Effects of Heat Stress on Production Performance and Protein Metabolism of Skeletal Muscle in Meat Rabbits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Sample Collection and Preparation
2.3. Determination of Indicators and Methods
2.3.1. Growth Performance
2.3.2. Slaughter Performance
2.3.3. Blood Indices
2.3.4. Meat Physical Characteristics
2.3.5. Identification and Bioinformatic Analysis of Protein
2.4. Statistical Analysis
3. Results and Analysis
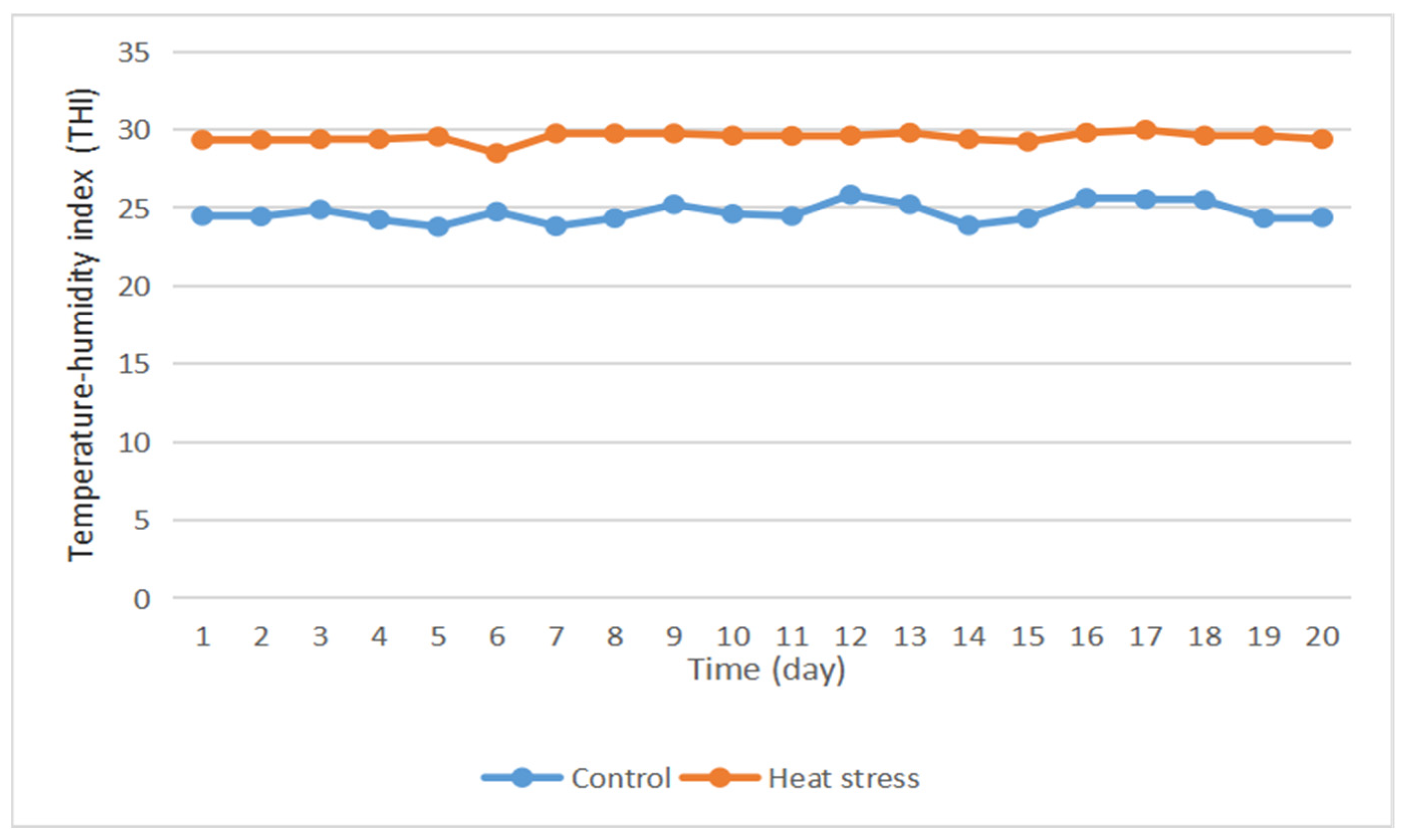
3.1. Temperature—Humidity Index
- Heat stress in rabbits can be divided into four grades: no heat stress at THI < 27.8; moderate heat stress at 27.8 < THI < 28.9; severe heat stress at 28.9 < THI < 30.0; and especially severe heat stress at THI > 30. The average daily THI of the control group was lower than 27.8 (Figure 1), indicating that the experimental rabbits in this group were in a state of no heat stress throughout the experiment. The average daily THI of the heat stress group was greater than 28.9, and thus, the rabbits were in a state of heat stress.
