Variations in the Fecal Microbiota of Red Deer in Relation to the Hunting Area in the Friuli-Venezia Giulia Region, Italy
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area Description
2.2. Sampling Procedure
2.3. DNA Extraction and Sequencing of 16S rRNA Gene Amplicons
2.4. Bioinformatic and Statistical Analysis
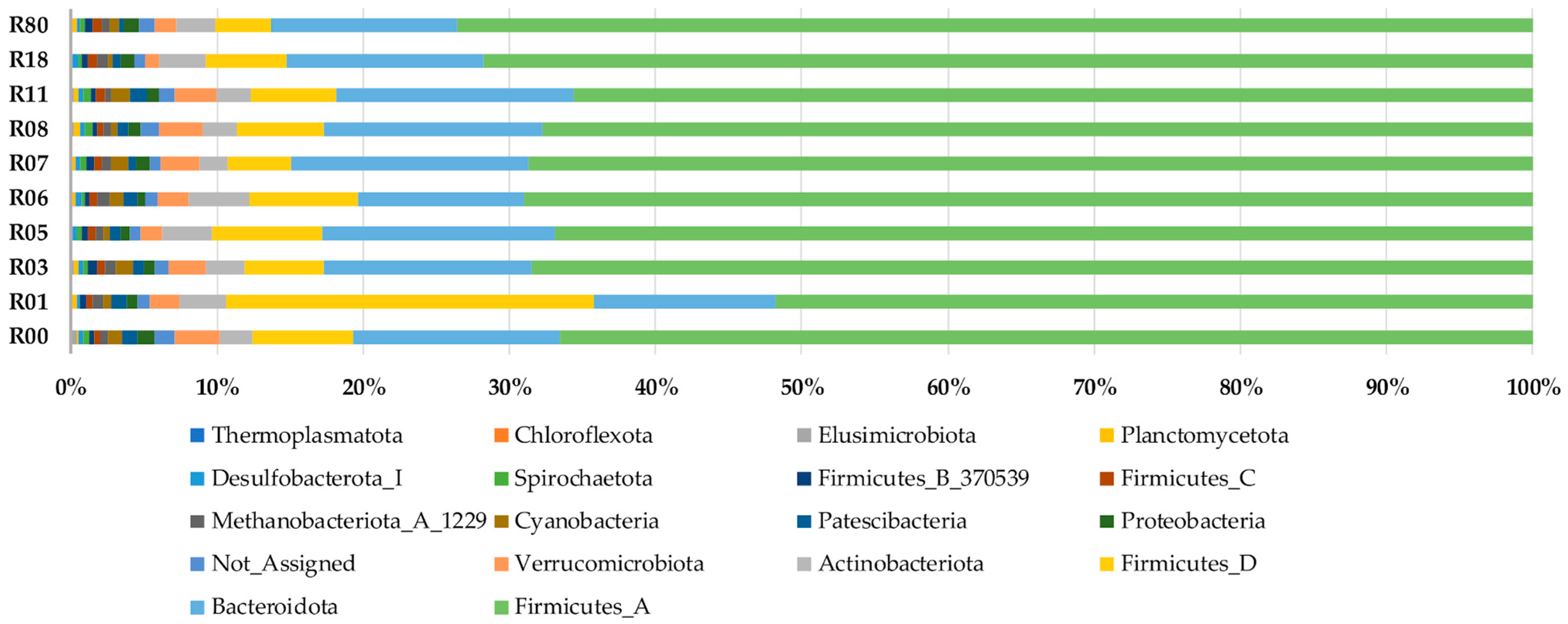
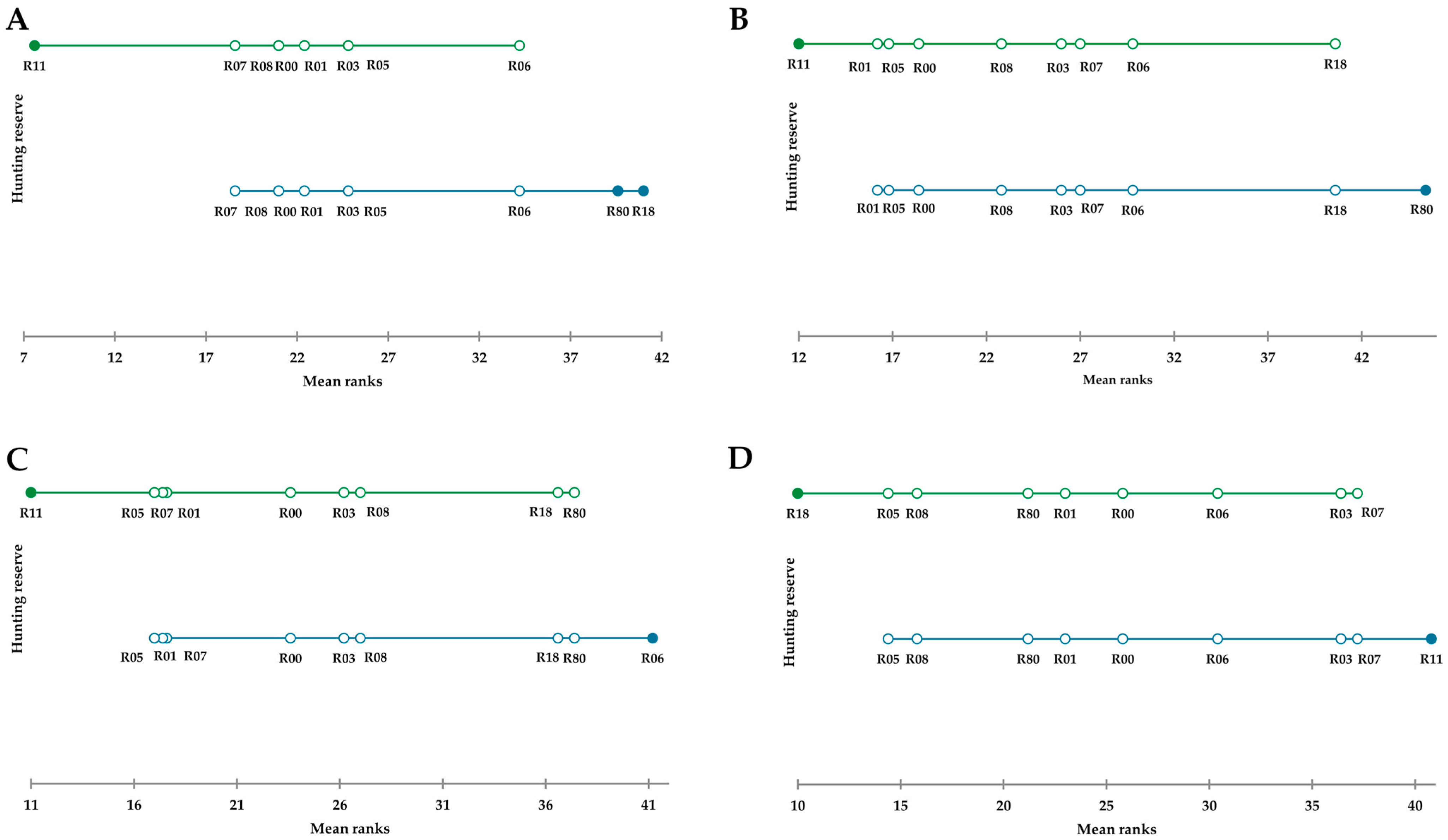
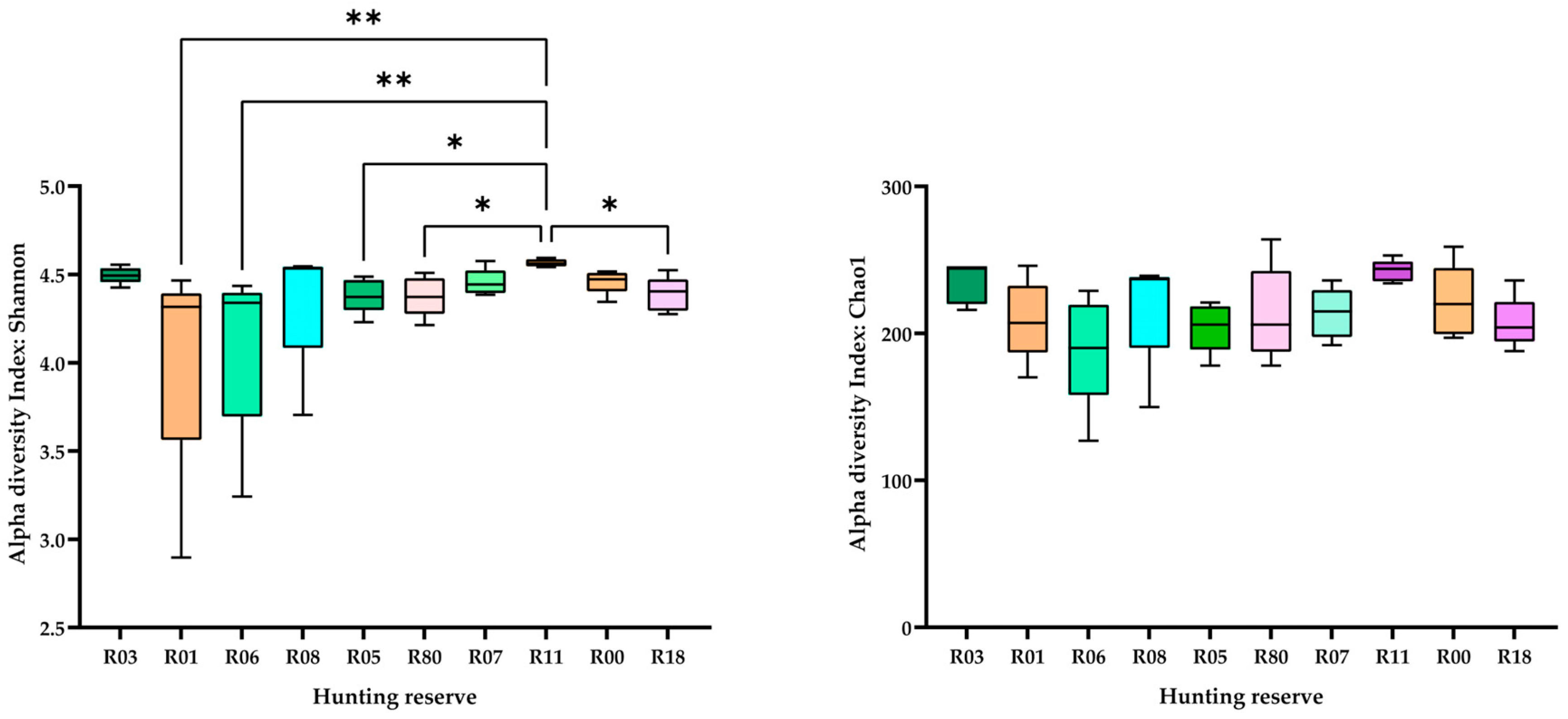
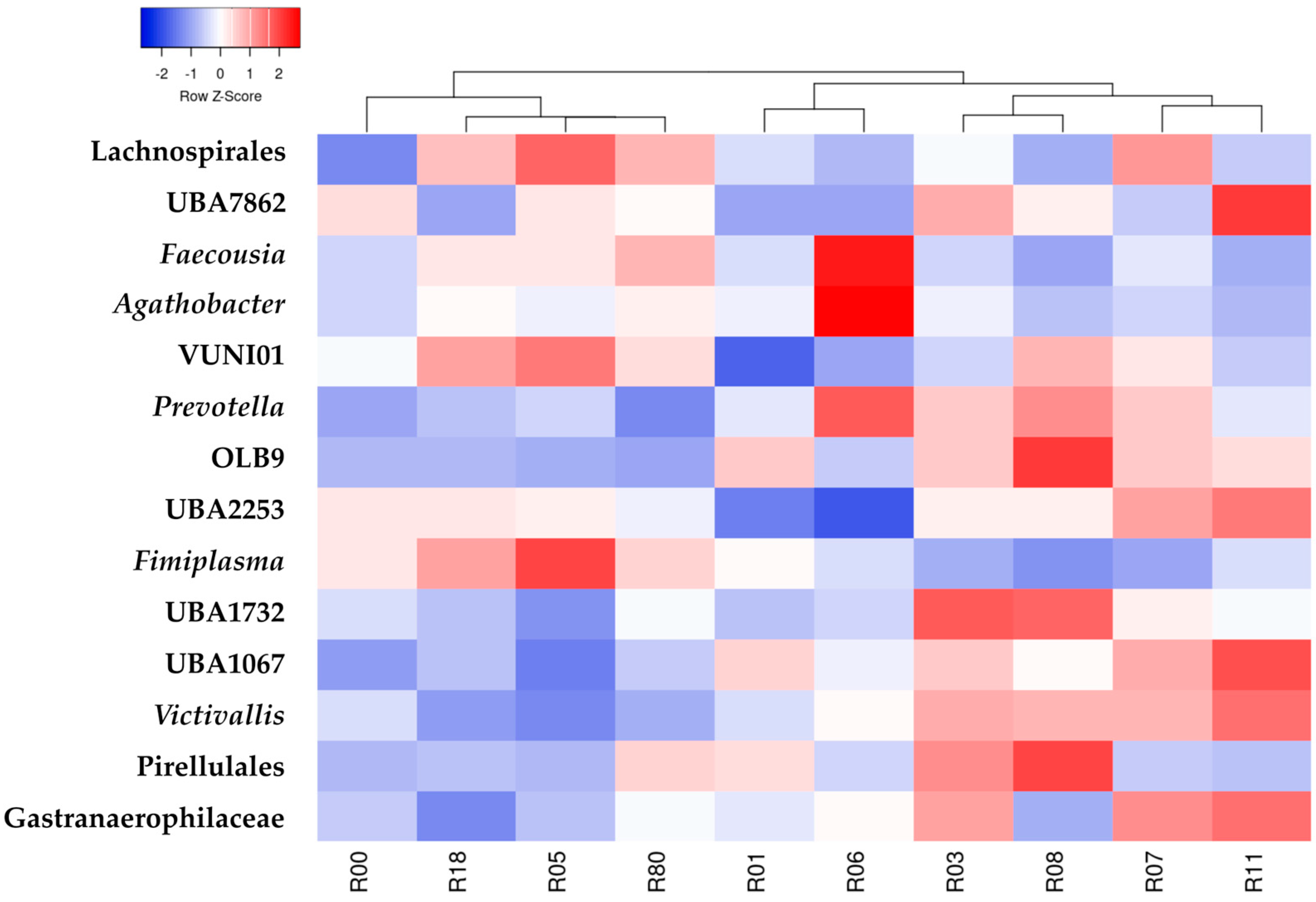
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| RA | Relative abundance |
References
- Víquez-R, L.; Henrich, M.; Riegel, V.; Bader, M.; Wilhelm, K.; Heurich, M.; Sommer, S. A Taste of Wilderness: Supplementary Feeding of Red Deer (Cervus Elaphus) Increases Individual Bacterial Microbiota Diversity but Lowers Abundance of Important Gut Symbionts. Anim. Microbiome 2024, 6, 28. [Google Scholar] [CrossRef]
