Hongiastoma zhangbuensis, a New Species of the Subfamily Acrossocheilinae (Teleostei: Cyprinidae) from South China †
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
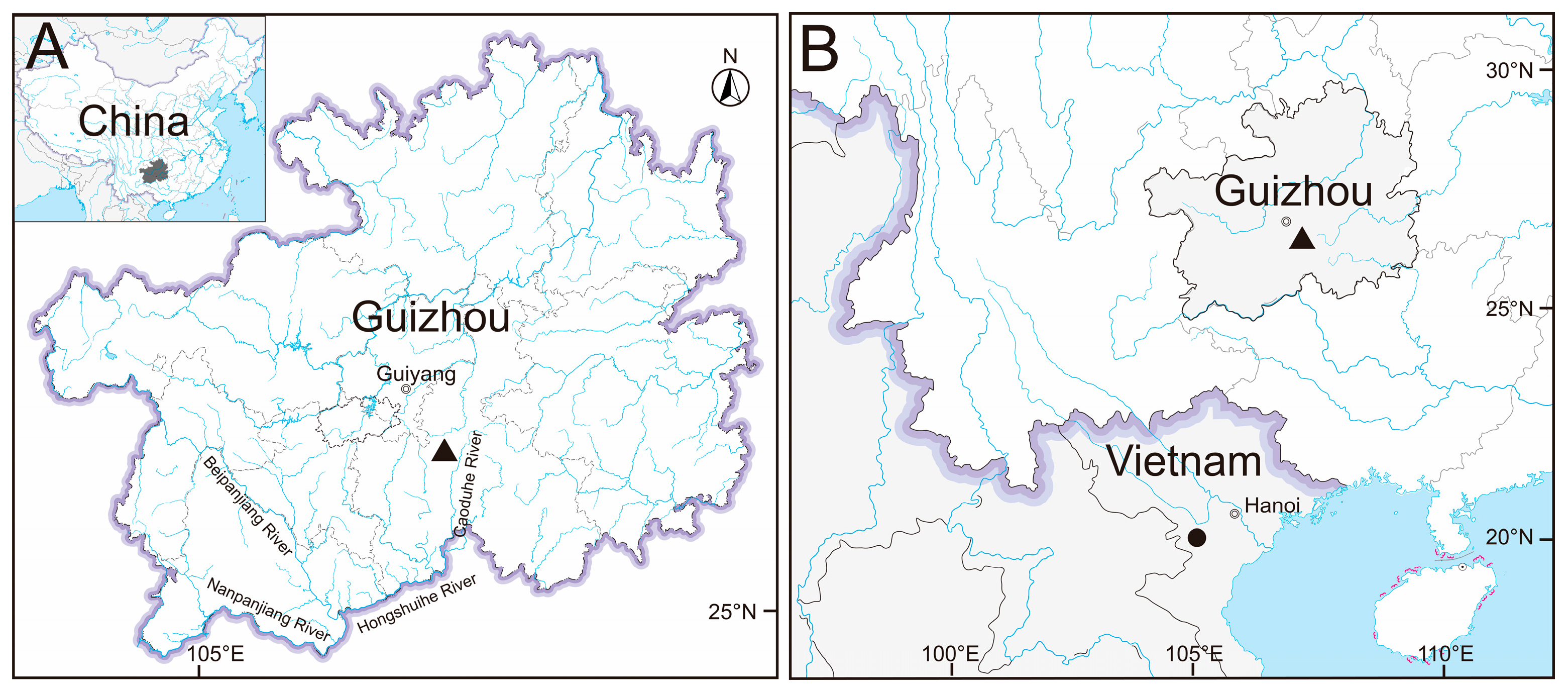
2.1. Sampling
2.2. Morphological Examination
2.3. Molecular Analysis
3. Results
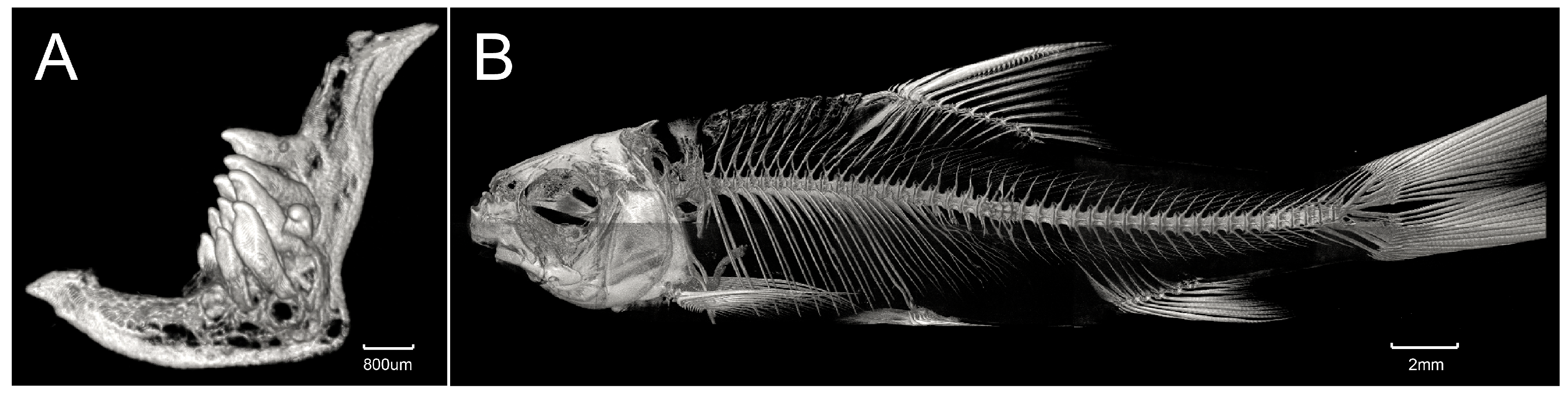
3.1. Taxonomy