3.2. Growth Performance
3.3. Slaughter Performance
3.4. Blood Indices
3.5. Physical Characteristics of the LTL Meat
3.6. Protein Metabolism of Skeletal Muscle
3.6.1. Data Quality Control and Identification
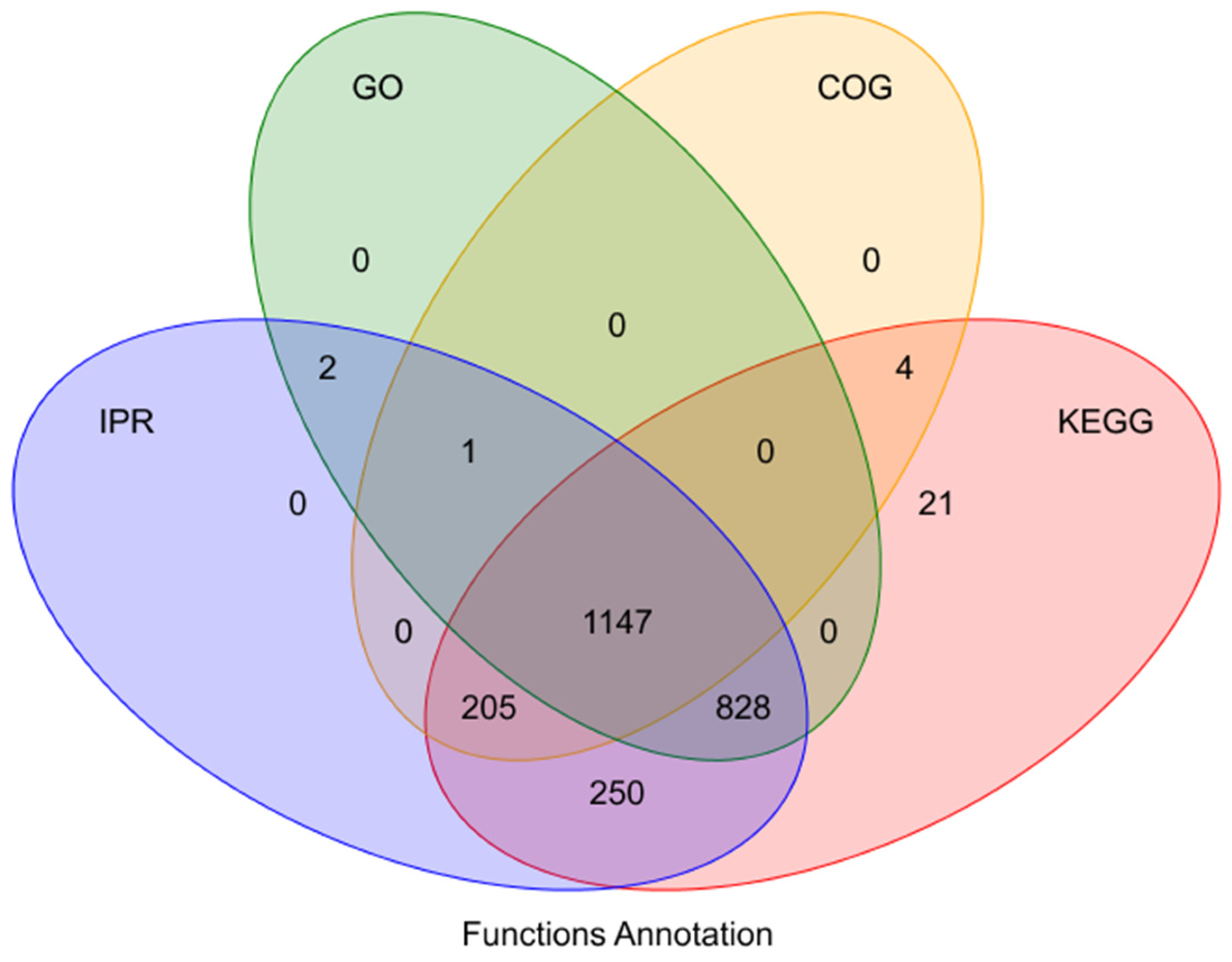
3.6.2. Protein Function Annotation
3.6.3. Protein Quantitative Analysis
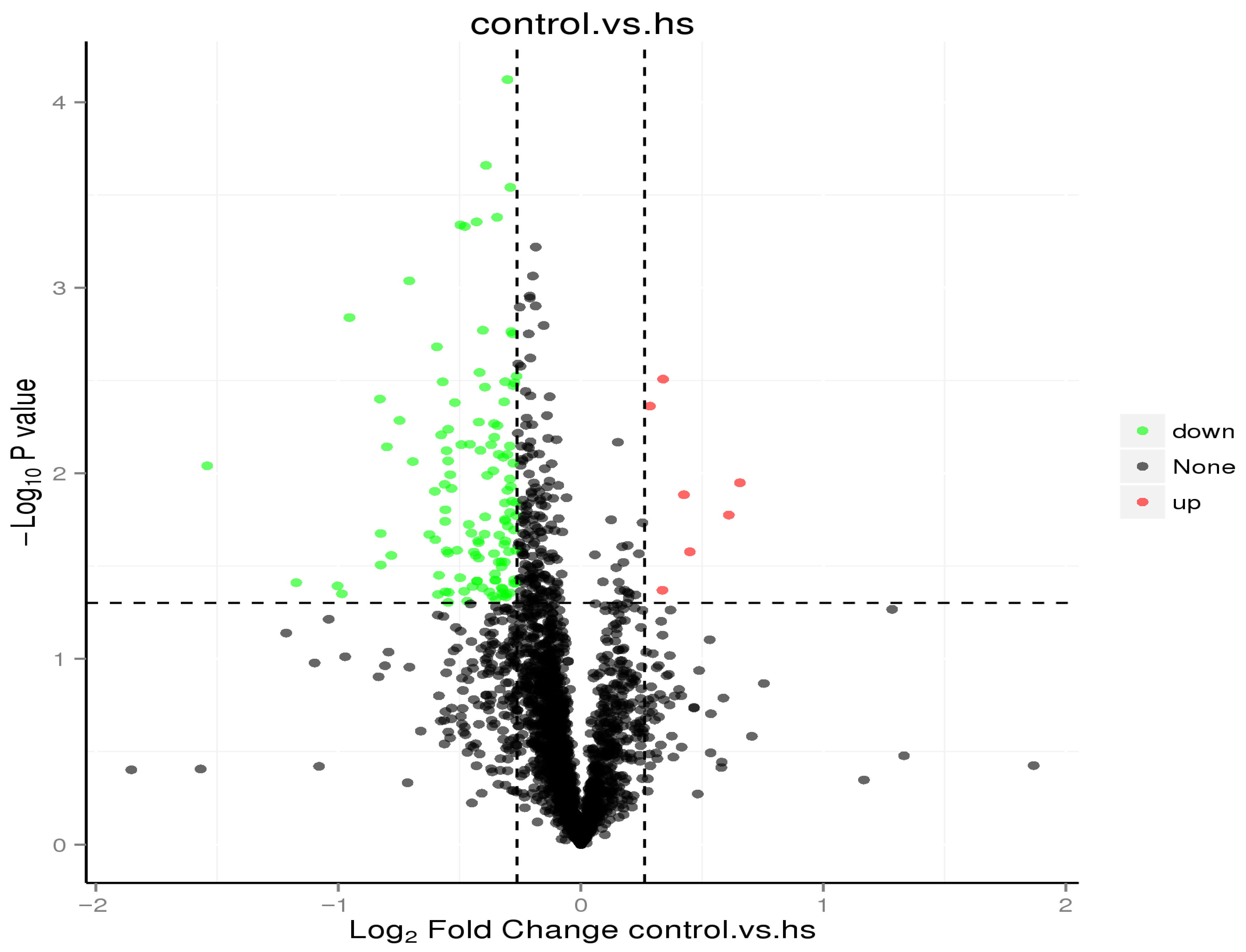
3.6.4. Differential Protein Analysis
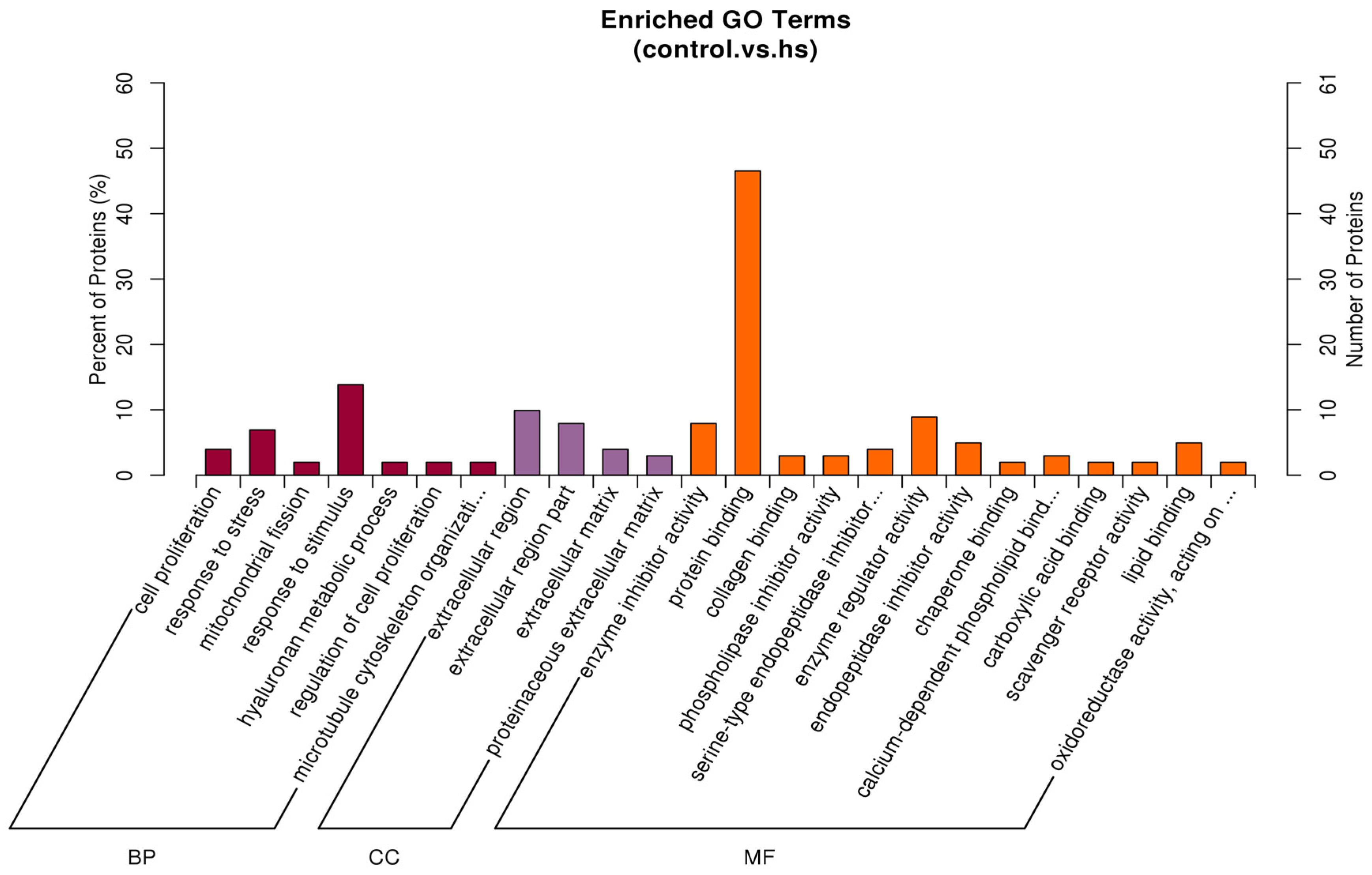
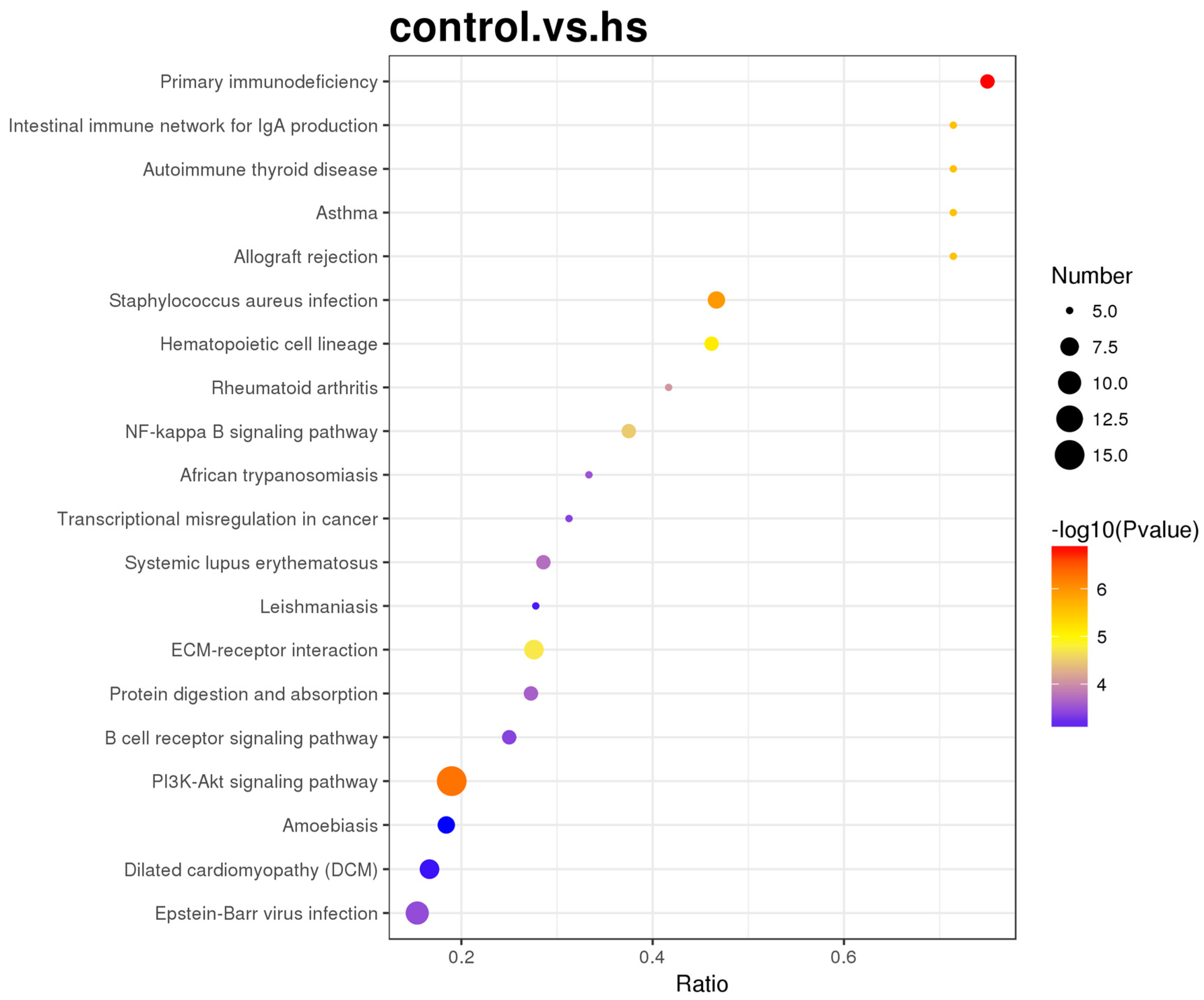
3.6.5. Enrichment Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chang-Fung-Martel, J.; Harrison, M.T.; Brown, J.N.; Rawnsley, R.; Smith, A.P.; Meinke, H. Negative relationship between dry matter intake and the temperature-humidity index with increasing heat stress in cattle: A global meta-analysis. Int. J. Biometeorol. 2021, 65, 2099–2109. [Google Scholar] [CrossRef]
- Asemota, O.D.; Aduba, P.; Mello, G.; Orheruata, A.M. Effect of temperature humidity index (THI) on the performance of rabbits (Oryctolagus cuniculus) in the humid tropics. Arch. De Zootec. 2017, 66, 257–261. [Google Scholar]
- Marco-Jiménez, F.; García-Diego, F.J.; Vicente, J.S. Effect of gestational and lactational exposure to heat stress on performance in rabbits. World Rabbit Sci. 2017, 25, 17–25. [Google Scholar] [CrossRef][Green Version]
- Brown-Brandl, T.M.; Nienaber, J.A.; Xin, H.; Gates, R.S. A literature review of swine heat production. Trans. ASABE 2004, 47, 259–270. [Google Scholar] [CrossRef]
- Jing, J.; Wang, J.; Wu, Q.; Yin, S.; He, Z.; Tang, J.; Jia, G.; Liu, G.; Chen, X.; Tian, G.; et al. Nano-Se exhibits limited protective effect against heat stress induced poor breast muscle meat quality of broilers compared with other selenium sources. J. Anim. Sci. Biotechno. 2024, 15, 2571–2588. [Google Scholar] [CrossRef] [PubMed]
- Pearce, S.C.; Gabler, N.K.; Ross, J.W.; Escobar, J.; Patience, J.F.; Rhoads, R.P.; Baumgard, L.H. The effects of heat stress and plane of nutrition on metabolism in growing pigs. J. Anim. Sci. 2013, 91, 2108–2118. [Google Scholar] [CrossRef] [PubMed]
- Wheelock, J.B.; Rhoads, R.P.; VanBaale, M.J.; Sanders, S.R.; Baumgard, L.H. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 2010, 93, 644–655. [Google Scholar] [CrossRef]
- Pearce, S.C.; Mani, V.; Boddicker, R.L.; Johnson, J.S.; Weber, T.E.; Ross, J.W.; Rhoads, R.P.; Baumgard, L.H.; Gabler, N.K. Heat stress reduces intestinal barrier integrity and favors intestinal glucose transport in growing pigs. PLoS ONE 2013, 8, e70215. [Google Scholar] [CrossRef]