- Wang, M.; Guo, Q.; Shan, Y.; Cheng, Z.; Zhang, Q.; Bai, J.; Dong, Y.; Zhong, Z. Effects of Probiotic Supplementation on Body Weight, Growth Performance, Immune Function, Intestinal Microbiota and Metabolites in Fallow Deer. Biology 2024, 13, 603. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xiao, L.; Liu, Z.; Deng, Y.; Zhu, J.; Yang, C.; Liu, Q.; Tian, D.; Cui, X.; Peng, J. Impacts of Captive Domestication and Geographical Divergence on the Gut Microbiome of Endangered Forest Musk Deer. Animals 2025, 15, 1954. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Z.; Jin, Y.; Sun, Y.; Wang, B.; Liu, X.; Yuan, Z.; Zhang, W.; Zhang, C.; Zhang, M. The Gut Microbial Differences between Pre-Released and Wild Red Deer: Firmicutes Abundance May Affect Wild Adaptation after Release. Front. Microbiol. 2024, 15, 1401373. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Li, Z.; Liu, X.; Jin, Y.; Sun, Y.; Yuan, Z.; Zhang, W.; Wang, J.; Zhang, M. Response of the Gut Microbiota to Changes in the Nutritional Status of Red Deer during Winter. Sci. Rep. 2024, 14, 24961. [Google Scholar] [CrossRef]
- Zhen, J.; Ren, Y.; Zhang, H.; Yuan, X.; Wang, L.; Shen, H.; Liu, P.; Chen, Y. Effect of Different Dietary Regimes on the Gut Microbiota and Fecal Metabolites of Père David’s Deer. Animals 2022, 12, 584. [Google Scholar] [CrossRef]
- Wei, L.; Zeng, B.; Li, B.; Guo, W.; Mu, Z.; Gan, Y.; Li, Y. Hybridization Alters Red Deer Gut Microbiome and Metabolites. Front. Microbiol. 2024, 15, 1387957. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, M.; Xu, S.; Zhang, H.; Li, Y.; Hu, D. Analysis on Changes and Influencing Factors