Effects of Genotype and Sex on Carcass Traits, Myosin Heavy Chain Isoforms and Meat Characteristics of Pigs
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Muscle Sampling
2.2. Determination of Gene Expression
2.2.1. RNA Extraction, cDNA Synthesis, and Quantitative Polymerase Chain Reaction (qPCR) Analysis
2.2.2. Myosin Heavy Chain Isoforms Expression
2.2.3. Calpain System Gene Expression Analysis
2.3. Meat Quality Analysis
2.3.1. pH
2.3.2. Color
2.3.3. Drip Loss
2.3.4. Cooking Loss and Shear Force
2.3.5. Muscle Fiber Diameter and Sarcomere Length
2.4. Chemical Composition
2.5. Fatty Acid Composition
2.6. Ribonucleotides
2.7. Statistical Analysis
3. Results
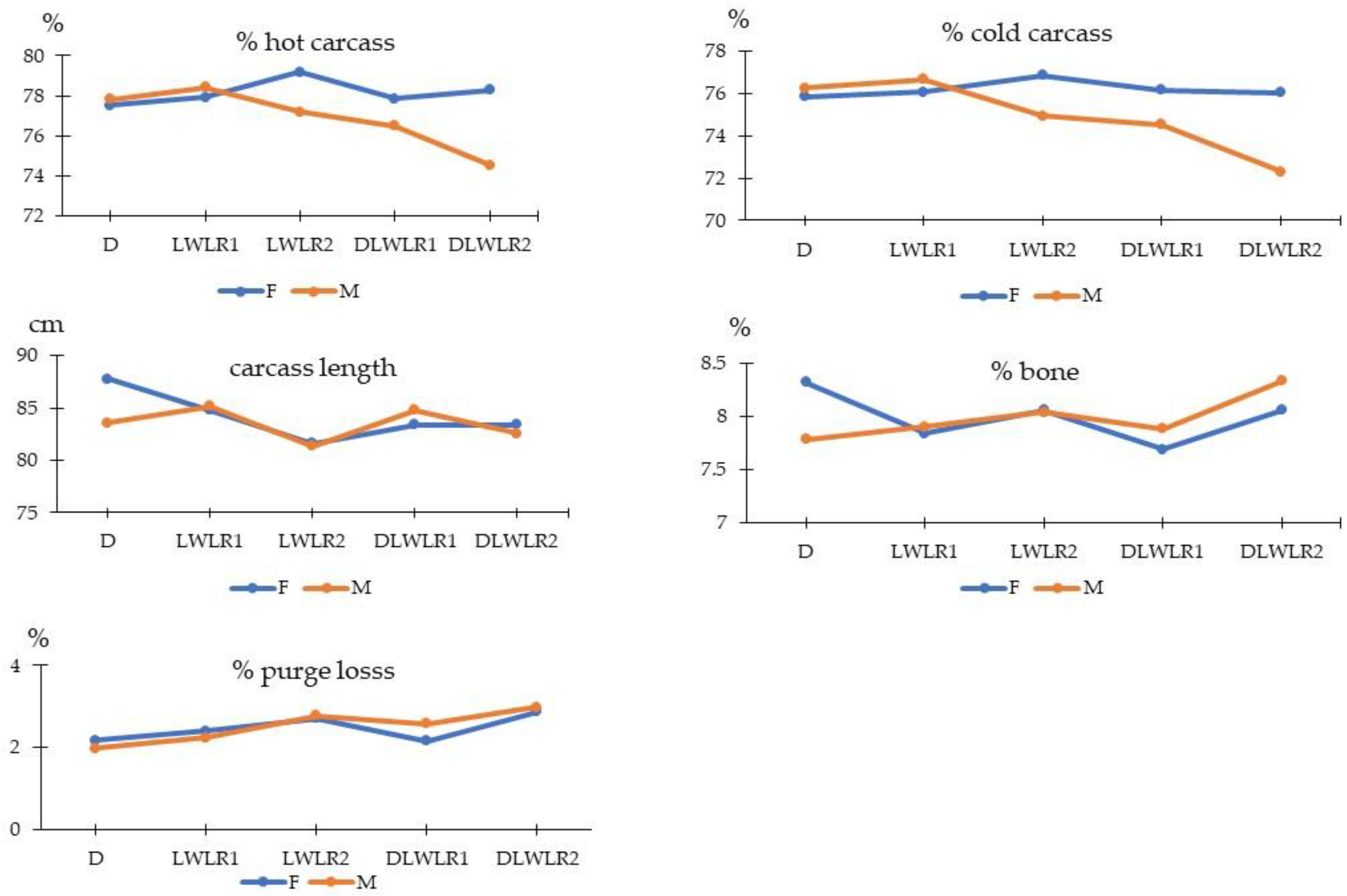
3.1. Carcass Traits
3.2. Myosin Heavy Chain Isoform Expression
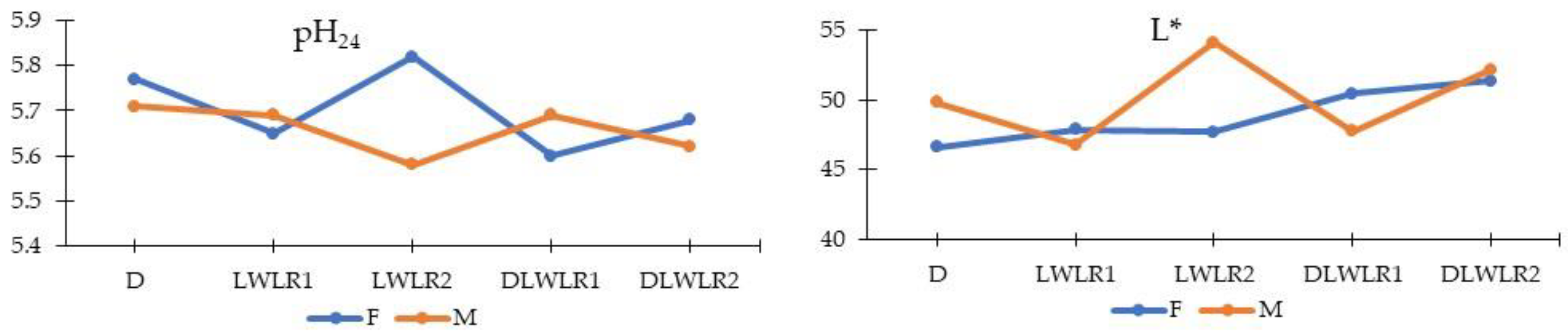
3.3. Meat Characteristics