- Pontalti, E.; Pirrone, F.; Zotte, D.A.; Eleonora, N.; Rina, V.; Simona, M.; Marco, B. Impact of heat stress on growth performance and carcass traits of fast- medium- and slow-growing broiler chicken genotypes. Poult. Sci. 2025, 104, 105509. [Google Scholar] [CrossRef]
- Genz, J.; West, C. Effects of rearing temperature on growth, energy reserves, and thermal plasticity of juvenile lake sturgeon. Fish Physiol. Biochem. 2025, 51, 124. [Google Scholar] [CrossRef]
- Li, M.; Cheng, J.B.; Shi, B.L.; Yang, H.J.; Zheng, N.; Wang, J.Q. Effects of heat stress on serum insulin, adipokines, AMP-activated protein kinase, and heat shock signal molecules in dairy cows. J. Zhejiang Univ.-Sci. Biotech. 2015, 16, 541–548. [Google Scholar]
- Baumgard, L.H.; Rhoads, R.P., Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 2013, 1, 311–337. [Google Scholar] [CrossRef]
- Wiśniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Zheng, X.; Ning, C.; Dong, Y.; Zhao, P.; Li, J.; Fan, Z.; Li, J.; Yu, Y.; Mrode, R.; Liu, J. Quantitative proteome analysis of bovine mammary gland reveals protein dynamic changes involved in peak and late lactation stages. Biochem. Biophys. Res. Commun. 2017, 494, 292–297. [Google Scholar] [CrossRef]
- Wang, X.; Shi, T.; Zhao, Z.; Hou, H.; Zhang, L. Proteomic analyses of sheep (ovis aries) embryonic skeletal muscle. Sci. Rep. 2020, 10, 1750. [Google Scholar] [CrossRef] [PubMed]
- Marai, I.F.M.; Habeeb, A.A.M.; Gad, A.E. Rabbits’ productive, reproductive and physiological performance traits as affected by heat stress: A review. Livest. Prod. Sci. 2002, 78, 71–90. [Google Scholar] [CrossRef]
- Blasco, A.; Ouhayoun, J. Harmonization of criteria and terminology in rabbit meat research. World Rabbit Sci. 1996, 4, 93–99. [Google Scholar] [CrossRef]
- Liu, G.; Bai, L.; Sun, H.; Liu, C.; Yang, L.; Jiang, W.; Zhang, Y.; Gao, S. The effect of conjugated linoleic acids on the growth performance, carcase composition and meat quality of fattening rabbits. Ital. J. Anim. Sci. 2022, 21, 1074–1083. [Google Scholar] [CrossRef]
- Baumgard, L.H.; Rhoads, R.P. Ruminant production and metabolic responses to heat stress. J. Anim. Sci. 2012, 90, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Ratel, E.T.I.; Mekawy, A.; Hassab, H.S.; Abdelnour, S. Enhancing growing rabbit heat stress resilience through dietary supplementation with natural antioxidants. BMC Vet. Res. 2025, 21, 28. [Google Scholar]