of the Intestinal Microbiota of Alpine Musk Deer between the Place of Origin and Migration. Animals 2023, 13, 3791. [Google Scholar] [CrossRef]
- Mahmoudi, M.; Almario, J.; Lutap, K.; Nieselt, K.; Kemen, E. Microbial Communities Living inside Plant Leaves or on the Leaf Surface Are Differently Shaped by Environmental Cues. ISME Commun. 2024, 4, ycae103. [Google Scholar] [CrossRef]
- Sun, Y.; Yu, Y.; Guo, J.; Zhong, L.; Zhang, M. Alterations in Fecal Microbiota Linked to Environment and Sex in Red Deer (Cervus Elaphus). Animals 2023, 13, 929. [Google Scholar] [CrossRef]
- Cronin, P.; Joyce, S.A.; O’Toole, P.W.; O’Connor, E.M. Dietary Fibre Modulates the Gut Microbiota. Nutrients 2021, 13, 1655. [Google Scholar] [CrossRef]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the Gut Microbiota in Nutrition and Health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef]
- Krajmalnik-Brown, R.; Ilhan, Z.-E.; Kang, D.-W.; DiBaise, J.K. Effects of Gut Microbes on Nutrient Absorption and Energy Regulation. Nutr. Clin. Pract. Off. Publ. Am. Soc. Parenter. Enter. Nutr. 2012, 27, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Fändriks, L. Roles of the Gut in the Metabolic Syndrome: An Overview. J. Intern. Med. 2017, 281, 319–336. [Google Scholar] [CrossRef] [PubMed]
- Oriolo, G.; Pingitore, G.; Strazzaboschi, L.; Laureti, L. Carta della Natura della Regione Friuli-Venezia Giulia—Standard nazionale: Carta degli habitat alla scala 1:25.000. Regione Friuli-Venezia Giulia, 2021, ISPRA. Available online: https://www.isprambiente.gov.it/it/servizi/sistema-carta-della-natura/cartografia/carta-della-natura-alla-scala-1-50.000/friuli-venezia-giulia-1 (accessed on 8 July 2025).
