Elimination of ASFV via Precise Culling in a Large-Scale Breeding Herd in China: A Field Experience
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Review
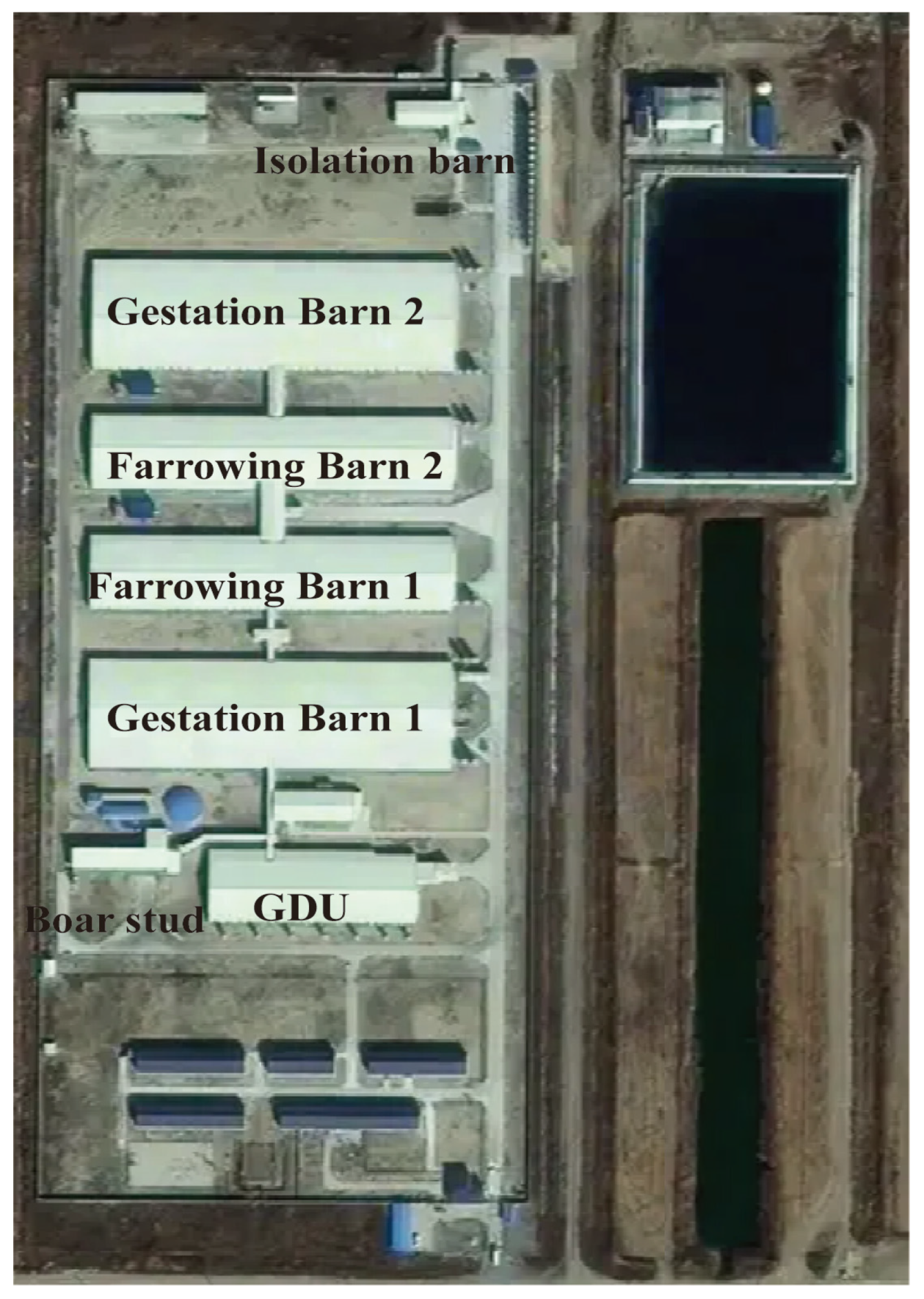
2.2. Farm Description
2.3. Biosecurity
2.4. Disease Monitoring
- Boars for semen collection: Sample boars one day before semen collection; all boars should be sampled at least once a week. If any boar shows abnormal behavior, it should not be utilized for semen collection and it needs be sampled and tested immediately.