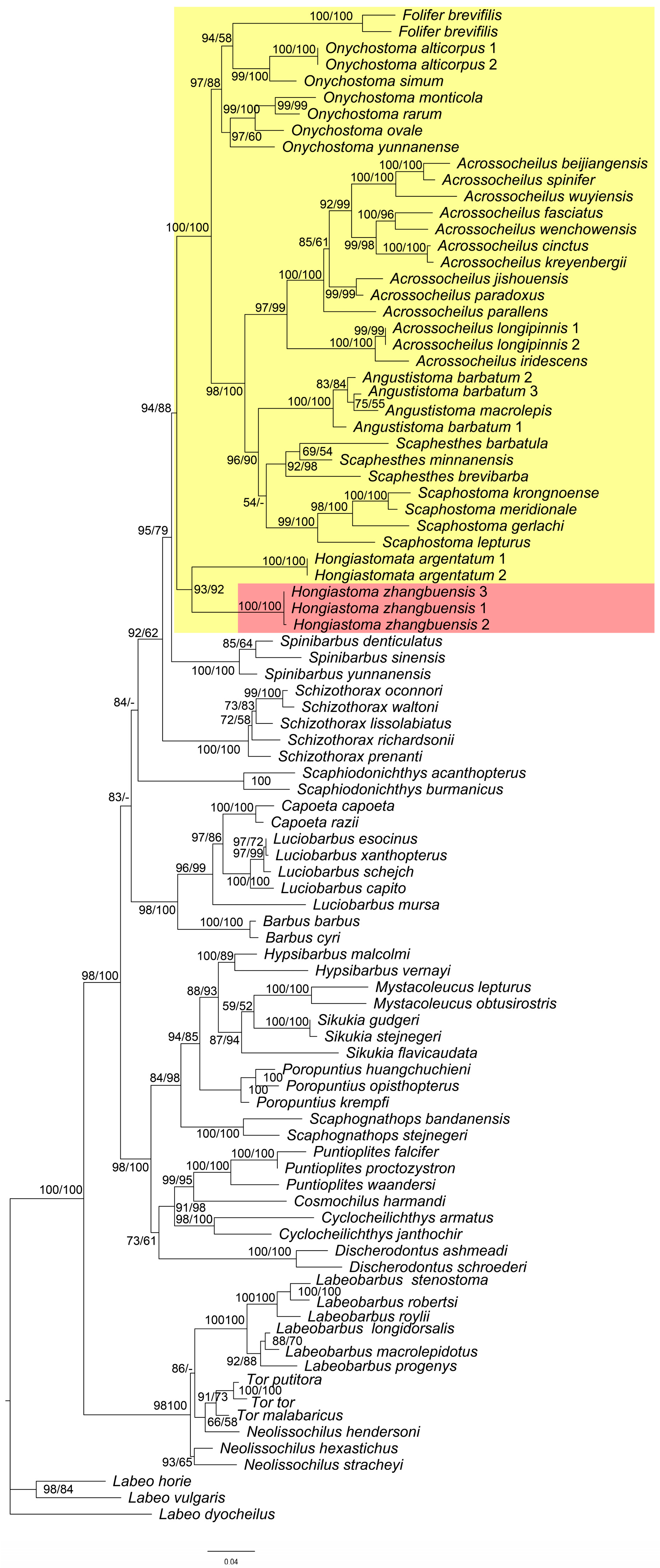
3.2. Molecular Results
4. Discussion
5. Conclusions
6. Nomenclatural Acts
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Eschmeyer, W.N. (Ed.) Catalog of Fishes. 2025. Available online: https://researcharchive.calacademy.org/research/ichthyology/catalog/SpeciesByFamily.asp (accessed on 15 March 2025).