- El-Ratel, I.T.; Al-Samarai, E.A.; El Basuini, M.F.; El-Kholy, K.H.; Gomaa, A.M.; Abdel-Khalek, A.M.; Fouda, S.F.; Hassan, M.A.E.; El-Raghi, A.A.; Momenah, M.A.; et al. Investigating the dose-response relationship of gum Arabic (Acacia senegal) in ameliorating heat stress responses in rabbits. J. Agricul. Food Res. 2025, 21, 101936. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Ayyat, M.S.; Abd El-Monem, U.M. Growth performance and reproductive traits at first parity of New Zealand White female rabbits as affected by heat stress and its alleviation under Egyptian conditions. Trop. Anim. Health Prod. 2001, 33, 451–462. [Google Scholar] [CrossRef]
- Okab, A.B.; El-Banna, S.G. Physiological and biochemical parameters in New-Zealand white male rabbits during spring and summer seasons. Egypt. J. Basic Appl. Physiol. 2003, 2, 289–300. [Google Scholar]
- Okab, A.B.; El-Banna, S.G.; Koriem, A.A. Influence of environmental temperatures on some physiological and biochemical parameters of male New-Zealand rabbits. Slovak J. Anim. Sci. 2008, 41, 12–19. [Google Scholar]
- Sirotkin, A.; Parkanyi, V.; Pivko, J. High temperature impairs rabbit viability, feed consumption, growth and fecundity: Examination of endocrine mechanisms. Domest. Anim. Endocrin. 2021, 74, 106478. [Google Scholar] [CrossRef]
- Ayyat, M.S.; Marai, I.F.M. Effects of heat stress on growth, carcass traits and blood components of New Zealand White rabbits fed various dietary energy-fiber levels, under Egyptian conditions. J. Arid Environ. 1997, 37, 557–568. [Google Scholar] [CrossRef]
- Ogunjimi, L.A.O.; Ogunwande, G.A.; Osunade, J.A. Rabbit weight gain, feed efficiency, rectal temperature and respiration rate as affected by building thermal environment in the humid tropical climate of Southwestern Nigeria. Agric. Int. CIGR E-J. 2008, 10, 1–14. [Google Scholar]
- Belhadj Slimen, I.; Najar, T.; Ghram, A.; Abdrrabba, M. Heat stress effects on livestock: Molecular, cellular and metabolic aspects, a review. J. Anim. Physiol. Anim. Nutr. 2016, 100, 401–412. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Jia, G.Q.; Zuo, J.J.; Zhang, Y.; Lei, J.; Ren, L.; Feng, D.Y. Effects of constant and cyclic heat stress on muscle metabolism and meat quality of broiler breast fillet and thigh meat. Poult. Sci. 2012, 91, 2931–2937. [Google Scholar] [CrossRef] [PubMed]