3.4. Chemical Composition and Ribonucleotides
3.5. Calpain System Gene Expression
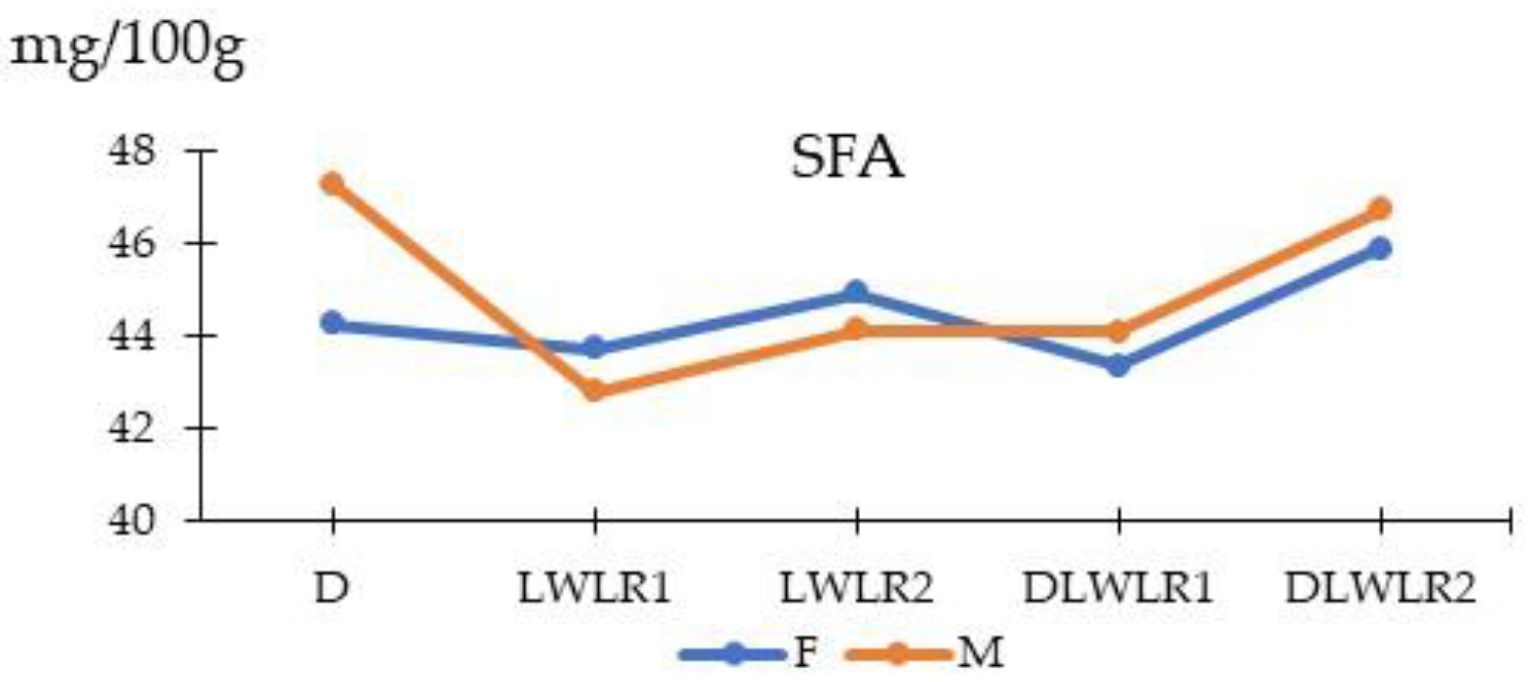
3.6. Fatty Acids
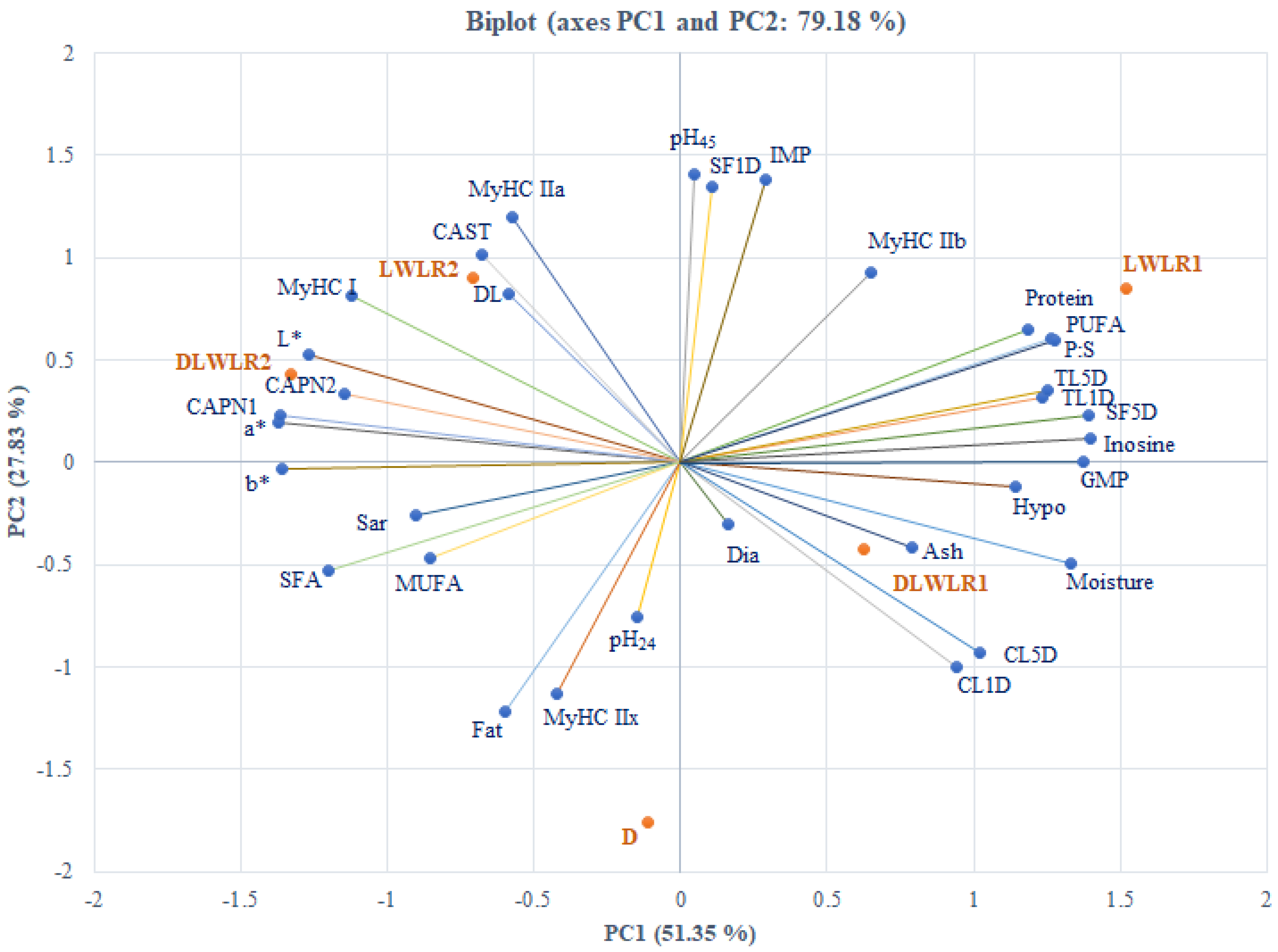
3.7. Principal Component Analysis
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wongmonta, S. An assessment of household food consumption patterns in Thailand. J. Asia Pac. Econ. 2022, 27, 289–309. [Google Scholar] [CrossRef]
- Thuannadee, A.; Noosuwan, C. Consumer meat preference and willingness to pay for local organic meat in Thailand: A case study of Taphao Thong-Kasetsart chicken. J. Agribus. Dev. Emerg. Econ. 2023, 15, 81–95. [Google Scholar] [CrossRef]
- Noidad, S.; Limsupavanich, R.; Suwonsichon, S.; Chaosap, C. Effect of visual marbling levels in pork loins on meat quality and Thai consumer acceptance and purchase intent. Asian-Australas. J. Anim. Sci. 2019, 32, 1923. [Google Scholar] [CrossRef]
- Chankong, J.; Chuaychu-Noo, N.; Maliwan, P.; Kakulpim, P. Factors affecting the frequency of consumers’ purchase of Thai native chicken in Nakhon Sri Thammarat Province. J. Agric. Res. Ext. 2023, 40, 11. [Google Scholar]
- Premashthira, A.; Photchanaprasert, N.; Sanglestsawai, S. Consumer preferences for pork safety characteristics in Thailand. Kasetsart J. Soc. Sci. 2022, 43, 653–660. [Google Scholar] [CrossRef]
- Visetnoi, S.; Nelles, W. Can organic pork help achieve sustainable development goals in Thailand? Agriculture 2023, 13, 1822. [Google Scholar] [CrossRef]
- Organization for Economic Co-Operation and Development (OECD). Meat Consumption (Indicator). Available online: https://data.oecd.org/agroutput/meat-consumption.htm (accessed on 22 August 2024).
