Simple Summary
Acute cold stress adversely affects dairy cow health and lactation, but the specific mechanism by which damages bovine mammary epithelial cells (BMECs) remains unclear. Our study shows that acute cold stress harms BMECs by increasing cell apoptosis and disrupting the balance of apoptosis-related genes. We further identified that alpha-enolase (ENO1), a key glycolytic enzyme with multiple non-glycolytic functions in pathophysiological processes including signal transduction and apoptosis, plays a critical protective role, since its downregulation exacerbates apoptosis. These findings explain how acute cold stress impairs the normal function of BMECs and suggest a new direction for protecting dairy cows from cold stress by targeting ENO1 as a potential target. This research could lead to better strategies for maintaining cow mammary health, ensuring stable milk production, and reducing economic losses for farmers in cold environments.
Abstract
Low-temperature environments in cold regions pose a significant threat to cattle farming. Bovine mammary epithelial cells (BMECs) are highly sensitive to cold stress, and acute cold stress can induce apoptosis, adversely affecting lactation performance and health. To explore the mechanism of acute cold stress-induced apoptosis in BMECs, we established an in vitro acute cold stress model. Results showed that mRNA levels of HSP90 increased significantly in a time-dependent manner after 2 h of cold stress, confirming successful model establishment. Following 4 h of cold stress, pro-apoptotic genes (Caspase-3, Bax) exhibited significantly elevated mRNA levels, while the anti-apoptotic gene (BCL-2) showed significantly reduced mRNA levels. Concurrently, the apoptosis rate increased significantly, indicating that acute cold stress induces apoptosis and suggesting the 4 h mark may represent a critical transition point. Integrated transcriptomic and functional analyses identified ENO1 as a core metabolic regulator counteracting acute cold stress-induced apoptosis in BMECs. As a multifunctional protein, ENO1 (alpha-enolase) acts as a central enzyme in glycolysis while exerting additional roles in cellular signaling and apoptotic processes, thereby participating in various pathophysiological regulations. Both mRNA and protein levels of ENO1 were significantly elevated in cold-stressed cells compared to untreated controls. Importantly, interference with ENO1 expression aggravated the extent of cold stress-induced apoptosis, demonstrating the regulatory role of ENO1 in this process. To our knowledge, this is the first report elucidating the core regulatory function of ENO1 in acute cold stress-induced apoptosis in BMECs. These findings provide a theoretical basis for understanding apoptotic mechanisms under stress.
1. Introduction
Against the backdrop of intensifying global climate change and frequent extreme cold events, the adverse effects of cold environments on the cattle industry have become increasingly prominent. In major dairy-producing regions such as Russia and Ontario, Canada, extreme winter cold has emerged as a critical constraint on dairy industry development. Studies indicate that winter cold waves can induce cold stress responses in dairy cows [1]. This response exerts multifaceted detrimental effects on production performance. For one thing, it causes significant declines in milk yield; research by Thompson et al. has confirmed that reduced mammary blood flow under cold conditions may be a key mechanism inhibiting milk secretion [2]. For another, it increases the incidence of diseases such as mastitis, elevating morbidity and mortality rates in calves, and ultimately reducing economic returns [3,4].
Cold stress refers to a series of defensive responses and functional adjustments initiated by the body when animals are exposed to temperatures below their temperature tolerance threshold [5,6]. As an important type of environmental stress parallel to heat stress, both can activate the neuro-endocrine-immune network, trigger oxidative stress and metabolic disorders, and affect the physiological health of animals [7,8,9]. However, there are significant differences in physiological mechanisms between cold stress and heat stress. Heat stress is centered on heat dissipation disorders, where cell damage stems from high-temperature-induced protein denaturation, and cell homeostasis is mainly maintained by activating heat shock proteins (HSPs) [10,11]. In contrast, cold stress focuses on the surge in heat production demand, and copes with low temperatures by enhancing metabolic heat production and activating cold resistance-related genes [12,13,14].
Under normal circumstances, animals maintain stable core body temperature by enhancing metabolic heat production. However, when the ambient temperature is excessively low, it leads to an imbalance between heat production and heat dissipation, disrupting the homeostasis of the body’s internal environment and subsequently triggering physiological dysfunction [15]. Studies have revealed that cold stimulation directly reduces blood flow in the mammary tissues of ruminants. On one hand, this is because low temperatures activate the sympathoadrenal medullary axis to release norepinephrine, and simultaneously activate oxidative stress-related pathways, which further exacerbate norepinephrine-mediated vasoconstriction. On the other hand, to maintain core body temperature, the body prioritizes blood distribution to core organs, thereby reducing mammary blood flow [16,17,18,19]. Reduced mammary blood flow results in insufficient nutrient supply and impaired excretion of metabolic waste, placing bovine mammary epithelial cells (BMECs) in an adverse metabolic environment. This leads to mitochondrial dysfunction, insufficient ATP production, and triggers the mitochondrial apoptotic pathway. Additionally, direct damage caused by low temperatures further exacerbates BMEC apoptosis [20,21,22]. The phenomenon of cold stress-induced cell apoptosis has been confirmed in various cell types, including mouse lymphocytes and bovine Sertoli cells [23,24,25,26]. As a genetically regulated programmed death process, cell apoptosis is of great significance in maintaining tissue homeostasis and responding to environmental stress [27,28]. However, excessive apoptosis leading to large-scale cell death can have adverse effects on the organism [29,30].