- Hoang, H.D.; Jang-Liaw, N.-H.; Pham, H.M.; Tran, N.T.; Durand, J.-D.; Nguyen, T.D.; Pfeiffer, J.; Page, L.M. Generic revision of the Southeast and East Asian torrent carp Subfamily Acrossocheilinae (Pisces: Teleostei) with description of three new genera and a new species from Vietnam. J. Zool. Syst. Evol. Res. 2025, 2025, 8895501. [Google Scholar] [CrossRef]
- Xin, Q.; Zhang, E.; Cao, W.X.; Xin, Q.; Zhang, E.; Cao, W.X. Onychostoma virgulatum, a new species of cyprinid fish (Pisces: Teleostei) from southern Anhui Province, South China. Ichthyol. Explor. Freshw. 2009, 20, 255–266. [Google Scholar]
- Kottelat, M. Freshwater Fishes of Northern Vietnam; World Bank: Washington, DC, USA, 2001; p. 123. [Google Scholar]
- Kottelat, M. The fishes of the inland waters of Southeast Asia: A catalogue and core bibliography of the fishes known to occur in freshwaters, mangroves and estuaries. Raffles Bull. Zool. 2013, 61 (Suppl. S27), 1–663. [Google Scholar]
- Roberts, T.R.; Catania, D. Designation of lectotypes and neotypes, systematic status, and biological remarks on Red River Cyprinidae described by Nguyen and Doan, 1969. Nat. Hist. Bull. Siam Soc. 2007, 55, 85–97. [Google Scholar]
- Shan, X.H.; Lin, R.D.; Yue, P.Q.; Chu, X.L. Barbinae. In Fauna Sinica (Osteichthyes: Cypriniformes III); Yue, P.Q., Ed.; Science Press: Beijing, China, 2000; pp. 126–147. (In Chinese) [Google Scholar]
- Song, X.L.; Cao, L.; Zhang, E. Onychostoma brevibarba, a new cyprinine fish (Pisces: Teleostei) from the middle Chang Jiang basin in Hunan Province, South China. Zootaxa 2018, 4410, 147–163. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Sado, T.; Vincent Hirt, M.; Pasco-Viel, E.; Arunachalam, M.; Li, J.; Wang, X.; Freyhof, J.; Saitoh, K.; Simons, A.M.; et al. Phylogeny and polyploidy: Resolving the classification of cyprinine fishes (Teleostei: Cypriniformes). Mol. Phylogenetics Evol. 2015, 85, 97–116. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.P.; Yang, J.X.; Chen, X.Y. Molecular phylogeny and systematics of the Barbinae (Teleostei: Cyprinidae) in China inferred from mitochondrial DNA sequences. Biochem. Syst. Ecol. 2016, 68, 250–259. [Google Scholar] [CrossRef]
- Kottelat, M. Fishes of Laos; Wildlife Heritage Trust Publications: Colombo, Sri Lanka, 2001; p. 198. [Google Scholar]
- Chu, X.L.; Chen, Y.R. The Fishes of Yunnan, China. Part I: Cyprinidae; Science Press: Beijing, China, 1989; p. 377. (In Chinese) [Google Scholar]
- Zheng, L.P.; Yang, J.X.; Chen, X.Y. Garra incisorbis, a new species of labeonine from Pearl River basin in Guangxi, China (Teleostei: Cyprinidae). Ichthyol. Explor. Freshw. 2016, 26, 299–303. [Google Scholar]
- Zheng, L.P.; Yang, J.X.; Chen, X.Y.; Wang, W.Y. Phylogenetic relationships of the Chinese Labeoninae (Teleostei, Cypriniformes) derived from two nuclear and three mitochondrial genes. Zool. Scr. 2010, 39, 559–571. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Capella-Gutiérrez, S.; Silla-Martínez, J.M.; Gabaldón, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Minh, B.Q.; Schmidt, H.A.; Chernomor, O.; Schrempf, D.; Woodhams, M.D.; von Haeseler, A.; Lanfear, R. IQ-TREE 2: New models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020, 37, 1530–1534. [Google Scholar] [CrossRef] [PubMed]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A. FigTree v1. 4.4. A Graphical Viewer of Phylogenetic Trees. 2014. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 25 November 2018).