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of general 16S ribosomal RNA gene PCR primers for classical and next-generation sequencing-based diversity studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Lu, Y.; Zhou, G.; Ewald, J.; Pang, Z.; Shiri, T.; Xia, J. MicrobiomeAnalyst 2.0: Comprehensive Statistical, Functional and Integrative Analysis of Microbiome Data. Nucleic Acids Res. 2023, 51, W310–W318. [Google Scholar] [CrossRef]
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic Biomarker Discovery and Explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Su, R.; Dalai, M.; Luvsantseren, B.; Chimedtseren, C.; Hasi, S. Comparative Study of the Function and Structure of the Gut Microbiota in Siberian Musk Deer and Forest Musk Deer. Appl. Microbiol. Biotechnol. 2022, 106, 6799–6817. [Google Scholar] [CrossRef]
- You, Z.; Deng, J.; Liu, J.; Fu, J.; Xiong, H.; Luo, W.; Xiong, J. Seasonal Variations in the Composition and Diversity of Gut Microbiota in White-Lipped Deer (Cervus albirostris). PeerJ 2022, 10, e13753. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, B.; Chen, H.; Yang, F.; Huang, J.; Jiao, X.; Zhang, Y. Environmental Factors and Gut Microbiota: Toward Better Conservation of Deer Species. Front. Microbiol. 2023, 14, 1136413. [Google Scholar] [CrossRef]
- Arahal, D.R.; Bull, C.T.; Christensen, H.; Chuvochina, M.; Dedysh, S.N.; Fournier, P.-E.; Konstantinidis, K.T.; Parker, C.T.; Ventosa, A.; Young, P.; et al. Judicial Opinion 129. Int. J. Syst. Evol. Microbiol. 2024, 74, 006064. [Google Scholar] [CrossRef]
- Pallen, M.J. Request for an Opinion on the Standing and Retention of Firmicutes as a Phylum Name. Int. J. Syst. Evol. Microbiol. 2023, 73, 005933. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhang, S.; Wu, J.; Ye, T.; Wang, S.; Wang, P.; Xing, D. Butyrate-Producing Bacteria and the Gut-Heart Axis in Atherosclerosis. Clin. Chim. Acta Int. J. Clin. Chem. 2020, 507, 236–241. [Google Scholar] [CrossRef] [PubMed]
- Dias, B.D.C.; Lamarca, A.P.; Machado, D.T.; Kloh, V.P.; de Carvalho, F.M.; Vasconcelos, A.T.R. Metabolic Pathways Associated with Firmicutes Prevalence in the Gut of Multiple Livestock Animals and Humans. Anim. Microbiome 2025, 7, 20. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef]
- Costa-Roura, S.; Villalba, D.; Balcells, J.; De la Fuente, G. First Steps into Ruminal Microbiota Robustness. Animals 2022, 12, 2366. [Google Scholar] [CrossRef]
- Magle, S.B.; Kardash, L.H.; Rothrock, A.O.; Chamberlin, J.C.; Mathews, N.E. Movements and Habitat Interactions of White-Tailed Deer: Implications for Chronic Wasting Disease Management. Am. Midl. Nat. 2015, 173, 267–282. [Google Scholar] [CrossRef]