- Thanapongtharm, W.; Linard, C.; Chinson, P.; Kasemsuwan, S.; Visser, M.; Gaughan, A.E.; Epprech, M.; Robinson, T.P.; Gilbert, M. Spatial analysis and characteristics of pig farming in Thailand. BMC Vet. Res. 2016, 12, 218. [Google Scholar] [CrossRef]
- Department of Livestock Development, Ministry of Agriculture and Cooperatives, Thailand. Number of Livestock Inventory in Thailand in 2017. Available online: https://drive.google.com/file/d/1YeRonvHZ5DYA5Ry5adLcFF0EJZ0NxtCy/view (accessed on 1 June 2024).
- Chan, R.; Chiemchaisri, C.; Chiemchaisri, W.; Boonsoongnern, A.; Tulayakul, P. Occurrence of antibiotics in typical pig farming and its wastewater treatment in Thailand. Emerg. Contam. 2022, 8, 21–29. [Google Scholar] [CrossRef]
- Kim, J.A.; Cho, E.S.; Jeong, Y.D.; Choi, Y.H.; Kim, Y.S.; woo Choi, J.; Kim, J.S.; Jang, A.; Hong, J.K.; Sa, S.J. The effects of breed and gender on meat quality of Duroc, Pietrain, and their crossbred. J. Anim. Sci. Technol. 2020, 62, 409. [Google Scholar] [CrossRef]
- Li, J.; Han, Q.; Liu, R.; Wen, P.; Ji, W.; Pan, L.; Wang, C.; Zhao, P.; Liu, H.; Bao, J. Effects of environment and breed on growth performance and meat quality of fattening pigs. Anim. Welf. 2020, 29, 177–184. [Google Scholar] [CrossRef]
- Lebret, B.; Čandek-Potokar, M. Pork quality attributes from farm to fork. Part I. Carcass and fresh meat. Animal 2022, 16, 100402. [Google Scholar] [CrossRef]
- Elbert, K.; Matthews, N.; Wassmuth, R.; Tetens, J. Effects of sire line, birth weight and sex on growth performance and carcass traits of crossbred pigs under standardized environmental conditions. Arch. Anim. Breed. 2020, 63, 367–376. [Google Scholar] [CrossRef]
- Zomeño, C.; Gispert, M.; Čandek-Potokar, M.; Mörlein, D.; Font-i-Furnols, M. A matter of body weight and sex type: Pig carcass chemical composition and pork quality. Meat Sci. 2023, 197, 109077. [Google Scholar] [CrossRef] [PubMed]
- Wimmers, K.; Ngu, N.; Jennen, D.; Tesfaye, D.; Murani, E.; Schellander, K.; Ponsuksili, S. Relationship between myosin heavy chain isoform expression and muscling in several diverse pig breeds. J. Anim. Sci. 2008, 86, 795–803. [Google Scholar] [CrossRef]
- Chaosap, C.; Parr, T.; Wiseman, J. Effect of compensatory growth on performance, carcass composition and plasma IGF-1 in grower finisher pigs. Animal 2011, 5, 749–756. [Google Scholar] [CrossRef][Green Version]
- Lindholm-Perry, A.; Rohrer, G.; Holl, J.; Shackelford, S.; Wheeler, T.; Koohmaraie, M.; Nonneman, D. Relationships among calpastatin single nucleotide polymorphisms, calpastatin expression and tenderness in pork longissimus 1. Anim. Genet. 2009, 40, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Čikoš, Š.; Bukovská, A.; Koppel, J. Relative quantification of mRNA: Comparison of methods currently used for real-time PCR data analysis. BMC Mol. Biol. 2007, 8, 113. [Google Scholar] [CrossRef]
- Hemmings, K.; Parr, T.; Daniel, Z.; Picard, B.; Buttery, P.; Brameld, J. Examination of myosin heavy chain isoform expression in ovine skeletal muscles. J. Anim. Sci. 2009, 87, 3915–3922. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chaosap, C.; Sivapirunthep, P.; Sitthigripong, R.; Tavitchasri, P.; Maduae, S.; Kusee, T.; Setakul, J.; Adeyemi, K. Meat quality, post-mortem proteolytic enzymes, and myosin heavy chain isoforms of different Thai native cattle muscles. Anim. Biosci. 2021, 34, 1514. [Google Scholar] [CrossRef]