- Angus, D.J.; Febbraio, M.A.; Lasini, D.; Hargreaves, M. Effect of carbohydrate ingestion on glucose kinetics during exercise in the heat. J. Appl. Physiol. 2001, 90, 601–605. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Bai, X.; Xu, K.; Zhang, C.; Chen, L. Effect of phloretin ongrowth performance, serum biochemical parameters and antioxidant profile in heat-stressed broilers. Poult. Sci. 2021, 100, 101217. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Baker, M.B.; Hecht, R.; Winters, D.; Boone, T. Effects of the obese gene product on body weight regulation in ob/ob mice. Science 1995, 269, 540–543. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef]
- Balthasar, N.; Coppari, R.; Mc, M.J.; Liu, S.M.; Lee, C.E.; Tang, V.; Kenny, C.D.; Robert, A.M.; Streamson, C.J.; Joel, K.E.; et al. Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 2004, 42, 983–991. [Google Scholar] [CrossRef]
- Farooqi, I.S.; Matarese, G.; Lord, G.M.; Keogh, J.M.; Lawrence, E.; Agwu, C.; Sanna, V.; Jebb, S.A.; Perna, F.; Fontana, S.; et al. Beneficial effects of leptin on obesity, T cell hyporesponsiveness, and neuroendocrine/metabolic dysfunction of human congenital leptin deficiency. J. Clin. Investig. 2002, 110, 1093–1103. [Google Scholar] [CrossRef]
- Norton, P.A. Affect of serum leptin on nutritional status in renal disease. J. Am. Diet. Assoc. 2002, 102, 1119–1125. [Google Scholar] [CrossRef]
- Thuahnai, S.T.; Lund-Katz, S.; Williams, D.L.; Phillips, M.C. Scavenger receptor class B, type I-mediated uptake of various lipids into cells. Influence of the nature of the donor particle interaction with the receptor. J. Biol. Chem. 2001, 276, 43801–43808. [Google Scholar] [CrossRef]
- Rinaldo, D.; Le, D.J. Effects of warm exposure on adipose tissue and muscle metabolism in growing pigs. Comp. Biochem. Phys. A 1991, 100, 995–1002. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, C.; Zhang, Y.; Zhao, X. Effects of heat stress on the muscle meat quality of Rainbow Trout. Fishes 2024, 9, 459. [Google Scholar] [CrossRef]
- Abu Hafsa, S.H.; Centoducati, G.; Hassan, A.A.; Maggiolino, A.; Elghandour, M.M.M.Y.; Salem, A.Z.M. Effects of dietary supplementations of Vitamin C, organic selenium, betaine, and pomegranate peel on alleviating the effect of heat stress on growing rabbits. Animals 2024, 14, 950. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Fan, Z.; Wu, D.; Wang, L.; Wang, C.; Liu, H.; Yang, Y.; Han, S. Effects of arginine supplementation in high-carbohydrate diets on the growth, hematological parameters, and hepatic and skeletal muscle glucose metabolism of juvenile mirror carp (Cyprinus carpio) based on PI3K/Akt signaling pathway. Aquac. Rep. 2024, 39, 102409. [Google Scholar] [CrossRef]