To counteract cold stress-induced damage, cells activate intrinsic cold resistance mechanisms. Alpha-enolase (ENO1), a key enzyme in the glycolytic pathway, also possesses diverse non-glycolytic functions [31,32]. It is involved in various pathophysiological processes such as cellular signal transduction and apoptosis [33,34]. Existing studies have suggested that under environmental stress conditions, the expression level and functional status of ENO1 may undergo adaptive changes, thereby affecting the process of cell apoptosis. For dairy cows, the number of mammary epithelial cells and their lactation capacity directly determine milk yield, and reducing their apoptosis rate is an important prerequisite for maintaining lactation performance [35].
However, research on the mechanisms underlying cold stress-induced apoptosis in bovine mammary epithelial cells (BMECs) remains limited, and the role of ENO1 in this process is yet to be defined. Therefore, this study employed BMECs to establish an in vitro model simulating acute cold stress. Integrating transcriptomic analysis and functional validation, we systematically investigated the signaling pathways of acute cold stress-induced apoptosis and the core regulatory function of ENO1. Our findings will provide a theoretical basis for deciphering the apoptotic mechanisms in bovine mammary cells under acute cold stress, while also offering foundational insights for breeding cold-resistant dairy cattle lines and informing the development of cold-wave prevention strategies for dairy farms.
2. Materials and Methods
2.1. Cell Culture and Treatment
The bovine mammary epithelial cell line (BMECs, MAC-T) was cultured in DMEM/F12 medium supplemented with 10% fetal bovine serum (Gibco, Carlsbad, CA, USA) and 1% penicillin-streptomycin (Corning, Corning, NY, USA) at 37 °C under 5% CO2. To investigate the effects of acute cold stress on BMECs, cells at 85–90% confluence were seeded into 6-well or 12-well plates (Corning, Corning, NY, USA) and incubated overnight at 37 °C according to established protocols. The following day, cells were transferred to a 4 °C environment and subjected to cold stress treatment for 0, 2, 4, 6, or 8 h in medium containing 0.1 M HEPES (pH 7.4; Thermo Fisher Scientific, Waltham, MA, USA). Cells were harvested post-treatment for subsequent experiments. Based on combined expression analysis of HSP90 and apoptosis-related genes, the optimal condition for establishing the acute cold stress model was determined to be 4 h of exposure at 4 °C.
2.2. Preparation of Sequencing Samples
RNA sequencing (RNA-seq) analysis was performed on bovine mammary epithelial cells. This included three biological control replicates (CON1, CON2, CON3; cultured at 37 °C for 4 h) and three biological replicates of the cold stress-treated group (LT1, LT2, LT3; treated at 4 °C for 4 h). All sequencing services were provided by LC-Bio Technology Co., Ltd. (Hangzhou, China).
2.3. Apoptosis Assay
Cell apoptosis rates were determined using an Annexin V-FITC Apoptosis Detection Kit (Beyotime, Nantong, China). Following the manufacturer’s protocol, cells were collected after cold stress treatment, stained with Annexin V-FITC and propidium iodide (PI), and incubated at room temperature for 15 min. DAPI (Beyotime, Nantong, China) was added to stain nuclei. Cells were observed under an inverted fluorescence microscope (Olympus, Tokyo, Japan): nuclei appeared blue (DAPI), early apoptotic cells fluoresced green (Annexin V-FITC positive only), and late apoptotic cells fluoresced red (double positive for Annexin V-FITC and PI). At least three random fields per group were captured. Quantification using ImageJ2 software (National Institutes of Health, Bethesda, MD, USA) involved: (1) converting images to 8-bit grayscale; (2) applying a uniform threshold to all images to distinguish signal from background; (3) counting positive cells using the “Analyze Particles” tool, with size and circularity parameters set to exclude debris (0.5–50 μm2, circularity 0.3–1.0). Results are expressed as the percentage of PI- and FITC-positive cells relative to the total number of DAPI-stained nuclei.
2.4. Total RNA Extraction and RT-qPCR
Total RNA was extracted using TRIzol reagent, and its concentration and purity were measured. RNA samples were deemed qualified when the A260/A230 and A260/A280 ratios both exceeded 1.80. RNA integrity was assessed via 1.5% agarose gel electrophoresis; qualified samples exhibited clear, bright 28S and 18S rRNA bands, with the 28S band intensity approximately twice that of the 18S band. Qualified RNA was stored at −80 °C. Reverse transcription was performed using an mRNA Reverse Transcription Kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions. Quantitative real-time PCR (RT-qPCR) was conducted using SYBR® Premix Ex Taq™ (Foregene, Chengdu, China) on a ForeQuant F4 Sequence Detection System. The thermal cycling protocol was: 95 °C for 10 min (initial denaturation); 40 cycles of 95 °C for 10 s (denaturation) and 60 °C for 20 s (annealing/extension); followed by melt curve analysis to verify primer specificity. All primer sequences used in this study are listed in Table 1. The relative expression level of each gene was calculated using the 2(−ΔΔCt) method, with β-actin as the internal reference gene.

Table 1.
Primer sequences.