- Pig with abnormal behavior: Pigs with signs of fever, anorexia, abortion, vomiting, lameness, or any abnormal appearance should be identified and sampled for an ASFV test. A throat swab sample, as a preferred method of sampling for ASFV [21], is collected using a long swab, as shown in Figure 2. Technicians should wear disposable long-arm gloves when sampling. Additional environmental samples are collected around the abnormal pig using cotton swabs and pooled in the same sampling tube with the throat swab. Abnormal pigs sampled per day typically account for approximately 1.5–2% of the herd as routine monitoring. Aborted sows should be sampled for three consecutive days and then tested again a week after to confirm a negative test result. Treatments for abnormal pigs are held until the day after the PCR results are available to avoid potential spread of disease during the treatment procedure.
- Gilt entry: Before integrating gilts from GDU into the herd, cotton rope samples from each gilt pen are required. Only transfer gilts after negative results are confirmed.
- Nurse-off sows: Sows that stopped nursing with feed refusal must have two negative tests within five days before they can be removed from farrow crates. Sows with insufficient milk production but with normal feed intake can be transferred after one negative test result.
- Environmental sampling: The non-production area should be sampled at least once a week, including the cafeteria, office, showers, dormitory rooms, etc. Environmental samples from inside each barn should be collected twice a week, including the euthanasia device, electronic carcass transporter, and boar carts for heat checking, etc. Dust samples from inside feed bins are collected to monitor the risk of virus transmission via feed. Cotton gauze is hung next to the barn air intake for environmental air monitoring.
- Mortality disposal: Any dead pig is assumed to be ASF-positive and should not be moved or disposed until the test results are available. This is to avoid potential transmission through contact.
2.5. Diagnosis
3. Results
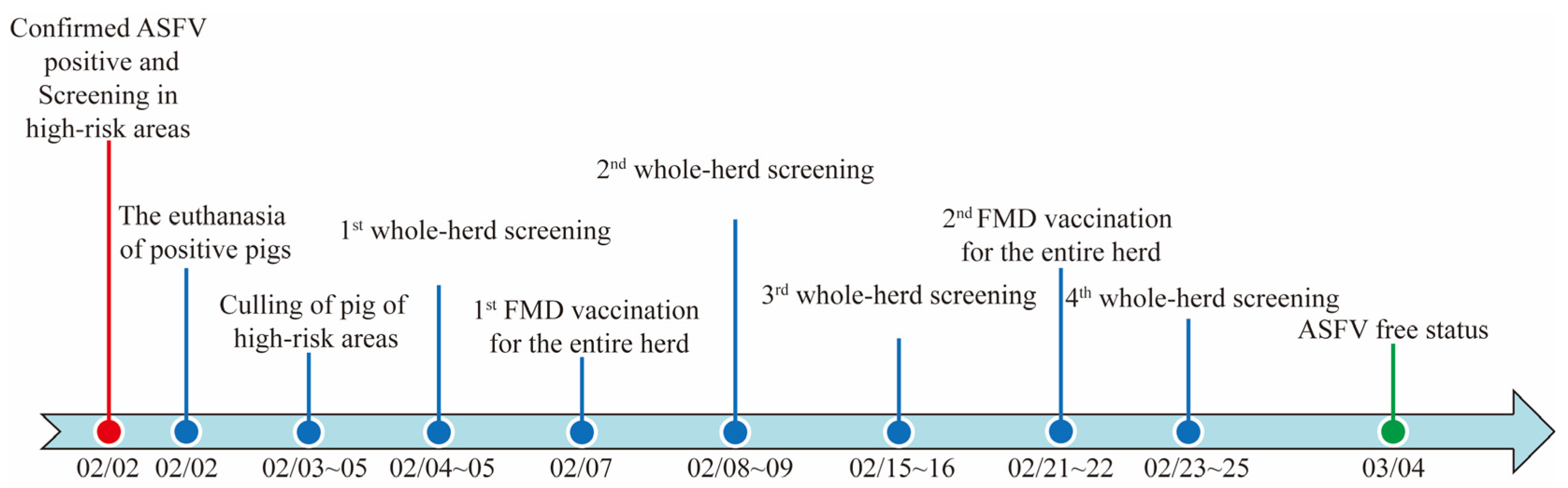
3.1. ASF Outbreak
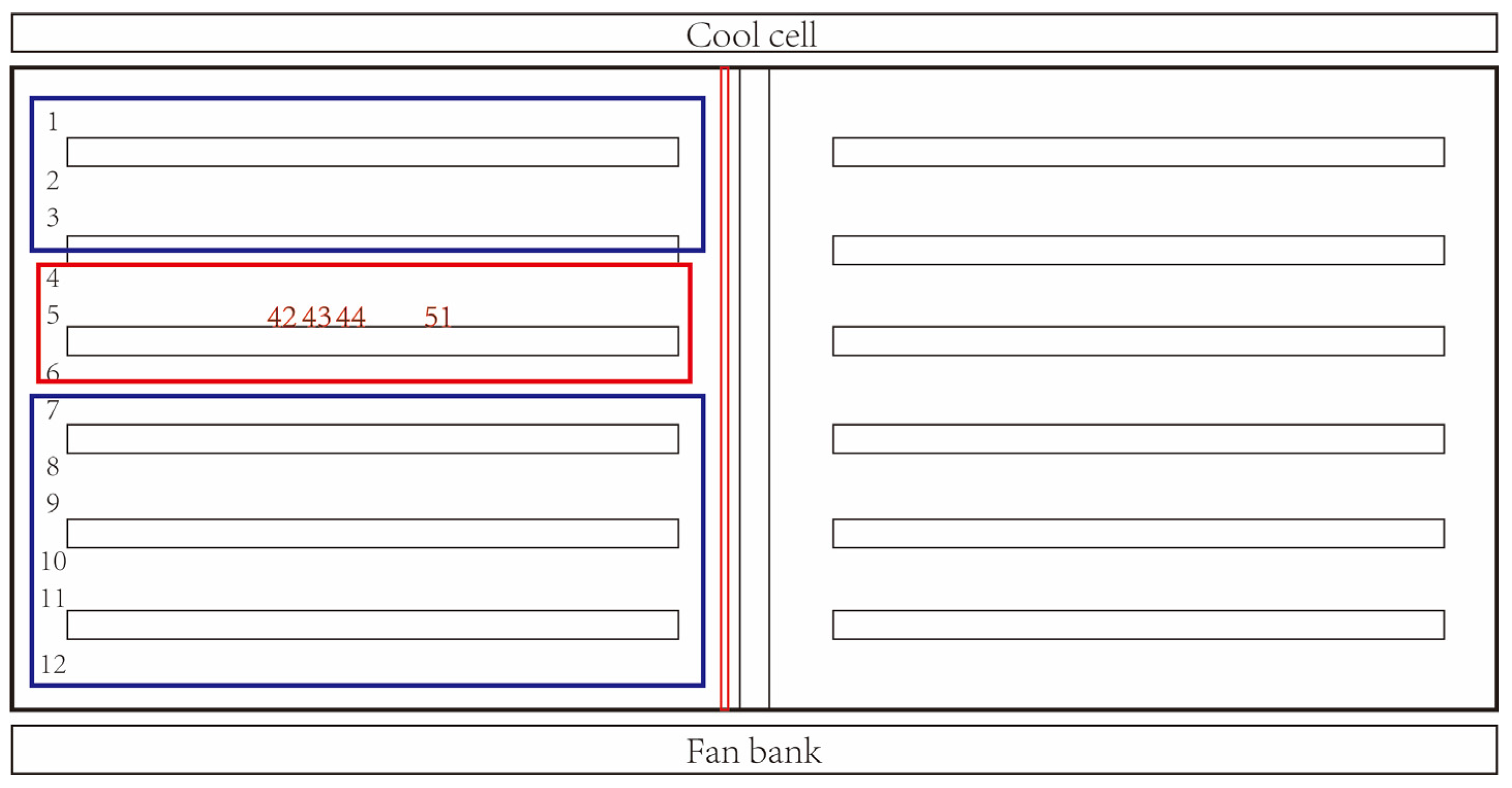
3.2. Epidemiological Investigation
- Was the infected pig recently moved within the barn? What was the route of movement?