- Qu, Y.; Xiong, W.; Zhou, R.; Song, N.; Qian, J. Dexmedetomidine mitigates oxidative stress in H9C2 cardiac myoblasts under a high-glucose environment via the PI3K/AKT signaling pathway. Mol. Med. Rep. 2025, 32, 251. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Y.; Yang, W.; Xiao, C.; Fu, S.; Deng, Q.; Ding, H.; Wang, Z.; Liu, Q.; Li, X. SREBP-1c overexpression induces triglycerides accumulation through increasing lipid synthesis and decreasing lipid oxidation and VLDL assembly in bovine hepatocytes. J. Steroid Biochem. 2014, 143, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Nayak, R.R.P.S.; Das, A.; Ramamurthy, K.; Pasupuleti, M.; Rajagopal, R.; Arockiaraj, J. Exposure to bisphenol A and sodium nitrate found in processed meat induces endocrine disruption and dyslipidemia through PI3K/AKT/SREBP pathway in zebrafish larvae. J. Nutr. Biochem. 2025, 140, 109887. [Google Scholar] [CrossRef] [PubMed]
| Raw Material Composition | Content | Nutrients (2) | Content |
|---|---|---|---|
| Corn | 17.0 | Digestible energy (MJ/kg) | 10.28 |
| Soybean meal | 10.0 | Crude protein | 16.20 |
| Wheat middling | 8.0 | Crude fibre | 17.47 |
| Wheat bran | 12.0 | Ash | 11.75 |
| Corn germ meal | 10.0 | Crude fat | 2.79 |
| Peanut straw powder | 25.0 | Lysine | 0.60 |
| Sunflower meal | 8.0 | Methionine | 0.27 |
| Soybean straw powder | 7.0 | Calcium | 0.97 |
| Premix (1) | 3.0 | Total phosphorus | 0.43 |
| Total | 100.0 |
| Items | Control | Heat Stress | Root Mean Square Error | p-Value |
|---|---|---|---|---|
| Initial body weight (g) | 2392.0 ± 68.7 | 2326.0 ± 56.1 | 198.3543 | 0.4665 |
| Average daily gain (g/d) | 62.90 ± 3.20 a | 51.70 ± 4.73 b | 10.8282 | 0.0201 |
| Average daily feed intake (g/d) | 215.31 ± 13.32 a | 185.76 ± 11.36 b | 20.6241 | 0.0362 |
| Feed–gain ratio | 4.07 ± 0.81 | 3.99 ± 0.67 | 2.3614 | 0.9369 |
| Items | Control | Heat Stress | Root Mean Square Error | p-Value |
|---|---|---|---|---|
| Preslaughter body weight (g) | 3650.0 ± 102.5 a | 3360.0 ± 96.7 b | 222.9132 | 0.0426 |
| Semiclean carcass weight (g) | 1964.0 ± 76.6 a | 1934.0 ± 78.6 b | 153.5178 | 0.0288 |
| Full clean carcass weight (g) | 1887.0 ± 74.1 a | 1865.4 ± 78.4 b | 152.5478 | 0.0440 |
| Semiclean slaughter ratio (%) | 57.72 ± 1.17 a | 54.05 ± 0.50 b | 2.1116 | 0.0173 |
| Full clean slaughter ratio (%) | 51.93 ± 1.13 a | 49.67 ± 0.63 b | 2.7265 | 0.0167 |
| Head weight (g) | 123.8 ± 3.1 | 125.7 ± 2.9 | 9.4331 | 0.6578 |
| Heart weight (g) | 6.1 ± 0.5 | 5.0 ± 0.2 | 1.1762 | 0.0510 |
| Liver weight (g) | 58.4 ± 2.1 a | 52.1 ± 1.6 b | 5.9503 | 0.0293 |