2.5. siRNA Synthesis and Transfection
Small interfering RNAs (siRNAs) and negative control (NC) siRNA were designed and synthesized by GenePharma (Shanghai, China). Their sequences are listed in Table 2. For transfection, cells were seeded into 6-well plates. Upon reaching 50–60% confluence, siRNA (final concentration: 50 nM) and Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) were mixed in Opti-MEM medium (Gibco, Grand Island, NY, USA) according to the manufacturer’s protocol. The mixture was incubated at room temperature for 15 min and then added to the cells. Cells were subsequently incubated at 37 °C for 48 h. Subsequent experiments were performed following verification of transfection efficiency by RT-qPCR or Western blotting.

Table 2.
Sequences of forward and reverse siRNA oligonucleotides targeting ENO1.
2.6. Protein Extraction and Western Blotting
Cells were lysed using radioimmunoprecipitation assay (RIPA) buffer (Beyotime, Nantong, China). Protein concentration was determined with a BCA protein assay kit (Beyotime, Nantong, China). Protein expression levels were detected by Western blotting, with β-actin serving as the internal reference for normalization. Protein samples were separated using SDS-polyacrylamide gel electrophoresis (SDS-PAGE). Subsequently, separated proteins were transferred onto polyvinylidene difluoride (PVDF) membranes (Beyotime, Nantong, China), followed by a 2 h blocking step. After blocking, membranes were incubated with appropriate primary antibodies at 4 °C overnight (antibody dilution concentrations are listed in Table 3). Following secondary antibody incubation, protein bands were visualized using Sparkjade ECLsuper (ED0015-A; Sparkjade Biotechnology Co., Jinan, China).

Table 3.
Antibody dilution concentration.
2.7. Statistical Analysis
All data are presented as mean ± standard error of the mean (SEM). Experiments were independently repeated at least three times. Comparisons between two groups were analyzed using Student’s t-test, while one-way ANOVA was employed for multiple comparisons. Statistical analyses were performed using GraphPad Prism 9. Significance levels are denoted as follows: * p < 0.05, ** p < 0.01.
3. Results
3.1. Establishment of Acute Cold Stress Model and Apoptosis Assessment
After acute cold stress treatment, apoptosis rates, cold adaptation responses, and expression levels of apoptosis-related genes in BMECs were analyzed, with the following results: Annexin V-FITC assays revealed a highly significant increase in apoptosis rates at 4, 6, and 8 h post-cold stress compared to controls (p < 0.01, Figure 1A,B), with apoptosis severity escalating over time. This indicates a positive correlation between cold stress duration and apoptotic progression. RT-qPCR analysis of relevant genes (Figure 1C–G) revealed: Heat shock protein 90 (HSP90, a canonical stress-response marker involved in cytoprotection and protein repair) and the pro-apoptotic gene Bax were upregulated in a time-dependent manner. Caspase3 mRNA levels increased significantly at 4 h and remained elevated thereafter, driving apoptotic progression. The anti-apoptotic gene Bcl-2 exhibited a contrasting trend: its expression decreased in a time-dependent manner starting from 4 h, opposite to the trend of pro-apoptotic genes. In addition, the mRNA expression of ZC3H10 (involved in the regulation of cellular cold acclimatization) peaked at 4 h.
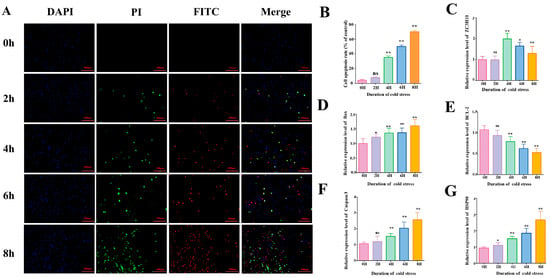
Figure 1.
Acute cold stress induces apoptosis. (A) Annexin V-FITC fluorescence apoptosis assay of BMECs treated at 4 °C (0 h, 2 h, 4 h, 6 h, 8 h); blue fluorescence represents DAPI-stained cells, green fluorescence represents PI-staining-positive early apoptotic cells, and red fluorescence represents FITC-staining-positive late apoptotic cells. (B) Percentage of apoptotic cells. Apoptosis rate = positive apoptotic cells/number of DAPI-stained cells × 100% (n = 3) (C–G) Relative mRNA expression levels of ZC3H10, Bax, BCL-2, Caspase-3, and HSP90 in 4–treated (0 h, 2 h, 4 h, 6 h, 8 h) BMECs (n = 6). Data are expressed as mean ± standard error. * p < 0.05; ** p < 0.01, ns (not significantly different).
Based on the above findings, subsequent experiments used a 4 h acute cold stress treatment to further investigate the molecular mechanisms underlying apoptosis induced by acute cold stress and the functions of related genes.
3.2. Analysis of Transcriptome Sequencing Results
Transcriptomic analysis identified 1205 differentially expressed genes (DEGs) between the control group (CON) and acute cold stress-treated group (LT), comprising 368 upregulated and 837 downregulated genes (Figure 2A). This provides a critical gene set for investigating cold stress effects on cells. To validate sample reliability, hierarchical clustering analysis of DEGs (Figure 2D) revealed pronounced distinctions between CON and LT groups. Principal component analysis (PCA) further demonstrated tight clustering of CON samples, while LT samples formed a separate cluster distinct from CON (Figure 2B). Validation of 10 randomly selected DEGs by RT-qPCR showed high concordance with sequencing data (Figure 2C), further confirming the accuracy of transcriptome sequencing.