- Were there personnel activities (e.g., breeding, health checking, treatment, body condition checking, etc.) or any equipment that had been in close contact with the index pigs?
- Location of the index pig within the barn (a map of crate layout is needed). How many pigs were housed in proximity, especially with a shared feed trough?
- Identify alleys in the neighborhood and public zones with frequent people and pig traffic.
3.3. Precise Culling
3.4. Whole-Herd Screening
3.5. Downstream Farms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ASF | African swine fever |
| ASFV | African swine fever virus |
| WOAH | World Organization for Animal Health |
| HAD | Hematopoietic adhesion |
| PRRS | Porcine Reproductive and Respiratory Syndrome |
| PCR | Polymerase chain reaction |
| GDU | Gilt development unit |
References
- Gallardo, M.C.; de la Torre Reoyo, A.; Fernández-Pinero, J.; Iglesias, I.; Muñoz, M.J.; Arias, M.L. African Swine Fever: A Global View of the Current Challenge. Porc. Health Manag. 2015, 1, 21. [Google Scholar] [CrossRef]
- Dixon, L.K.; Sun, H.; Roberts, H. African Swine Fever. Antivir. Res. 2019, 165, 34–41. [Google Scholar] [CrossRef]
- Li, Z.; Chen, W.; Qiu, Z.; Li, Y.; Fan, J.; Wu, K.; Li, X.; Zhao, M.; Ding, H.; Fan, S.; et al. African Swine Fever Virus: A Review. Life 2022, 12, 1255. [Google Scholar] [CrossRef] [PubMed]
- Penrith, M.-L.; Vosloo, W.; Jori, F.; Bastos, A.D.S. African Swine Fever Virus Eradication in Africa. Virus Res. 2013, 173, 228–246. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA); Boklund, A.; Cay, B.; Depner, K.; Földi, Z.; Guberti, V.; Masiulis, M.; Miteva, A.; More, S.; Olsevskis, E.; et al. Epidemiological Analyses of African Swine Fever in the European Union (November 2017 until November 2018). EFS2 2018, 16, e05494. [Google Scholar] [CrossRef]
- Sauter-Louis, C.; Conraths, F.J.; Probst, C.; Blohm, U.; Schulz, K.; Sehl, J.; Fischer, M.; Forth, J.H.; Zani, L.; Depner, K.; et al. African Swine Fever in Wild Boar in Europe—A Review. Viruses 2021, 13, 1717. [Google Scholar] [CrossRef]
- Wen, X.; He, X.; Zhang, X.; Zhang, X.; Liu, L.; Guan, Y.; Zhang, Y.; Bu, Z. Genome Sequences Derived from Pig and Dried Blood Pig Feed Samples Provide Important Insights into the Transmission of African Swine Fever Virus in China in 2018. Emerg. Microbes Infect. 2019, 8, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Mason-D’Croz, D.; Bogard, J.R.; Herrero, M.; Robinson, S.; Sulser, T.B.; Wiebe, K.; Willenbockel, D.; Godfray, H.C.J. Modelling the Global Economic Consequences of a Major African Swine Fever Outbreak in China. Nat. Food 2020, 1, 221–228. [Google Scholar] [CrossRef]