- Sun, Y.; Sun, Y.; Shi, Z.; Liu, Z.; Zhao, C.; Lu, T.; Gao, H.; Zhu, F.; Chen, R.; Zhang, J.; et al. Gut Microbiota of Wild and Captive Alpine Musk Deer (Moschus chrysogaster). Front. Microbiol. 2019, 10, 3156. [Google Scholar] [CrossRef]
- Khatoon, M.; Patel, S.H.; Pandit, R.J.; Jakhesara, S.J.; Rank, D.N.; Joshi, C.G.; Kunjadiya, A.P. Rumen and Fecal Microbial Profiles in Cattle Fed High Lignin Diets Using Metagenome Analysis. Anaerobe 2022, 73, 102508. [Google Scholar] [CrossRef]
- Morotomi, M.; Nagai, F.; Watanabe, Y. Description of Christensenella minuta Gen. Nov., Sp. Nov., Isolated from Human Faeces, Which Forms a Distinct Branch in the Order Clostridiales, and Proposal of Christensenellaceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2012, 62, 144–149. [Google Scholar] [CrossRef]
- Zoetendal, E.G.; Plugge, C.M.; Akkermans, A.D.L.; de Vos, W.M. Victivallis Vadensis Gen. Nov., Sp. Nov., a Sugar-Fermenting Anaerobe from Human Faeces. Int. J. Syst. Evol. Microbiol. 2003, 53, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Gomes Carvalho Alves, K.L.; Granja-Salcedo, Y.T.; Messana, J.D.; Carneiro de Souza, V.; Generoso Ganga, M.J.; Detogni Colovate, P.H.; Kishi, L.T.; Berchielli, T.T. Rumen Bacterial Diversity in Relation to Nitrogen Retention in Beef Cattle. Anaerobe 2021, 67, 102316. [Google Scholar] [CrossRef] [PubMed]
- Shang, W.; Zhang, S.; Qian, H.; Huang, S.; Li, H.; Liu, J.; Chen, D. Gut Microbiota and Sepsis and Sepsis-Related Death: A Mendelian Randomization Investigation. Front. Immunol. 2024, 15, 1266230. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Yang, Y.; Xu, B.; Song, P.; Jiang, F.; Gao, H.; Cai, Z.; Gu, H.; Zhang, T. Comparative Macrogenomics Reveal Plateau Adaptation of Gut Microbiome in Cervids. BMC Biol. 2025, 23, 154. [Google Scholar] [CrossRef]
- Iversen, K.N.; Dicksved, J.; Zoki, C.; Fristedt, R.; Pelve, E.A.; Langton, M.; Landberg, R. The Effects of High Fiber Rye, Compared to Refined Wheat, on Gut Microbiota Composition, Plasma Short Chain Fatty Acids, and Implications for Weight Loss and Metabolic Risk Factors (the RyeWeight Study). Nutrients 2022, 14, 1669. [Google Scholar] [CrossRef]
- Zhao, G.; Ma, T.; Tang, W.; Li, D.; Mishra, S.K.; Xu, Z.; Wang, Q.; Jie, H. Gut Microbiome of Chinese Forest Musk Deer Examined across Gender and Age. BioMed Res. Int. 2019, 2019, 9291216. [Google Scholar] [CrossRef]