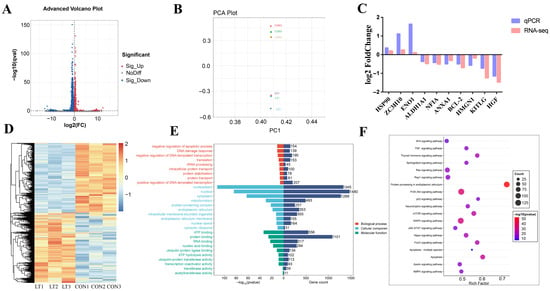
Figure 2.
Analysis of differentially expressed genes. (A) Volcano plot of differentially expressed genes (DEGs) between the control group (CON) and the 4 h acute cold stress-treated group (LT). (B) Principal component analysis (PCA) of the CON group and the 4 h acute cold stress-treated group (LT). (C) Validation of 10 DEGs by RT-qPCR. (D) Heatmap of DEGs between the CON group and the 4 h acute cold stress-treated group (LT). (E) Gene Ontology (GO) enrichment bar plot of DEGs in the CON group versus the 4 h acute cold stress-treated group (LT). (F) Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment bubble plot of DEGs in the CON group versus the 4 h acute cold stress-treated group (LT).
Functionally, Gene Ontology (GO) enrichment analysis (Figure 2E) elucidated cold stress impacts across three domains: In the Biological process dimension, genes were significantly enriched in entries such as “negative regulation of apoptotic process”. In the Cellular component dimension, differentially expressed genes were significantly enriched in nucleoplasm and other subcellular structures. The differential distribution of genes involved in processes such as rRNA processing, intracellular protein transport, and protein stabilization was observed in different subcellular structures. In the Molecular function dimension, genes related to functions such as “ATP binding” and “protein binding” were significantly enriched.
KEGG enrichment analysis (Figure 2F) showed that “Apoptosis” and “Apoptosis—multiple species” pathways were significantly enriched, which directly confirmed that the apoptosis pathway was activated in BMECs under cold stress. apoptosis pathway was activated under cold stress, and a large number of differentially expressed genes were involved in it, which was direct evidence of the cellular initiation of apoptosis program. At the same time, PI3K-Akt, MAPK, p53, and other signaling pathways indirectly related to apoptosis were also activated, which intertwined with each other to constitute the apoptosis regulatory network of BMECs under acute cold stress. Through multi-faceted analysis, functional annotation, and literature research, the key differential gene ENO1 was identified, and the multi-level apoptotic regulatory network of bovine mammary epithelial cells (BMECs) under acute cold stress was clarified.
3.3. ENO1 Regulates Cold Stress-Induced Apoptosis in BMECs
To investigate the role of the ENO1 gene in cold stress-induced apoptosis, this study detected the mRNA and protein levels of ENO1 using RT-qPCR and Western blot. The results showed that compared with the non-interfered control group, the mRNA and protein expression levels of ENO1 in both the control group (37 °C) and cold stress-treated group (4 °C) were significantly decreased in BMECs with ENO1 expression interference (p < 0.05, Figure 3A–C,H,J), indicating successful ENO1 interference.
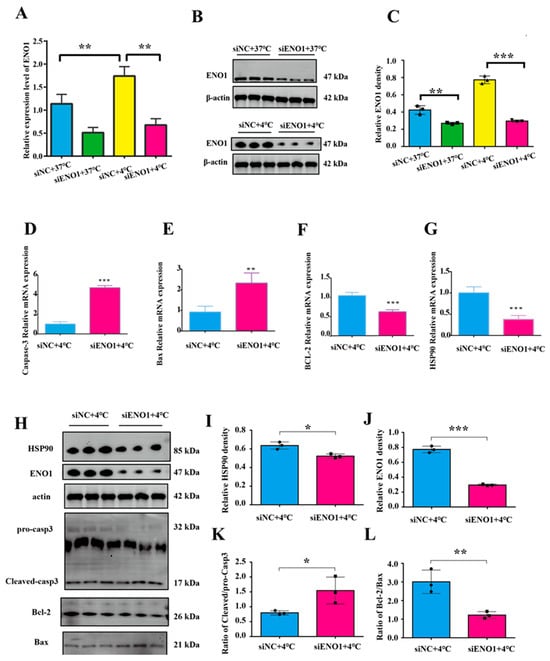
Figure 3.
Interference with ENO1 and acute cold stress co-treatment promotes apoptosis in BMECs. (A–C) Transfection efficiency of ENO1 in BMECs. (D–G) Relative mRNA expression levels of Caspase3, Bax, BCL-2, and HSP90 in BMECs. (H–L) Relative protein expression levels of HSP90, ENO1 and apoptosis-related genes in BMECs. The original Western blot (WB) images are available in the Supplementary Materials. (n = 3). Data are expressed as mean ± standard error. * p < 0.05; ** p < 0.01, *** p < 0.001, ns (not significantly different).