| Holotype | Range (n = 5) | Mean ± SD | |
|---|---|---|---|
| In percent of standard length | |||
| Body depth | 26.1 | 26.1–29.0 | 27.6 ± 1.40 |
| Head length | 21.6 | 21.6–23.2 | 22.6 ± 0.62 |
| Head depth | 19.9 | 19.7–21.6 | 20.6 ± 0.87 |
| Head width | 15.2 | 14.2–15.7 | 14.9 ± 0.54 |
| Dorsal-fin length | 31.3 | 25.2–31.3 | 27.7 ± 2.46 |
| Pectoral-fin length | 21.0 | 20.2–21.8 | 21.2 ± 0.66 |
| Pelvic-fin length | 19.7 | 18.5–20.9 | 20.0 ± 0.95 |
| Anal-fin length | 22.5 | 19.1–22.5 | 21.3 ± 1.28 |
| Caudal peduncle length | 21.5 | 20.1–21.8 | 20.9 ± 0.72 |
| Caudal peduncle depth | 8.9 | 8.9–10.5 | 10.1 ± 0.67 |
| Predorsal length | 44.7 | 44.7–49.3 | 47.0 ± 1.70 |
| Prepectoral length | 21.4 | 21.4–24.3 | 22.7 ± 1.20 |
| Prepelvic length | 48.6 | 48.1–49.6 | 48.7 ± 0.59 |
| Preanal length | 70.1 | 70.1–72.7 | 72.0 ± 1.06 |
| In percent of lateral head length | |||
| Snout length | 38.7 | 35.5–42.4 | 39.8 ± 2.64 |
| Head depth | 92.2 | 86.1–94.1 | 91.4 ± 3.06 |
| Eye diameter | 24.7 | 24.7–28.4 | 26.3 ± 1.50 |
| Interorbital width | 51.7 | 47.4–51.7 | 49.6 ± 1.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, L.-P.; Chen, W.-T. Hongiastoma zhangbuensis, a New Species of the Subfamily Acrossocheilinae (Teleostei: Cyprinidae) from South China. Animals 2025, 15, 2563. https://doi.org/10.3390/ani15172563
Zheng L-P, Chen W-T. Hongiastoma zhangbuensis, a New Species of the Subfamily Acrossocheilinae (Teleostei: Cyprinidae) from South China. Animals. 2025; 15(17):2563. https://doi.org/10.3390/ani15172563
Chicago/Turabian StyleZheng, Lan-Ping, and Wei-Tao Chen. 2025. "Hongiastoma zhangbuensis, a New Species of the Subfamily Acrossocheilinae (Teleostei: Cyprinidae) from South China" Animals 15, no. 17: 2563. https://doi.org/10.3390/ani15172563
APA StyleZheng, L.-P., & Chen, W.-T. (2025). Hongiastoma zhangbuensis, a New Species of the Subfamily Acrossocheilinae (Teleostei: Cyprinidae) from South China. Animals, 15(17), 2563. https://doi.org/10.3390/ani15172563