- Cross, H.; West, R.; Dutson, T. Comparison of methods for measuring sarcomere length in beef semitendinosus muscle. Meat Sci. 1981, 5, 261–266. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaitherburg, MD, USA, 2006. [Google Scholar]
- Chaosap, C.; Sahatsanon, K.; Sitthigripong, R.; Sawanon, S.; Setakul, J. The effects of using pineapple stem starch as an alternative starch source and ageing period on meat quality, texture profile, ribonucleotide content, and fatty acid composition of longissimus thoracis of fattening dairy steers. Foods 2021, 10, 2319. [Google Scholar] [CrossRef] [PubMed]
- Martins, J.M.; Varino, R.; Charneca, R.; Albuquerque, A.; Garrido, N.; Neves, J.; Freitas, A.; Costa, F.; Marmelo, C.; Ramos, A. Outdoor Finishing of Intact Male Portuguese Alentejano Pigs on a Sustainable High-Fiber Diet: Impacts on Blood, Growth, Carcass, Meat Quality and Boar Taint Compounds. Animals 2023, 13, 2221. [Google Scholar] [CrossRef]
- Lertpatarakomol, R.; Chaosap, C.; Chaweewan, K.; Sitthigripong, R.; Limsupavanich, R. Carcass characteristics and meat quality of purebred Pakchong 5 and crossbred pigs sired by Pakchong 5 or Duroc boar. Asian-Australas. J. Anim. Sci. 2018, 32, 585. [Google Scholar] [CrossRef] [PubMed]
- Peinado, J.; Medel, P.; Fuentetaja, A.; Mateos, G. Influence of sex and castration of females on growth performance and carcass and meat quality of heavy pigs destined for the dry-cured industry. J. Anim. Sci. 2008, 86, 1410–1417. [Google Scholar] [CrossRef][Green Version]
- Xia, J.Q.; Liu, D.Y.; Liu, J.; Jiang, X.P.; Wang, L.; Yang, S.; Liu, D. Sex effects on carcass characteristics, meat quality traits and meat amino acid and fatty acid compositions in a novel Duroc line pig. J. Anim. Physiol. Anim. Nutr. 2023, 107, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-M.; Lee, S.-H.; Ryu, Y.-C. Comparisons of meat quality and muscle fibre characteristics on multiple pig breeds and sexes using principal component analysis. Anim. Prod. Sci. 2017, 58, 2091–2099. [Google Scholar] [CrossRef]
- Jeong, J.-Y.; Kim, G.-D.; Ha, D.-M.; Park, M.-J.; Park, B.-C.; Joo, S.-T.; Lee, C. Relationships of muscle fiber characteristics to dietary energy density, slaughter weight, and muscle quality traits in finishing pigs. J. Anim. Sci. Technol. 2012, 54, 175–183. [Google Scholar] [CrossRef][Green Version]
- Chaosap, C.; Sitthigripong, R.; Sivapirunthep, P.; Pungsuk, A.; Adeyemi, K.D.; Sazili, A.Q. Myosin heavy chain isoforms expression, calpain system and quality characteristics of different muscles in goats. Food Chem. 2020, 321, 126677. [Google Scholar] [CrossRef]
- Kim, G.-D.; Jeong, J.-Y.; Jung, E.-Y.; Yang, H.-S.; Lim, H.-T.; Joo, S.-T. The influence of fiber size distribution of type IIB on carcass traits and meat quality in pigs. Meat Sci. 2013, 94, 267–273. [Google Scholar] [CrossRef]
- Chang, K.; Da Costa, N.; Blackley, R.; Southwood, O.; Evans, G.; Plastow, G.; Wood, J.; Richardson, R. Relationships of myosin heavy chain fibre types to meat quality traits in traditional and modern pigs. Meat Sci. 2003, 64, 93–103. [Google Scholar] [CrossRef]
- Kaur, L.; Hui, S.X.; Morton, J.D.; Kaur, R.; Chian, F.M.; Boland, M. Endogenous proteolytic systems and meat tenderness: Influence of post-mortem storage and processing. Food Sci. Anim. Resour. 2021, 41, 589. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef] [PubMed]
- Gandolfi, G.; Pomponio, L.; Ertbjerg, P.; Karlsson, A.; Costa, L.N.; Lametsch, R.; Russo, V.; Davoli, R. Investigation on CAST, CAPN1 and CAPN3 porcine gene polymorphisms and expression in relation to post-mortem calpain activity in muscle and meat quality. Meat Sci. 2011, 88, 694–700. [Google Scholar] [CrossRef]