To clarify the correlation between ENO1 and apoptosis in BMECs under acute cold stress conditions, BMECs were co-treated with acute cold stress (4 °C) and ENO1 interference. results showed that after co-treatment, the mRNA expression of the pro-apoptotic genes Bax and Caspase3 was significantly increased (Figure 3D,E), and the mRNA expression of anti-apoptotic gene Bcl-2 and cold stress-related gene HSP90 was significantly decreased (p < 0.05, Figure 3F,G). The results of protein level detection showed that the expression level of HSP90 was significantly reduced (Figure 3I); the ratios of Cleaved/procasp3 and Bax/Bcl-2 were significantly elevated (Figure 3K,L).
4. Discussion
In this study, a reliable in vitro model of acute cold stress in bovine mammary epithelial cells (BMECs) was established to investigate the molecular mechanism of apoptosis induced by acute cold stress. mRNA expression of HSP90, a classical stress biomarker [36,37], was upregulated with the increase in the cold stress treatment time, which indicated that the model was successfully constructed. Based on the successfully constructed model, in order to further investigate the effect of acute cold stress on apoptosis, we further detected apoptosis-related indexes. Given the significant dysregulation of apoptosis-related genes and the elevated apoptosis rate, we identified 4 h as the critical time point for apoptosis initiation, which provides a key reference for exploring the early apoptotic events triggered by cold stress.
Under acute cold stress, excessive accumulation of intracellular reactive oxygen species (ROS) causes oxidative damage and mitochondrial dysfunction [38]. Mitochondria are the main source of intracellular ROS and play a key role in initiating and executing cell apoptosis. When mitochondrial dysfunction occurs, it triggers the mitochondria-dependent apoptotic pathway, leading to the release of cytochrome c, formation of apoptosomes, and subsequent activation of downstream caspase-3, thereby initiating cell apoptosis [39]. Early studies have demonstrated that acute cold stress can induce apoptosis in various mammalian cells [23,24,25,26], and excessive apoptosis of BMECs exerts negative effects on milk production efficiency and mammary gland health [40]. Consistent with these early findings, sequencing results showed that acute cold stress treatment significantly increased the activation of apoptotic pathways. KEGG functional analysis revealed that the “Apoptosis” and “Apoptosis-multiple species” pathways were significantly enriched, which directly confirms that the apoptotic pathways of BMECs are activated under cold stress. A large number of differentially expressed genes are involved in these pathways, providing direct evidence that cells initiate apoptotic programs. Meanwhile, signaling pathways indirectly related to apoptosis, such as PI3K–Akt, MAPK, and p53, are also activated. These pathways interact with each other, collectively forming a regulatory network for BMEC apoptosis under acute cold stress.
Studies by Ma J et al. have indicated that ENO1 can reduce intracellular ROS production and cell apoptosis by regulating mitochondrial homeostasis [41]. As a multifunctional protein, ENO1 participates in the regulation of apoptotic pathways through multiple mechanisms, and its action mechanisms exhibit significant differences in different cell types and pathological conditions. A study on pancreatic ductal adenocarcinoma (PDAC) found that ENO1 mediates cell apoptosis by regulating ERK activation [42]. In a study on hypoxic pulmonary hypertension models, it was observed that ENO1 improves mitochondrial function in endothelial cells and reduces apoptosis through the PI3K-Akt-mTOR pathway [43]. It is thus hypothesized that ENO1 may play an important role in coping with mitochondrial damage caused by acute cold stress by affecting mitochondrial function or related apoptotic pathways.
Based on previous reports and sequencing results, we identified ENO1 as a key regulatory factor. After treating BMECs at 4 °C for 4 h, the expression level of ENO1 was significantly upregulated, indicating that cells have an active compensatory mechanism. GO annotation revealed that ENO1 has both core glycolytic enzyme activity and apoptosis regulatory potential. From the perspective of cellular metabolism, ATP depletion caused by cold stress further exacerbates the apoptotic process. As a key glycolytic enzyme, the upregulation of ENO1 may enhance the glycolytic process and maintain ATP production, thereby alleviating the energy crisis caused by cold stress. This suggests its important role in linking energy metabolism and cell survival. In addition, previous studies have confirmed that ENO1 not only promotes cell survival by regulating glycolysis in breast cancer but also may interact with HSP70 to protect hepatocytes from heat stress [44,45]. Meanwhile, our experimental results confirmed that interfering with ENO1 expression exacerbated acute cold stress-induced apoptosis of BMECs, and these results clarified the core regulatory role of ENO1 in the survival of BMECs under acute cold stress conditions.
Although our research is insightful, there are limitations, including the singularity of the cell model, the failure to consider the interaction between cells in mammary tissues in vivo, the lack of involvement in differences in cellular stress responses under different physiological states, and the failure to explore the role of ENO1 in other types of stress (such as oxidative stress). Subsequent studies need to verify the results in in vivo models (such as cold-stressed lactating dairy cows). Meanwhile, the precise molecular interactions (such as binding partners and pathway crosstalk) through which ENO1 inhibits apoptosis have not been clarified.
In conclusion, we confirmed that acute cold stress induces apoptosis of BMECs and identified ENO1 as a key metabolic factor against this damage. This work not only deepens the understanding of the cold stress response mechanism in bovine mammary epithelial cells but also pioneeringly proposes ENO1 as a potential target for improving the cold resistance of dairy cows.