- Zhou, X.; Li, N.; Luo, Y.; Liu, Y.; Miao, F.; Chen, T.; Zhang, S.; Cao, P.; Li, X.; Tian, K.; et al. Emergence of African Swine Fever in China, 2018. Transbound. Emerg. Dis. 2018, 65, 1482–1484. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, R.; Zhang, X.; Li, F.; Wang, J.; Zhang, J.; Liu, X.; Wang, L.; Zhang, J.; Wu, X.; et al. Replication and Virulence in Pigs of the First African Swine Fever Virus Isolated in China. Emerg. Microbes Infect. 2019, 8, 438–447. [Google Scholar] [CrossRef]
- Wang, T.; Sun, Y.; Qiu, H.-J. African Swine Fever: An Unprecedented Disaster and Challenge to China. Infect. Dis. Poverty 2018, 7, 111. [Google Scholar] [CrossRef]
- Wang, Z.; Qi, C.; Ge, S.; Li, J.; Hu, Y.; Zhang, X.; Lv, Y.; Han, N.; Wu, X.; Wang, Z.; et al. Genetic Variation and Evolution of Attenuated African Swine Fever Virus Strain Isolated in the Field: A Review. Virus Res. 2022, 319, 198874. [Google Scholar] [CrossRef]
- Vuono, E.; Ramirez-Medina, E.; Silva, E.; Rai, A.; Pruitt, S.; Espinoza, N.; Valladares, A.; Velazquez-Salinas, L.; Gladue, D.P.; Borca, M.V. Deletion of the H108R Gene Reduces Virulence of the Pandemic Eurasia Strain of African Swine Fever Virus with Surviving Animals Being Protected against Virulent Challenge. J. Virol. 2022, 96, e0054522. [Google Scholar] [CrossRef]
- Chandana, M.S.; Nair, S.S.; Chaturvedi, V.K.; Abhishek; Pal, S.; Charan, M.S.S.; Balaji, S.; Saini, S.; Vasavi, K.; Deepa, P. Recent Progress and Major Gaps in the Vaccine Development for African Swine Fever. Braz. J. Microbiol. 2024, 55, 997–1010. [Google Scholar] [CrossRef] [PubMed]
- Tran, X.H.; Phuong, L.T.T.; Huy, N.Q.; Thuy, D.T.; Nguyen, V.D.; Quang, P.H.; Ngôn, Q.V.; Rai, A.; Gay, C.G.; Gladue, D.P.; et al. Evaluation of the Safety Profile of the ASFV Vaccine Candidate ASFV-G-ΔI177L. Viruses 2022, 14, 896. [Google Scholar] [CrossRef] [PubMed]
- Nga, B.T.T.; Auer, A.; Padungtod, P.; Dietze, K.; Globig, A.; Rozstalnyy, A.; Hai, T.M.; Depner, K. Evaluation of Selective Culling as a Containment Strategy for African Swine Fever at a Vietnamese Sow Farm. Pathogens 2024, 13, 567. [Google Scholar] [CrossRef] [PubMed]
- Li, M. Technical Specifications for Early Tooth Extraction of African Swine Fever in Dongkou County. ACRPVM 2024, 13, 64–68. [Google Scholar] [CrossRef]
- Chuong, V.D.; Schambow, R.A.; Diep, N.T.; Minh, P.Q.; Long, N.V.; To Nga, B.T.; Perez, A.M. Epidemiology and Control of African Swine Fever in Vietnam: A Scoping Review. Pathogens 2025, 14, 329. [Google Scholar] [CrossRef]
- Nga, B.T.T. Partial culling: Effectiveness and challenges in controlling African swine fever in Viet Nam. In Global Consultation on African Swine Fever; FAO: Rome, Italy, 2024; ISBN 978-92-5-138925-6. [Google Scholar]
- Wu, F.; Cochrane, R.; Yaros, J.; Zhang, C.; Tsai, S.-Y.; Spronk, G. Interventions to Reduce Porcine Epidemic Diarrhea Virus Prevalence in Feed in a Chinese Swine Production System: A Case Study. Transbound. Emerg. Dis. 2022, 69, 57–65. [Google Scholar] [CrossRef]