| Kidney weight (g) | 12.5 ± 0.5 | 11.5 ± 0.9 | 2.3688 | 0.3577 |
| Items | Control | Heat Stress | Root Mean Square Error | p-Value |
|---|---|---|---|---|
| Serum hormone indices | ||||
| Insulin (μIU/mL) | 5.12 ± 1.00 | 6.06 ± 0.93 | 3.0460 | 0.4974 |
| Glucagon (μIU/mL) | 270.33 ± 6.55 | 263.12 ± 8.77 | 24.4779 | 0.5186 |
| Leptin (ng/mL) | 13.71 ± 0.79 b | 21.09 ± 0.79 a | 2.5105 | 0.0001 |
| Serum biochemical indices (mmol/L) | ||||
| Glucose | 2.62 ± 0.44 | 2.73 ± 0.27 | 1.1491 | 0.6526 |
| Total protein | 60.28 ± 1.94 a | 50.08 ± 1.78 b | 5.8900 | 0.0041 |
| Cholesterol | 1.08 ± 0.16 b | 1.71 ± 0.21 a | 0.5882 | 0.0285 |
| Triglyceride | 3.20 ± 0.92 | 3.68 ± 0.31 | 2.1696 | 0.6227 |
| High-density lipoprotein (HDL) | 0.53 ± 0.09 b | 0.96 ± 0.13 a | 0.3571 | 0.0160 |
| Low-density lipoprotein (LDL) | 0.51 ± 0.08 b | 0.89 ± 0.08 a | 0.2497 | 0.0034 |
| Serum immune indices | ||||
| Immunoglobulin G (IgG, μg/mL) | 8.11 ± 0.14 a | 6.87 ± 0.28 b | 0.7141 | 0.0011 |
| Immunoglobulin M (IgM, ng/mL) | 687.66 ± 11.73 a | 654.52 ± 9.29 b | 33.4621 | 0.0399 |
| Immunoglobulin A (IgA, ng/mL) | 124.97 ± 0.87 a | 118.09 ± 2.86 b | 6.6971 | 0.0338 |
| Items | Control | Heat Stress | Root Mean Square Error | p-Value |
|---|---|---|---|---|
| pHu value | 6.17 ± 0.06 | 6.10 ± 0.05 | 0.1785 | 0.4475 |
| Lightness (L*) | 49.45 ± 1.06 | 50.66 ± 0.83 | 5.2146 | 0.3712 |
| Redness (a*) | 11.26 ± 0.66 | 11.04 ± 0.75 | 3.8607 | 0.8277 |
| Yellowness (b*) | 6.79 ± 0.38 b | 8.02 ± 0.35 a | 2.0105 | 0.0214 |
| Shear force (N) | 32.35 ± 2.53 | 31.22 ± 2.10 | 7.3544 | 0.7354 |
| Drip loss ratio (%) | 2.60 ± 0.32 | 3.45 ± 0.35 | 1.0508 | 0.0876 |
| Cooking loss ratio (%) | 36.74 ± 0.67 | 35.14 ± 0.65 | 2.0777 | 0.1022 |
| Compared Samples | Quantifiable Protein | Regulated Type | Fold-Change > 1.2 or ≤0.84 | Fold-Change > 1.3 or ≤0.77 | Fold-Change > 1.5 or ≤0.67 | Fold-Change > 2.0 or ≤0.50 |
|---|---|---|---|---|---|---|
| Control vs. Heat Stress (hs) | 2460 | Up | 7 | 4 | 2 | 0 |
| Down | 122 | 64 | 19 | 3 |
| Protein | Description | Gene | Fold-Change | p-Value | log2 Fold-Change | Regulated Type |
|---|---|---|---|---|---|---|
| G1SUJ3 | Histone H2A | H2AFV | 1.5757 | 0.0113 | 0.6560 | up |
| B7NZF9 | Nucleophosmin 1 isoform 1 (predicted) | NPM1 | 1.5266 | 0.0168 | 0.6104 | up |
| P01870 | Ig gamma chain C region | Unmatched | 0.3437 | 0.0091 | −1.5406 | down |
| A0A1Y1B8B3 | IgG heavy chain VDJ region (fragment) | Unmatched | 0.4434 | 0.0389 | −1.1732 | down |
| A0A1Y1BG72 | IgG heavy chain VDJ region (fragment) | Unmatched | 0.4989 | 0.0405 | −1.0031 | down |