5. Conclusions
In this study, an in vitro cold stress model of bovine mammary epithelial cells (BMECs) was established (treated at 4 °C), confirming that 4 h is the key time point for apoptosis activation. This is the first study to reveal that α-enolase (ENO1), as a key regulatory factor, plays a central role in resisting apoptosis of BMECs induced by acute cold stress. This discovery provides an important theoretical basis for in-depth understanding of the apoptotic mechanism of BMECs under acute cold stress, clarifies the value of ENO1 as a potential target in improving cellular cold resistance, and lays a foundation for subsequent research on cold resistance breeding of dairy cows. Subsequent animal experiments are required to further verify the regulatory role of ENO1 in vivo and explore its specific molecular mechanism. However, this study has provided a new direction for the improvement strategy of cold resistance traits in dairy cow gene regulation. The findings are expected to offer theoretical support for pastures to formulate more scientific early-warning and intervention plans against cold waves, thereby helping reduce production losses in winter and further facilitating the sustainable development of animal husbandry.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ani15172559/s1, Figure S1: Original Western blot images.
Author Contributions
N.S.: writing—review and editing. J.W.: methodology. J.L.: methodology. H.Y.: data curation. X.J.: data curation. W.S.: investigation. S.L.: funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Innovation in High-quality Cattle Breeding Materials and Methods, and New Breed Selection, Key R&D Program of Sichuan Province, 2021YFYZ0001; Molecular Mechanism of p73 in Regulating Ferroptosis of Bovine Ovarian Granulosa Cells, National Natural Science Foundation of China, 32202670; Exploration of Germplasm Resources and Key Technologies for Efficient Breeding of Yingjing Yellow Cattle, kczx2023-2025-02.
Institutional Review Board Statement
All experiments in the present study involving animals were performed under the direction of the Institutional Animal Care and Use Committee from the College of Animal Science and Technology, Sichuan Agricultural University, China (Certification No. SYXK2019-187; Approval time: 29 January 2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Abbreviations
| BMECs | Bovine Mammary Epithelial Cells |
| ENO1 | α-enolase 1 |
| β-actin | Beta-Actin |
| LT | Low Temperature |
| HSP90 | Heat Shock Protein 90 |
| Caspase3 | Cysteine Aspartyl Protease 3 |
| Bax | BCL-2-associated X protein (BAX) |
| BCL-2 | B-cell lymphoma 2 |
| ZC3H10 | zinc finger CCCH—type containing 10 |
| ALDH1A1 | Aldehyde Dehydrogenase 1 Family Member A1 |
| HGF | Hepatocyte Growth Factor |
| NFIA | Nuclear Factor I A |
| ANXA1 | Annexin A1 |
| HMGN1 | High Mobility Group Nucleosome-Binding Protein 1 |
| KITLG | Kit Ligand |
References
- Angrecka, S.; Herbut, P. Conditions for Cold Stress Development in Dairy Cattle Kept in Free Stall Barn During Severe Frosts. Czech J. Anim. Sci. 2015, 60, 81–87. [Google Scholar] [CrossRef]
- Thompson, G.E.; Thomson, E.M. Effect of Cold Exposure on Mammary Circulation Oxygen Consumption and Milk Secretion in the Goat. J. Physiol. 1977, 272, 187–196. [Google Scholar] [CrossRef]
- Vander Zaag, A.; Le Riche, E.; Baldé, H.; Kallil, S.; Ouellet, V.; Charbonneau, É.; Coates, T.; Wright, T.; Luimes, P.; Gordon, R. Comparing Thermal Conditions inside and Outside Lactating Dairy Cattle Barns in Canada. J. Dairy. Sci. 2023, 106, 4738–4758. [Google Scholar] [CrossRef]
- Lim, D.H.; Mayakrishnan, V.; Ki, K.S.; Kim, Y.; Kim, T.I. The Effect of Seasonal Thermal Stress on Milk Production and Milk Compositions of Korean Holstein and Jersey Cows. Anim. Biosci. 2021, 34, 567–574. [Google Scholar] [CrossRef]
- Zhang, H.W.; Wang, Y.N.; Qiu, D.R. Key Points of Feeding and Management Techniques for Dairy Cows Under Cold Stress Conditions. Breed. Feed. 2022, 21, 47–49. [Google Scholar]
- Su, S.H.; Hu, Y.C.; Wang, Y.; An, X.P.; Qi, J.W. Research Progress on Cold Stress and Intelligent Moni-toring in Ruminants. Chin. J. Anim. Sci. 2024, 60, 47–49. [Google Scholar]