- Dinh, T.T.; To, K.V.; Schilling, M.W. Fatty acid composition of meat animals as flavor precursors. Meat Muscle Biol. 2021, 5, 1–16. [Google Scholar] [CrossRef]
- Chaosap, C.; Chaweewan, K.; Adeyemi, K.D.; Phonkate, N.; Sitthigripong, R. Meat Characteristics, Expression of Myosin Heavy Chain and Metabolism-Related Genes in Thai Native Pigs. Foods 2024, 13, 1502. [Google Scholar] [CrossRef] [PubMed]
- Listrat, A.; Lebret, B.; Louveau, I.; Astruc, T.; Bonnet, M.; Lefaucheur, L.; Picard, B.; Bugeon, J. How muscle structure and composition influence meat and flesh quality. Sci. World J. 2016, 2016, 3182746. [Google Scholar] [CrossRef]
- Ntambi, J.M. Regulation of stearoyl-CoA desaturase by polyunsaturated fatty acids and cholesterol. J. Lipid Res. 1999, 40, 1549–1558. [Google Scholar] [CrossRef]
- Smith, S.B.; Gill, C.A.; Lunt, D.K.; Brooks, M.A. Regulation of fat and fatty acid composition in beef cattle. Asian-Australas. J. Anim. Sci. 2009, 22, 1225–1233. [Google Scholar] [CrossRef]
- Tikk, M.; Tikk, K.; Tørngren, M.A.; Meinert, L.; Aaslyng, M.D.; Karlsson, A.H.; Andersen, H.J. Development of inosine monophosphate and its degradation products during aging of pork of different qualities in relation to basic taste and retronasal flavor perception of the meat. J. Agric. Food Chem. 2006, 54, 7769–7777. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, J.; Gu, Y.; Cai, Z.; Feng, X.; Yang, C.; Xin, G. Research progress on inosine monophosphate deposition mechanism in chicken muscle. Crit. Rev. Food Sci. Nutr. 2022, 62, 1062–1078. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Li, H.; Guo, T.; Jia, J.; Zhang, P.; Wang, L.; Xia, N.; Qian, Q.; Peng, H. Comparison of nutrition and flavor characteristics of five breeds of pork in China. Foods 2022, 11, 2704. [Google Scholar] [CrossRef] [PubMed]
| Gene * | Primer Sequence (5′ to 3′) | Annealing Temperature (°C) | Product Size (bp) | Accession No. | Reference | |
|---|---|---|---|---|---|---|
| MYH7 | Forward | AAGGGCTTGAACGAGGAGTAGA | 60 | 115 | AB053226 | [16] |
| Reverse | TTATTCTGCTTCCTCCAAAGGG | |||||
| MYH2 | Forward | GCTGAGCGAGCTGAAATCC | 60 | 137 | AB025260 | |
| Reverse | ACTGAGACACCAGAGCTTCT | |||||
| MYH1 | Forward | AGAAGATCAACTGAGTGAACT | 57 | 149 | AB025262 | |
| Reverse | AGAGCTGAGAAACTAACGTG | |||||
| MYH4 | Forward | ATGAAGAGGAACCACATTA | 55 | 166 | AB025261 | |
| Reverse | TTATTGCCTCAGTAGCTTG | |||||
| CAPN1 | Forward | GACACCCTCCTGCACCGA | 55 | 101 | AF263610 | [17] |
| Reverse | TCCACCCACTCCCCAAACT | |||||
| CAPN2 | Forward | ACATGCACACCATCGGCTTT | 55 | 101 | U01181 | |
| Reverse | CGCTCTGTGCGTCAGGAAG | |||||
| CAST | Forward | AGGCTGTAAAAACAGAACCTG | 55 | 201 | M20160 | [18] |
| Reverse | ATTTCTCTGATGTTGGCTGCTC | |||||
| GAPDH | Forward | TCACTGCCACCCAGAAGA | 65 | 229 | ABO38240 | |
| Reverse | TACCAGGAAATGAGCTTGAC | |||||
| Trait | Genotype (G) | Sex (S) | RMSE | p-value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| Live Weight (kg) | 112.20 | 110.80 | 108.50 | 110.70 | 112.40 | 110.84 | 111.00 | 3.83 | 0.178 | 0.883 | 0.656 |
| Hot Carcass (kg) | 87.14 | 86.62 | 84.84 | 85.45 | 85.86 | 86.62 | 85.34 | 3.17 | 0.511 | 0.160 | 0.241 |
| % Hot Carcass | 77.66 | 78.18 | 78.18 | 77.18 | 76.41 | 78.15 | 76.89 | 1.02 | 0.001 | <0.0001 | 0.000 |
| Cold Carcass (kg) | 85.32 | 84.60 | 82.35 | 83.41 | 83.34 | 84.44 | 83.17 | 3.04 | 0.231 | 0.146 | 0.223 |