- Li, X.; Li, Y.; Fan, M.; Fan, S.; Gao, W.; Ren, J.; Liu, Q.; Li, J.; Wu, W.; Li, J.; et al. Inguinal Lymph Node Sample Collected by Minimally Invasive Sampler Helps to Accurately Diagnose ASF in Dead Pigs without Necropsy. Front. Vet. Sci. 2022, 9, 1000969. [Google Scholar] [CrossRef]
- Muzykina, L.; Barrado-Gil, L.; Gonzalez-Bulnes, A.; Crespo-Piazuelo, D.; Cerón, J.J.; Alonso, C.; Montoya, M. Overview of Modern Commercial Kits for Laboratory Diagnosis of African Swine Fever and Swine Influenza A Viruses. Viruses 2024, 16, 505. [Google Scholar] [CrossRef]
- Yang, J.; Zhu, R.; Li, N.; Zhang, Y.; Zhou, X.; Yue, H.; Li, Q.; Wang, Y.; Miao, F.; Chen, T.; et al. Protection Evaluation of a New Attenuated ASFV by Deletion of the L60L and CD2v Genes against Homologous Challenge. Viruses 2024, 16, 1464. [Google Scholar] [CrossRef]
- Koltsov, A.; Krutko, S.; Kholod, N.; Sukher, M.; Belov, S.; Korotin, A.; Koltsova, G. Deletion of the CD2 Gene in the Virulent ASFV Congo Strain Affects Viremia in Domestic Swine, but Not the Virulence. Animals 2023, 13, 2002. [Google Scholar] [CrossRef]
- Le, V.P.; Lan, N.T.; Canevari, J.T.; Villanueva-Cabezas, J.P.; Padungtod, P.; Trinh, T.B.N.; Nguyen, V.T.; Pfeiffer, C.N.; Oberin, M.V.; Firestone, S.M.; et al. Estimation of a Within-Herd Transmission Rate for African Swine Fever in Vietnam. Animals 2023, 13, 571. [Google Scholar] [CrossRef]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef] [PubMed]
- Walczak, M.; Żmudzki, J.; Mazur-Panasiuk, N.; Juszkiewicz, M.; Woźniakowski, G. Analysis of the Clinical Course of Experimental Infection with Highly Pathogenic African Swine Fever Strain, Isolated from an Outbreak in Poland. Aspects Related to the Disease Suspicion at the Farm Level. Pathogens 2020, 9, 237. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.; Zhang, Z.; Wang, Z.; He, X.; Zhang, X.; Wang, L.; Wang, W.; Huang, L.; Xi, F.; Huangfu, H.; et al. Emergence and Prevalence of Naturally Occurring Lower Virulent African Swine Fever Viruses in Domestic Pigs in China in 2020. Sci. China Life Sci. 2021, 64, 752–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Kang, W.; Yang, W.; Zhang, J.; Li, D.; Zheng, H. Structure of African Swine Fever Virus and Associated Molecular Mechanisms Underlying Infection and Immunosuppression: A Review. Front. Immunol. 2021, 12, 715582. [Google Scholar] [CrossRef]
- Sánchez-Torres, C.; Gómez-Puertas, P.; Gómez-del-Moral, M.; Alonso, F.; Escribano, J.M.; Ezquerra, A.; Domínguez, J. Expression of Porcine CD163 on Monocytes/Macrophages Correlates with Permissiveness to African Swine Fever Infection. Arch. Virol. 2003, 148, 2307–2323. [Google Scholar] [CrossRef]
