % | 87.70 |
| Soybean meal | 17.0 | Net energy, MJ/kg | 5.84 |
| DDGS | 8.0 | Crude protein, % | 18.50 |
| Corn germ meal | 6.2 | Crude fat, % | 3.50 |
| Rice bran meal | 6.0 | Crude fiber, % | 6.20 |
| Puffed corn | 5.0 | Crude ash, % | 5.90 |
| Soybean skin | 4.0 | Calcium, % | 1.10 |
| Beet pulp pellet | 4.0 | Total phosphorus, % | 0.50 |
| Puffed soybean powder | 3.0 | Lysine, % | 1.25 |
| Fermented soybean meal | 3.0 | Methionine, % | 0.48 |
| Mountain flour | 2.2 | Met + Cys, % | 0.83 |
| Salt | 0.6 | Threonine, % | 0.66 |
| Compound premix 1 | 1.0 | Tryptophan, % | 0.20 |
| Total | 100.0 |
| Official Symbol | GenBank ID | Primer Sequences (5′–3′) | Amplified Fragment Size (bp) | Annealing Temperature (°C) |
|---|---|---|---|---|
| HEPH | 101113655 | 5′-TTTATTCTCAACCAAGGCTAT-3′ 5′-GGAAATAAGTAGGAGTGAGGTT-3′ | 134 | 48 |
| NADHB5R | 101123639 | 5′-ACAGACAACGCAGAGGACGAG-3′ 5′-CTGGCTGAGGACAAAGAAGGT-3′ | 135 | 58 |
| CAT | 100307035 | 5′-TGCTCTACTGTTTCCGTCCTT-3′ 5′-GAACGGATATGGATCGCATAC-3′ | 186 | 60 |
| LDHB | 101118315 | 5′-GTCCGTTGTGGATCTGACCTG-3′ 5′-GTGTAGCCTAGAATGCCCTTG-3′ | 111 | 55 |
| TFR1 | 100885768 | 5′-CAAAGTTTCTGCCAGTCCGC-3′ 5′-GATCCAGTTGCTGTCCCGAT-3′ | 106 | 58 |
| GAPDH | 443005 | 5′-TTTATTCTCAACCAAGGCTAT-3′ 5′-GGAAATAAGTAGGAGTGAGGTT-3′ | 78 | 55 |
| Days | L-Carnitine (ng/mL) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C:L | C:H | L:H | ||
| 15 | 46.89 | 47.69 | 45.76 | 4.30 | 0.85 | 0.80 | 0.68 |
| 30 | 48.88 | 53.92 | 52.98 | 1.47 | <0.05 | <0.05 | 0.95 |
| 45 | 52.53 | 56.79 | 60.41 | 1.65 | <0.05 | <0.01 | <0.05 |
| Items | L-Carnitine (Gram/Sheep/Day) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01%(L) | 0.05% (H) | C:L | C:H | L:H | ||
| Initial Wt. (kg) | 15.53 | 14.93 | 15.39 | 1.48 | 0.69 | 0.92 | 0.76 |
| Final Wt. (kg) | 17.55 | 16.95 | 16.94 | 2.37 | 0.80 | 0.79 | 0.99 |
| ADFI (kg) | 0.4482 | 0.4684 | 0.4606 | 0.0109 | 0.12 | 0.30 | 0.50 |
| ADG (kg) | 0.0669 | 0.0667 | 0.0671 | 0.0152 | 0.29 | 0.68 | 0.50 |
| F/G | 6.71 | 6.82 | 6.81 | 0.19 | 0.67 | 0.87 | 0.11 |
| Items | L-Carnitine (Gram/Sheep/Day) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C:L | C:H | L:H | ||
| Carcass weight (kg) | 8.43 | 7.81 | 8.43 | 0.67 | 0.38 | 1.00 | 0.38 |
| Backfat thickness (cm) | 0.27 | 0.17 | 0.23 | 0.08 | 0.27 | 0.70 | 0.45 |
| Loin muscle area (cm2) | 4.13 | 2.63 | 3.49 | 0.92 | 0.15 | 0.51 | 0.39 |
| GR value (cm) | 0.40 | 0.28 | 0.32 | 0.11 | 0.28 | 0.78 | 0.41 |
| Shear force (N) | 5.38 | 4.68 | 4.84 | 0.86 | 0.45 | 0.55 | 0.86 |
| pH value | 5.94 | 6.03 | 6.26 | 0.11 | 0.47 | <0.05 | 0.08 |
| Drip loss (%) | 39.22 | 35.71 | 36.12 | 0.98 | <0.05 | <0.05 | 0.69 |
| Items | L-Carnitine (Gram/Sheep/Day) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C:L | C:H | L:H | ||
| a* value | 16.88 | 18.06 | 19.07 | 0.40 | <0.01 | <0.01 | <0.05 |
| b* value | 10.74 | 10.98 | 10.04 | 0.58 | 0.69 | 0.24 | 0.12 |
| L* value | 37.51 | 38.67 | 38.37 | 1.89 | 0.47 | 0.59 | 0.85 |
| Meat color saturation | 20.04 | 21.14 | 21.58 | 0.49 | <0.05 | <0.05 | 0.38 |
| Chroma angle | 0.56 | 0.54 | 0.48 | 0.02 | 0.43 | <0.01 | <0.05 |
| Items | Myoglobin Concentrations (mg/g) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C:L | C:H | L:H | ||