| % Cold Carcass | 76.04 | 76.36 | 75.89 | 75.35 | 74.17 | 76.18 | 74.94 | 0.96 | <0.0001 | <0.0001 | <0.0001 |
| %Purge Loss | 2.08 | 2.32 | 2.75 | 2.37 | 2.93 | 2.47 | 2.52 | 0.22 | <0.0001 | 0.410 | 0.024 |
| % Lean | 44.71 a | 42.25 b | 45.67 a | 44.94 a | 45.62 a | 44.89 | 44.25 | 1.57 | <0.0001 | 0.172 | 0.501 |
| % Fat | 10.86 b | 12.71 a | 11.82 ab | 11.13 ab | 10.88 b | 11.27 | 11.69 | 1.11 | 0.002 | 0.184 | 0.075 |
| % Bone | 8.05 | 7.87 | 8.05 | 7.78 | 8.18 | 7.99 | 7.98 | 0.29 | 0.034 | 0.971 | 0.027 |
| Lean/Fat | 4.19 a | 3.34 b | 3.89 ab | 4.07 a | 4.25 a | 4.08 | 3.92 | 0.52 | 0.002 | 0.096 | 0.126 |
| Carcass Length (cm) | 85.70 | 85.00 | 81.50 | 84.10 | 83.00 | 84.20 | 83.52 | 1.82 | <0.0001 | 0.194 | 0.017 |
| Trait | Genotype (G) | Sex (S) | RMSE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| MyHC I | 0.16 b | 0.17 b | 1.28 a | 0.12 b | 1.09 a | 0.58 | 0.55 | 0.39 | <0.0001 | 0.979 | 0.183 |
| MyHC IIA | 2.71 b | 4.11 a | 4.11 a | 2.55 b | 4.37 a | 3.67 | 3.65 | 0.95 | <0.0001 | 0.786 | 0.118 |
| MyHC IIX | 70.4 a | 32.67 c | 47.30 b | 38.38 bc | 39.68 bc | 47.59 | 43.79 | 12.28 | <0.0001 | 0.212 | 0.343 |
| MyHC IIB | 26.9 c | 67.27 a | 46.25 b | 64.96 a | 55.03 ab | 50.46 | 53.71 | 13.85 | <0.0001 | 0.374 | 0.269 |
| Trait | Genotype (G) | Sex (S) | RMSE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| pH45 | 6.36 c | 6.80 a | 6.77 ab | 6.54 bc | 6.71 ab | 6.66 | 6.61 | 0.27 | 0.003 | 0.560 | 0.970 |
| pH24 | 5.74 | 5.67 | 5.70 | 5.65 | 5.65 | 5.70 | 5.66 | 0.10 | 0.209 | 0.093 | 0.008 |
| Lightness (L*) | 48.22 | 47.34 | 50.91 | 49.09 | 51.79 | 48.80 | 50.14 | 2.28 | 0.000 | 0.047 | 0.001 |
| Redness (a*) | 7.90 | 7.33 | 8.39 | 7.91 | 8.69 | 8.22 | 7.87 | 1.51 | 0.108 | 0.291 | 0.602 |
| Yellowness (b*) | 1.64 a | 0.63 b | 2.12 a | 1.50 a | 2.19 a | 1.49 | 1.74 | 0.80 | 0.001 | 0.275 | 0.235 |
| Sarcomere Length (u) | 1.76 | 1.72 | 1.74 | 1.78 | 1.82 | 1.74 | 1.79 | 0.11 | 0.351 | 0.136 | 0.650 |
| Muscle Fiber Diameter (u) | 73.73 | 72.84 | 74.82 | 74.33 | 71.16 | 74.21 | 72.55 | 7.75 | 0.848 | 0.473 | 0.922 |
| Drip Loss (%) | 2.01 | 2.22 | 3.35 | 2.77 | 2.62 | 2.55 | 2.64 | 0.94 | 0.057 | 0.744 | 0.052 |
| Thawing Loss Day 1 (%) | 5.33 bc | 8.44 a | 6.65 ab | 8.24 a | 3.72 c | 6.50 | 6.46 | 2.34 | 0.000 | 0.951 | 0.934 |
| Thawing Loss Day 5 (%) | 7.02 b | 10.09 a | 7.53 b | 9.93 a | 7.17 b | 8.08 | 8.61 | 2.42 | 0.010 | 0.455 | 0.771 |
| Cooking Loss Day 1 (%) | 24.45 a | 22.65 ab | 20.26 bc | 24.89 a | 18.91 c | 22.68 | 21.79 | 2.75 | 0.000 | 0.286 | 0.121 |
| Cooking Loss Day 5 (%) | 22.74 ab | 21.24 b | 18.63 c | 23.08 a | 17.16 c | 20.88 | 20.26 | 1.91 | <0.0001 | 0.278 | 0.103 |
| Shear Force Day 1 (kg) | 4.56 c | 6.01 a | 5.92 a | 4.90 bc | 5.33 ab | 5.31 | 5.37 | 0.76 | 0.001 | 0.791 | 0.127 |
| Shear Force Day 5 (kg) | 4.48 b | 5.67 a | 4.40 b | 5.09 a | 4.15 b | 4.88 | 4.64 | 0.63 | <0.0001 | 0.201 | 0.135 |
| Trait | Genotype (G) | Sex (S) | RMSE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| Chemical Composition (%) | |||||||||||
| Moisture | 73.86 | 74.13 | 73.02 | 73.97 | 72.77 | 73.65 | 73.45 | 1.22 | 0.111 | 0.620 | 0.760 |
| Ether Extract | 4.02 a | 1.69 d | 1.95 cd | 2.37 bc | 3.00 b | 2.46 | 2.75 | 0.734 | <0.0001 | 0.169 | 0.285 |
| Crude Protein | 22.94 cd | 24.46 a | 23.66 b | 23.62 bc | 22.56 d | 23.61 | 23.29 | 0.80 | <0.0001 | 0.163 | 0.570 |
| Ash | 1.17 b | 1.16 bc | 1.17 b | 1.25 a | 1.10 c | 1.18 | 1.16 | 0.07 | 0.003 | 0.359 | 0.187 |
| Ribonucleotide (mg/100 g) | |||||||||||