- Rimaniol, A.-C.; Gras, G.; Verdier, F.; Capel, F.; Grigoriev, V.B.; Porcheray, F.; Sauzeat, E.; Fournier, J.-G.; Clayette, P.; Siegrist, C.-A.; et al. Aluminum Hydroxide Adjuvant Induces Macrophage Differentiation towards a Specialized Antigen-Presenting Cell Type. Vaccine 2004, 22, 3127–3135. [Google Scholar] [CrossRef]
- Sánchez-Cordón, P.J.; Chapman, D.; Jabbar, T.; Reis, A.L.; Goatley, L.; Netherton, C.L.; Taylor, G.; Montoya, M.; Dixon, L. Different Routes and Doses Influence Protection in Pigs Immunised with the Naturally Attenuated African Swine Fever Virus Isolate OURT88/3. Antivir. Res. 2017, 138, 1–8. [Google Scholar] [CrossRef] [PubMed]

| First Positive Sow | IDEXX 1 | Lijian (p72/CD2v Gene) 2 | |
|---|---|---|---|
| P72 | P72 | CD2v | |
| The 1st test of the original sample | 28.27 | 33.47 | Negative |
| The 2nd test of the original sample | 29.97 | 32.38 | Negative |
| The 1st test of re-sampling sample | 27.36 | 30.89 | Negative |
| The 2nd test of re-sampling sample | 27.99 | 31.63 | Negative |
| Location | CT Value |
|---|---|
| G1-5-42 | 36.08 |
| G1-5-44 | 37.12 |
| G1-5-51 | 40.99 |
| Category | Depopulation | Precise Culling |
|---|---|---|
| Animal loss 1 | ||
| Sow, n | 6000 | 1097 |
| Replacement gilt, n | 2011 | 0 |
| Piglet, n | 8958 | 0 |
| Boar, n | 136 | 0 |
| Total animal value, RMB | ¥25,264,756 | ¥3,291,000 |
| Mortality handling cost, RMB | ¥81,470 | ¥10,970 |
| Testing costs, RMB | ||
| FMD vaccination (2 times) | - | ¥28,200 |
| Whole-herd PCR tests (4 times) | - | ¥142,417 |
| Cleaning & disinfection costs, RMB | ¥85,000 | ¥17,000 |
| Restocking costs, RMB 2 | ||
| Replacement gilt | ¥15,000,000 | ¥2,742,500 |
| Boar | ¥408,000 | - |
| Production interruption (opportunity loss) | ||
| Wean pig (6 months throughput), n | 74,520 | 13,625 |
| Piglet profit loss, RMB | ¥11,178,000 | ¥2,043,711 |
| Total economic loss, RMB | ¥40,839,226 | ¥6,232,087 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Liu, Y.; Duan, L.; Tsai, S.-Y.; Yaros, J.P.; Wu, F. Elimination of ASFV via Precise Culling in a Large-Scale Breeding Herd in China: A Field Experience. Animals 2025, 15, 2521. https://doi.org/10.3390/ani15172521
Du X, Liu Y, Duan L, Tsai S-Y, Yaros JP, Wu F. Elimination of ASFV via Precise Culling in a Large-Scale Breeding Herd in China: A Field Experience. Animals. 2025; 15(17):2521. https://doi.org/10.3390/ani15172521
Chicago/Turabian StyleDu, Xingqian, Yuan Liu, Lianmao Duan, Shih-Yi Tsai, Joseph P. Yaros, and Fangzhou Wu. 2025. "Elimination of ASFV via Precise Culling in a Large-Scale Breeding Herd in China: A Field Experience" Animals 15, no. 17: 2521. https://doi.org/10.3390/ani15172521
APA StyleDu, X., Liu, Y., Duan, L., Tsai, S.-Y., Yaros, J. P., & Wu, F. (2025). Elimination of ASFV via Precise Culling in a Large-Scale Breeding Herd in China: A Field Experience. Animals, 15(17), 2521. https://doi.org/10.3390/ani15172521