| G1T4P1 | Fibrinogen C-terminal domain-containing protein | ANGPTL7 | 0.5052 | 0.0447 | −0.9852 | down |
| A0A1Y1B9K3 | IgM light chain (fragment) | Unmatched | 0.5162 | 0.0014 | −0.9539 | down |
| G1TRK9 | Ig-like domain-containing protein | Unmatched | 0.5630 | 0.0040 | −0.8287 | down |
| G1TMM0 | Inter-alpha-trypsin inhibitor heavy chain H3 | ITIH3 | 0.5643 | 0.0211 | −0.8255 | down |
| G1SM64 | Uncharacterized protein | ITIH2 | 0.5644 | 0.0312 | −0.8252 | down |
| G1TVZ5 | Ig-like domain-containing protein | Unmatched | 0.5744 | 0.0072 | −0.7998 | down |
| G1T5B1 | LRRNT domain-containing protein | KERA | 0.5817 | 0.0277 | −0.7816 | down |
| G1U2T3 | Uncharacterized protein | STIMATE | 0.5957 | 0.0052 | −0.7473 | down |
| A0A1Y1B9M1 | IgM light chain (fragment) | Unmatched | 0.6125 | 0.0009 | −0.7072 | down |
| A0A1Y1B8Z0 | IgG light chain (fragment) | Unmatched | 0.6187 | 0.0087 | −0.6927 | down |
| G1SEK8 | Uncharacterized protein | FETUB | 0.6488 | 0.0214 | −0.6242 | down |
| P01687 | Ig kappa chain V region BS-5 | Unmatched | 0.6588 | 0.0125 | −0.6020 | down |
| G1T3Z1 | C-type lectin domain-containing protein | MBL2 | 0.6597 | 0.0228 | −0.6001 | down |
| G1THZ6 | Uncharacterized protein | Unmatched | 0.6627 | 0.0021 | −0.5936 | down |
| A0A1Y1BDX9 | IgG light chain (fragment) | Unmatched | 0.6649 | 0.0450 | −0.5888 | down |
| P01697 | Ig kappa chain V region AH80-5 | Unmatched | 0.6666 | 0.0355 | −0.5851 | down |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, G.; Liu, C.; Sun, H.; Bai, L.; Yang, L.; Zhang, Y.; Gao, S. Effects of Heat Stress on Production Performance and Protein Metabolism of Skeletal Muscle in Meat Rabbits. Animals 2025, 15, 2560. https://doi.org/10.3390/ani15172560
Liu G, Liu C, Sun H, Bai L, Yang L, Zhang Y, Gao S. Effects of Heat Stress on Production Performance and Protein Metabolism of Skeletal Muscle in Meat Rabbits. Animals. 2025; 15(17):2560. https://doi.org/10.3390/ani15172560
Chicago/Turabian StyleLiu, Gongyan, Ce Liu, Haitao Sun, Liya Bai, Liping Yang, Yin Zhang, and Shuxia Gao. 2025. "Effects of Heat Stress on Production Performance and Protein Metabolism of Skeletal Muscle in Meat Rabbits" Animals 15, no. 17: 2560. https://doi.org/10.3390/ani15172560
APA StyleLiu, G., Liu, C., Sun, H., Bai, L., Yang, L., Zhang, Y., & Gao, S. (2025). Effects of Heat Stress on Production Performance and Protein Metabolism of Skeletal Muscle in Meat Rabbits. Animals, 15(17), 2560. https://doi.org/10.3390/ani15172560