- Bai, H.; Kawahara, M.; Kusama, K.; Sakurai, T.; Pfarrer, C.; Takahashi, M. Heat Stress Induces Oxidative Stress and Activates the KEAP1-NFE2L2-ARE Pathway in Reproduction-Related Cells. Anim. Sci. J. 2025, 96, e70023. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.Q.; Zhang, Z.W.; Qu, J.P.; Yao, H.D.; Li, M.; Li, S.; Xu, S.W. Cold Stress Induces Antioxidants and Hsps in Chicken Immune Organs. Cell Stress. Chaperones 2014, 19, 635–648. [Google Scholar] [CrossRef]
- Gujar, G.; Tiwari, M.; Yadav, N.; Monika, D. Heat Stress Adaptation in Cows—Physiological Responses and Underlying Molecular Mechanisms. J. Therm. Biol. 2023, 118, 103740. [Google Scholar] [CrossRef]
- Becker, C.A.; Stone, A.E. Graduate Student Literature Review: Heat Abatement Strategies Used to Reduce Negative Effects of Heat Stress in Dairy Cows. J. Dairy Sci. 2020, 103, 9667–9675. [Google Scholar] [CrossRef]
- Bettaieb, A.; Averill-Bates, D.A. Thermotolerance Induced at a Mild Temperature of 40 °C Alleviates Heat Shock-Induced ER Stress and Apoptosis in HeLa Cells. Biochim. Biophys. Acta 2015, 1853, 52–62. [Google Scholar] [CrossRef]
- Bornstein, M.R.; Neinast, M.D.; Zeng, X.; Chu, Q.; Axsom, J.; Thorsheim, C.; Li, K.; Blair, M.C.; Rabinowitz, J.D.; Arany, Z. Comprehensive Quantification of Metabolic Flux During Acute Cold Stress in Mice. Cell Metab. 2023, 35, 2077–2092.e6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Xiao, J.; Jiang, M.; Phillips, C.J.C.; Shi, B. Thermogenesis and Energy Metabolism in Brown Adipose Tissue in Animals Experiencing Cold Stress. Int. J. Mol. Sci. 2025, 26, 3233. [Google Scholar] [CrossRef]
- Yudin, N.S.; Larkin, D.M. Candidate Genes for Domestication and Resistance to Cold Climate According to Whole Genome Sequencing Data of Russian Cattle and Sheep Breeds. Vavilovskii Zhurnal Genet. Sel. 2023, 27, 463–470. [Google Scholar] [CrossRef]
- Ma, H.J.; Wang, P.C.; Yu, Y.; Ren, C.H.; Zhang, Z.J.; Wang, Q.J. Mechanisms and intervention measures of cold stress-induced thermogenesis in brown adipose tissue of lambs. Chin. J. Anim. Sci. 2025, 61, 61–67. [Google Scholar]
- Broucek, J.; Letkovicová, M.; Kovalcuj, K. Estimation of Cold Stress Effect on Dairy Cows. Int. J. Biometeorol. 1991, 35, 29–32. [Google Scholar] [CrossRef]
- Ameka, M.; Markan, K.R.; Morgan, D.A.; BonDurant, L.D.; Idiga, S.O.; Naber, M.C.; Zhu, Z.; Zingman, L.V.; Grobe, J.L.; Rahmouni, K.; et al. Liver-Derived FGF21 Maintains Core Body Temperature During Acute Cold Exposure. Sci. Rep. 2019, 9, 630. [Google Scholar] [CrossRef]
- Zha, S.; Ao, R.G. Effects of cold stress on antioxidant function and blood indices of grazing beef cattle. Contemp. Livest. Poult. Breed. Ind. 2021, 6, 12–14. [Google Scholar]
- Fu, K.; Li, Z.Y.; Cui, M.J. Research progress on heat and cold stress in dairy cow production. Today’s Anim. Husb. Vet. Med. 2020, 36, 65. [Google Scholar]
- Davis, S.R.; Collier, R.J. Mammary Blood Flow and Regulation of Substrate Supply for Milk Synthesis. J. Dairy. Sci. 1985, 68, 1041–1058. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Liu, H.; He, X.; Sun, Y.; Ge, W. Unraveling the Mystery of Cold Stress-Induced Myocardial Injury. Front. Physiol. 2020, 11, 580811. [Google Scholar] [CrossRef]
- Li, T.; Bai, H.; Yang, L.; Hao, W.; Wei, S.; Yan, P. Low Temperature Exposure Inhibits Proliferation and Induces Apoptosis of Bovine Subcutaneous Preadipocytes via P38 MAPK/JNK Activation. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2023, 264, 110813. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. The Protective Effects of Taurine on Oxidative Stress, Cytoplasmic Free-Ca(2+) and Apoptosis of Pufferfish (Takifugu obscurus) under Low Temperature Stress. Fish. Shellfish. Immunol. 2018, 77, 457–464. [Google Scholar] [CrossRef]
- Chen, L.; Chen, H. Effect of Mahuang Gancao Ganjiang Decoction on Fusion and Fission of Mitochondria and Apoptosis of Lymphocytes in Mice under Cold Stress. Evid. Based Complement Altern. Med. 2017, 2017, 5132963. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, H.; Zheng, P.; Feng, R.; Wang, X.; Huang, F.; Ma, M.; Tian, Y.; Zhang, G. The Alleviative Effect of Thyroid Hormone on Cold Stress-Induced Apoptosis Via Hsp70 and Mitochondrial Apoptosis Signal Pathway in Bovine Sertoli Cells. Cryobiology 2022, 105, 63–70. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Grune, T.; de Groot, H.; Rauen, U. Cold-Induced Apoptosis of Rat Liver Endothelial Cells: Involvement of the Proteasome. Transplantation 2003, 75, 1946–1953. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, Y.; Nakajima, Y.I.; Kuranaga, E. Apoptosis in Cellular Society: Communication between Apoptotic Cells and Their Neighbors. Int. J. Mol. Sci. 2016, 17, 2144. [Google Scholar] [CrossRef] [PubMed]