| Hypoxanthine | 8.80 | 9.64 | 7.96 | 8.55 | 8.39 | 8.69 | 8.64 | 1.36 | 0.098 | 0.900 | 0.516 |
| Inosine | 49.00 c | 66.00 a | 44.76 cd | 58.47 b | 42.39 d | 51.43 | 52.82 | 6.72 | <0.0001 | 0.475 | 0.412 |
| IMP | 360.3 c | 444.96 a | 438.40 ab | 399.14 bc | 410.56 ab | 405.62 | 415.72 | 49.55 | 0.004 | 0.481 | 0.844 |
| GMP | 4.17 b | 5.10 a | 3.66 c | 4.48 b | 3.74 c | 4.27 | 4.19 | 0.40 | <0.0001 | 0.503 | 0.747 |
| Trait | Genotype (G) | Sex (S) | RMSE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| CAPN1 | 0.62 c | 0.34 d | 0.79 b | 0.36 d | 1.08 a | 0.61 | 0.66 | 0.16 | <0.0001 | 0.299 | 0.800 |
| CAPN2 | 0.87 c | 0.84 bc | 1.24 ab | 1.10 bc | 1.49 a | 1.03 | 1.18 | 0.40 | 0.006 | 0.184 | 0.853 |
| CAST | 0.82 b | 0.93 b | 1.30 a | 0.75 b | 1.01 ab | 0.98 | 0.94 | 0.32 | 0.008 | 0.718 | 0.944 |
| Trait | Genotype (G) | Sex (S) | RMSE | p-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| D | LWLR1 | LWLR2 | DLWLR1 | DLWLR2 | F | M | G | S | G × S | ||
| C14:0 | 1.67 a | 1.37 c | 1.60 ab | 1.47 bc | 1.65 a | 1.60 | 1.50 | 0.16 | 0.0005 | 0.039 | 0.561 |
| C15:0 | 0.63 c | 1.57 a | 1.37 a | 0.97 b | 0.92 bc | 1.15 | 1.04 | 0.36 | <0.0001 | 0.302 | 0.573 |
| C16:0 | 27.66 ab | 25.46 d | 26.78 bc | 26.30 cd | 27.81 a | 26.76 | 26.85 | 0.95 | <0.0001 | 0.751 | 0.143 |
| C16:1 | 3.70 b | 3.48 b | 4.45 a | 3.80 b | 3.44 b | 3.84 | 3.70 | 0.71 | 0.021 | 0.498 | 0.569 |
| C18:0 | 14.53 a | 11.72 c | 11.88 c | 13.02 b | 14.19 a | 12.58 | 13.55 | 1.08 | <0.0001 | 0.003 | 0.067 |
| C18:1n9c | 41.53 | 39.80 | 41.29 | 42.25 | 41.92 | 41.17 | 41.55 | 2.00 | 0.083 | 0.506 | 0.104 |
| C18:2n6c | 7.85 bc | 12.01 a | 8.66 bc | 9.15 b | 7.25 c | 9.36 | 8.61 | 1.88 | <0.0001 | 0.166 | 0.371 |
| C20:1 | 0.80 ab | 0.96 a | 0.68 b | 0.68 b | 0.72 b | 0.78 | 0.75 | 0.18 | 0.005 | 0.534 | 0.527 |
| C18:3n3 | 0.33 b | 0.49 a | 0.40 ab | 0.38 b | 0.35 b | 0.40 | 0.37 | 0.10 | 0.014 | 0.333 | 0.698 |
| C23:0 | 1.29 b | 3.13 a | 2.88 a | 1.97 b | 1.72 b | 2.34 | 2.05 | 0.84 | <0.0001 | 0.231 | 0.311 |
| ΣMUFA | 46.03 | 44.25 | 46.42 | 46.74 | 46.08 | 45.80 | 46.00 | 2.11 | 0.097 | 0.728 | 0.088 |
| ΣPUFA | 8.18 bc | 12.49 a | 9.06 bc | 9.53 b | 7.60 c | 9.76 | 8.98 | 1.96 | <0.0001 | 0.169 | 0.387 |
| ΣSFA | 45.78 | 43.26 | 44.53 | 43.73 | 46.31 | 44.44 | 45.01 | 1.27 | <0.0001 | 0.121 | 0.007 |
| P:S | 0.18 bc | 0.29 a | 0.20 bc | 0.22 b | 0.16 bc | 0.22 | 0.20 | 0.04 | <0.0001 | 0.142 | 0.399 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chaosap, C.; Buajoom, W.; Pothising, N.; Kongtasorn, C.; Adeyemi, K.D. Effects of Genotype and Sex on Carcass Traits, Myosin Heavy Chain Isoforms and Meat Characteristics of Pigs. Animals 2025, 15, 2535. https://doi.org/10.3390/ani15172535
Chaosap C, Buajoom W, Pothising N, Kongtasorn C, Adeyemi KD. Effects of Genotype and Sex on Carcass Traits, Myosin Heavy Chain Isoforms and Meat Characteristics of Pigs. Animals. 2025; 15(17):2535. https://doi.org/10.3390/ani15172535
Chicago/Turabian StyleChaosap, Chanporn, Wuttikorn Buajoom, Numfon Pothising, Chananya Kongtasorn, and Kazeem D. Adeyemi. 2025. "Effects of Genotype and Sex on Carcass Traits, Myosin Heavy Chain Isoforms and Meat Characteristics of Pigs" Animals 15, no. 17: 2535. https://doi.org/10.3390/ani15172535
APA StyleChaosap, C., Buajoom, W., Pothising, N., Kongtasorn, C., & Adeyemi, K. D. (2025). Effects of Genotype and Sex on Carcass Traits, Myosin Heavy Chain Isoforms and Meat Characteristics of Pigs. Animals, 15(17), 2535. https://doi.org/10.3390/ani15172535