- Delgado, M.L.; Singh, P.; Funk, J.A.; Moore, J.A.; Cannell, E.M.; Kanesfsky, J.; Manning, S.D.; Scribner, K.T. Intestinal Microbial Community Dynamics of White-Tailed Deer (Odocoileus virginianus) in an Agroecosystem. Microb. Ecol. 2017, 74, 496–506. [Google Scholar] [CrossRef]
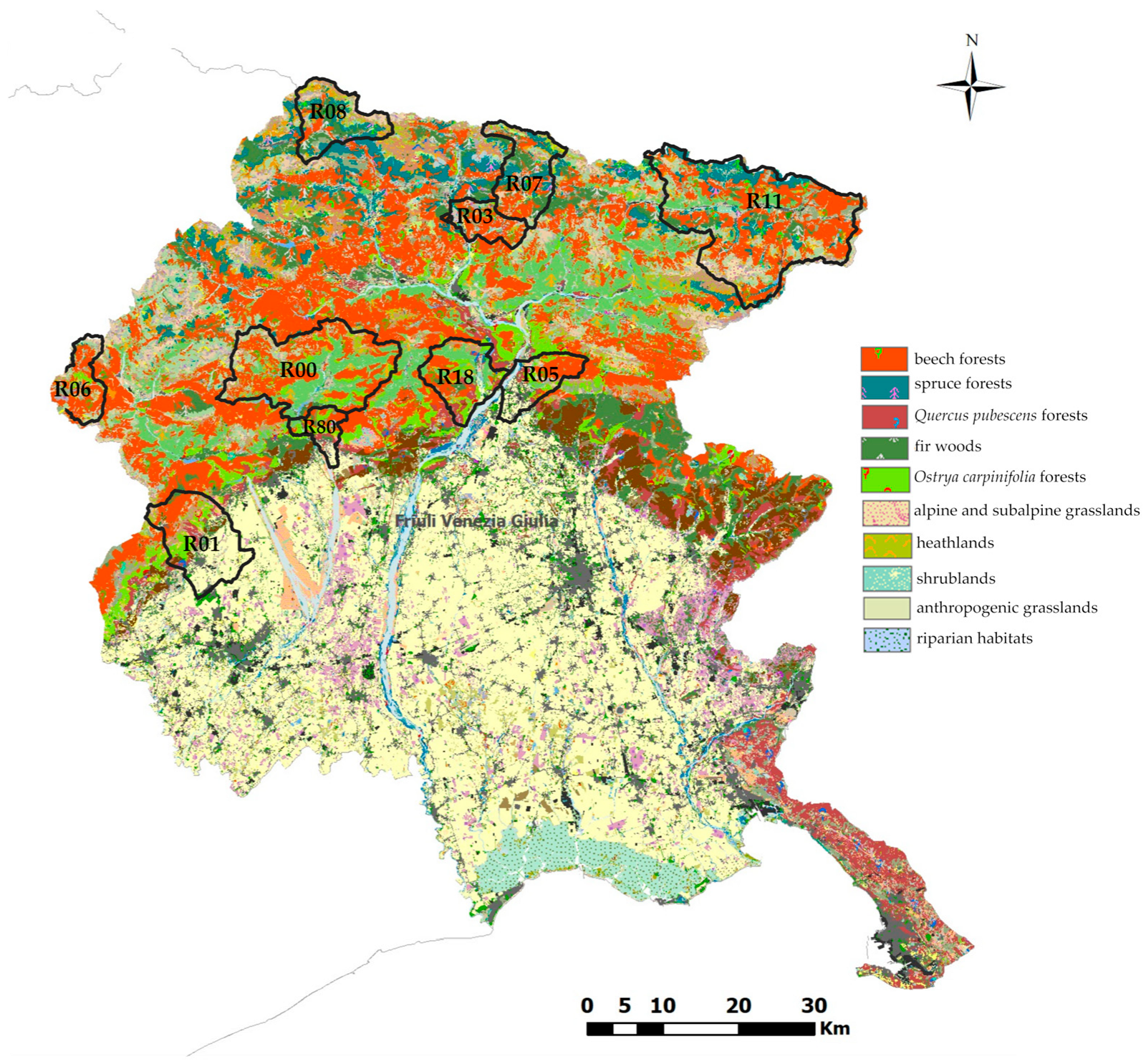

| Code | Hunting Reserve Name | Rainfall, mm/y | Altitude (a.s.l.), m | Average T, °C | Vegetation |
|---|---|---|---|---|---|
| R00 | Tramonti | 1600–2400 | 448–1558 | 0–12 | Fir woods |
| R01 | Aviano | 1500–2500 | 168–414 | 0–14 | Ostrya carpinifolia forests, fir woods, anthropogenic grasslands |
| R03 | Arta Terme | 1400–2300 | 993–1318 | 0–12 | Beech forest, alpine and subalpine grasslands |
| R05 | Gemona del Friuli | 1500–2300 | 619–1175 | 0–14 | Beech forest, Quercus pubescens and Ostrya carpinifolia forests, anthropogenic grasslands |
| R06 | Erto e Casso | 1600–2400 | 711–853 | 0–12 | Fir woods, spruce forests and beech forests heathlands, shrublands |
| R07 | Paularo | 1500–3400 | 665–1373 | 0–12 | Fir woods, spruce forests and beech forests |
| R08 | Forni Avoltri | 1400–2300 | 993–1198 | 0–12 | Fir woods, spruce forests, alpine and subalpine grasslands, heathlands, shrublands |
| R11 | Tarvisio-Malborghetto | 1500–3400 | 1070–1420 | 0–12 | Fir woods, spruce forests and beech forests |
| R18 | Trasaghis | 1500–2300 | 182–427 | 0–14 | Beech forest, Quercus pubescens and Ostrya carpinifolia, Pinus nigra, Pinus silvestris forests, riparian habitats |
| R80 | Meduno | 1500–2500 | 299–1009 | 0–14 | Ostrya carpinifolia forests, fir woods, anthropogenic grasslands |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefanon, B.; Cecchini, V.; Sgorlon, S.; Colitti, M. Variations in the Fecal Microbiota of Red Deer in Relation to the Hunting Area in the Friuli-Venezia Giulia Region, Italy. Animals 2025, 15, 2517. https://doi.org/10.3390/ani15172517
Stefanon B, Cecchini V, Sgorlon S, Colitti M. Variations in the Fecal Microbiota of Red Deer in Relation to the Hunting Area in the Friuli-Venezia Giulia Region, Italy. Animals. 2025; 15(17):2517. https://doi.org/10.3390/ani15172517
Chicago/Turabian StyleStefanon, Bruno, Valentina Cecchini, Sandy Sgorlon, and Monica Colitti. 2025. "Variations in the Fecal Microbiota of Red Deer in Relation to the Hunting Area in the Friuli-Venezia Giulia Region, Italy" Animals 15, no. 17: 2517. https://doi.org/10.3390/ani15172517
APA StyleStefanon, B., Cecchini, V., Sgorlon, S., & Colitti, M. (2025). Variations in the Fecal Microbiota of Red Deer in Relation to the Hunting Area in the Friuli-Venezia Giulia Region, Italy. Animals, 15(17), 2517. https://doi.org/10.3390/ani15172517