- Taabazuing, C.Y.; Okondo, M.C.; Bachovchin, D.A. Pyroptosis and Apoptosis Pathways Engage in Bidirectional Crosstalk in Monocytes and Macrophages. Cell Chem. Biol. 2017, 24, 507–514.e4. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, X.; Zhang, H. Excessive Apoptosis of RIP1-Deficient T Cells Leads to Premature Aging. EMBO Rep. 2023, 24, e57925. [Google Scholar] [CrossRef]
- Green, K.A.; Streuli, C.H. Apoptosis Regulation in the Mammary Gland. Cell Mol. Life Sci. 2004, 61, 1867–1883. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Li, B. Role of ENO1 and Its Targeted Therapy in Tumors. J. Transl. Med. 2024, 22, 1025. [Google Scholar] [CrossRef]
- Díaz-Ramos, A.; Roig-Borrellas, A.; García-Melero, A.; López-Alemany, R. A-Enolase, a Multifunctional Protein: Its Role on Pathophysiological Situations. J. Biomed. Biotechnol. 2012, 2012, 156795. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Qiao, H.; Hao, J.; Deng, C.; Zhou, N.; Yang, L.; Zeng, M.; Guan, Q. RNA-Binding Protein ENO1 Promotes the Tumor Progression of Gastric Cancer by Binding to and Regulating Gastric Cancer-Related Genes. J. Gastrointest. Oncol. 2023, 14, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Aziguli, T.; Xiao, S.Y.; Yang, Y.; Musitaba, M. ENO1 Promotes PDAC Progression by Inhibiting CD8(+) T Cell Infiltration through Upregulating PD-L1 Expression Via HIF-1α Signaling. Transl. Oncol. 2025, 52, 102261. [Google Scholar] [CrossRef]
- Herve, L.; Quesnel, H.; Lollivier, V.; Boutinaud, M. Regulation of Cell Number in the Mammary Gland by Controlling the Exfoliation Process in Milk in Ruminants. J. Dairy Sci. 2016, 99, 854–863. [Google Scholar] [CrossRef]
- Wang, Y.; Qu, P. Cold stress promotes HSP90 expression and disease progression in PCOS rats. Chin. J. Birth Health Hered. 2022, 30, 597–601. [Google Scholar]
- Sajad, S.; Jiang, S.; Anwar, M.; Dai, Q.; Luo, Y.; Hassan, M.A.; Tetteh, C.; Song, J. Genome-Wide Study of HSP90 Gene Family in Cabbage (Brassica oleracea Var. Capitata, L.) and Their Imperative Roles in Response to Cold Stress. Front. Plant Sci. 2022, 13, 908511. [Google Scholar] [CrossRef]
- Blagojevic, D.P.; Grubor-Lajsic, G.N.; Spasic, M.B. Cold Defence Responses: The Role of Oxidative Stress. Front. Biosci. 2011, 3, 416–427. [Google Scholar] [CrossRef]
- Lee, J.H.; Won, Y.S.; Park, K.H.; Lee, M.K.; Tachibana, H.; Yamada, K.; Seo, K.I. Celastrol Inhibits Growth and Induces Apoptotic Cell Death in Melanoma Cells Via the Activation ROS-Dependent Mitochondrial Pathway and the Suppression of PI3K/Akt Signaling. Apoptosis 2012, 17, 1275–1286. [Google Scholar] [CrossRef]
- Liu, L.; Lu, O.; Li, D.; Tian, Y.; Liu, Z.; Wen, Y.; Peng, T.; Song, Y.; Du, X.; Wang, Z.; et al. Sirtuin 3 Mitigates Oxidative-Stress-Induced Apoptosis in Bovine Mammary Epithelial Cells. J. Dairy Sci. 2023, 106, 7266–7280. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Zhu, J.; Li, J.; Liu, J.; Kang, X.; Yu, J. Enhanced E6AP-Mediated Ubiquitination of ENO1 Via Linc00663 Contributes to Radiosensitivity of Breast Cancer by Regulating Mitochondrial Homeostasis. Cancer Lett. 2023, 560, 216118. [Google Scholar] [CrossRef]
- Sun, H.; Mo, J.; Cheng, R.; Li, F.; Li, Y.; Guo, Y.; Li, Y.; Zhang, Y.; Bai, X.; Wang, Y.; et al. ENO1 Expression and ERK Phosphorylation in PDAC and Their Effects on Tumor Cell Apoptosis in a Hypoxic Microenvironment. Cancer Biol. Med. 2022, 19, 1598–1616. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, J.; Zhang, R.; Zhang, M.; Cui, H.; Wang, L.; Cui, Y.; Wang, W.; Sun, Y.; Wang, C. Targeting Endothelial ENO1 (Alpha-Enolase) PI3K-Akt-mTOR Axis Alleviates Hypoxic Pulmonary Hypertension. Hypertension 2023, 80, 1035–1047. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, H.; Miao, L.; Ding, J. Silencing of ENO1 Inhibits the Proliferation, Migration and Invasion of Human Breast Cancer Cells. J. BUON 2020, 25, 696–701. [Google Scholar] [PubMed]
- Zeng, T.; Cao, Y.; Gu, T.; Chen, L.; Tian, Y.; Li, G.; Shen, J.; Tao, Z.; Lu, L. Alpha-Enolase Protects Hepatocyte against Heat Stress through Focal Adhesion Kinase-Mediated Phosphatidylinositol 3-Kinase/Akt Pathway. Front. Genet. 2012, 12, 693780. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).