| Longissimus dorsi muscle | 2.84 | 3.29 | 3.52 | 0.07 | <0.01 | <0.01 | <0.05 |
| Biceps femoris | 2.90 | 3.34 | 3.55 | 0.05 | <0.01 | <0.01 | <0.05 |
| Triceps brachii | 2.98 | 3.28 | 3.60 | 0.06 | <0.01 | <0.01 | <0.05 |
| Items | Relative Concentration Ratio (%) | SEM | p-Value | |||||
|---|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C:L | C:H | L:H | |||
| Longissimus dorsi | MMb | 28.46 | 23.93 | 22.66 | 0.72 | <0.01 | <0.01 | 0.07 |
| OMb | 42.77 | 46.70 | 57.11 | 1.49 | <0.05 | <0.01 | <0.01 | |
| DMb | 20.07 | 19.97 | 20.70 | 0.66 | 0.86 | 0.27 | 0.25 | |
| Biceps femoris | MMb | 28.30 | 24.13 | 22.46 | 0.72 | <0.01 | <0.01 | 0.06 |
| OMb | 44.58 | 46.23 | 55.11 | 1.26 | 0.24 | <0.01 | <0.01 | |
| DMb | 20.89 | 20.83 | 20.51 | 0.54 | 0.92 | 0.48 | 0.20 | |
| Musculus triceps brachii | MMb | 29.27 | 24.07 | 22.40 | 1.03 | <0.01 | <0.01 | 0.20 |
| OMb | 42.55 | 46.27 | 55.42 | 1.47 | <0.05 | <0.01 | <0.01 | |
| DMb | 20.65 | 20.55 | 20.09 | 0.73 | 0.90 | 0.47 | 0.66 | |
| Items | L-Carnitine (Gram/Sheep/Day) | SEM | p-Value | ||||
|---|---|---|---|---|---|---|---|
| 0 (C) | 0.01% (L) | 0.05% (H) | C: L | C:H | L:H | ||
| SOD (U/L) | 223.15 | 343.53 | 275.73 | 14.60 | <0.01 | <0.01 | <0.01 |
| LDH (U/L) | 3.66 | 4.83 | 4.45 | 0.23 | <0.01 | <0.01 | 0.11 |
| NADHB5R (U/L) | 94.65 | 126.14 | 106.71 | 3.35 | <0.01 | <0.01 | <0.01 |
| MRA (ng/mL) | 2.25 | 3.53 | 4.21 | 0.08 | <0.01 | <0.01 | <0.01 |
| L-Carnitine | a* | MRA | SOD | LDH | NADHBR5 | Myoglobin | MMb | OMb | |
|---|---|---|---|---|---|---|---|---|---|
| L-Carnitine (45d) | 1 | ||||||||
| a* | 0.912 ** | 1 | |||||||
| MRA | 0.939 ** | 0.943 ** | 1 | ||||||
| SOD | 0.236 | 0.436 | 0.487 | 1 | |||||
| LDH | 0.267 | 0.497 | 0.582 * | 0.783 ** | 1 | ||||
| NADHB5R | 0.377 | 0.482 | 0.547 * | 0.938 ** | 0.834 ** | 1 | |||
| Myoglobin | 0.950 ** | 0.943 ** | 0.978 ** | 0.411 | 0.570 * | 0.472 | 1 | ||
| MMb | −0.874 ** | −0.917 ** | −0.961 ** | −0.628 * | −0.717 ** | −0.682 ** | −0.953 ** | 1 | |
| OMb | 0.974 ** | 0.863 ** | 0.891 ** | 0.143 | 0.305 | 0.146 | 0.923 ** | −0.796 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, X.; Liu, W.; Xin, J.; Zhang, Y.; Zhang, M.; Liang, W.; Li, J.; Hu, J. Effect of L-Carnitine on Muscle Quality and Antioxidant Capacity of Hybrid Sheep at an Early Stage. Animals 2025, 15, 2564. https://doi.org/10.3390/ani15172564
Qin X, Liu W, Xin J, Zhang Y, Zhang M, Liang W, Li J, Hu J. Effect of L-Carnitine on Muscle Quality and Antioxidant Capacity of Hybrid Sheep at an Early Stage. Animals. 2025; 15(17):2564. https://doi.org/10.3390/ani15172564
Chicago/Turabian StyleQin, Xia, Wenjie Liu, Jiaqi Xin, Yidan Zhang, Mingxi Zhang, Weiwei Liang, Jiantao Li, and Jianmin Hu. 2025. "Effect of L-Carnitine on Muscle Quality and Antioxidant Capacity of Hybrid Sheep at an Early Stage" Animals 15, no. 17: 2564. https://doi.org/10.3390/ani15172564
APA StyleQin, X., Liu, W., Xin, J., Zhang, Y., Zhang, M., Liang, W., Li, J., & Hu, J. (2025). Effect of L-Carnitine on Muscle Quality and Antioxidant Capacity of Hybrid Sheep at an Early Stage. Animals, 15(17), 2564. https://doi.org/10.3390/ani15172564





