Mitochondrial-Targeted Protective Potential of Elamipretide for the In Vitro Production of Porcine Embryos
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Collection and In Vitro Culture of Porcine Oocytes
2.3. Analysis of Oocyte Nuclear Maturation
2.4. Assessment of ROS and GSH Levels in Oocyte Cytoplasm After Maturation Culture
2.5. Assessment of Mitochondrial Membrane Potential in Oocytes After Maturation Culture
2.6. Detection of DNA-Fragmented Oocytes After Maturation Culture
2.7. In Vitro Fertilization and In Vitro Culture
2.8. Experimental Design
2.8.1. Experiment 1. Effects of SS-31 Supplementation on the IVM of Oocytes and Subsequent Embryonic Development
2.8.2. Experiment 2. Effects of SS-31 Supplementation on Antioxidant Capacity and Mitochondrial Membrane Potential After IVM
2.8.3. Experiment 3. Effect of SS-31 Supplementation on Oocyte DNA Fragmentation After IVM
2.9. Statistical Analysis
3. Results
3.1. Experiment 1. Effects of SS-31 Supplementation on the IVM of Oocytes and Subsequent Embryonic Development
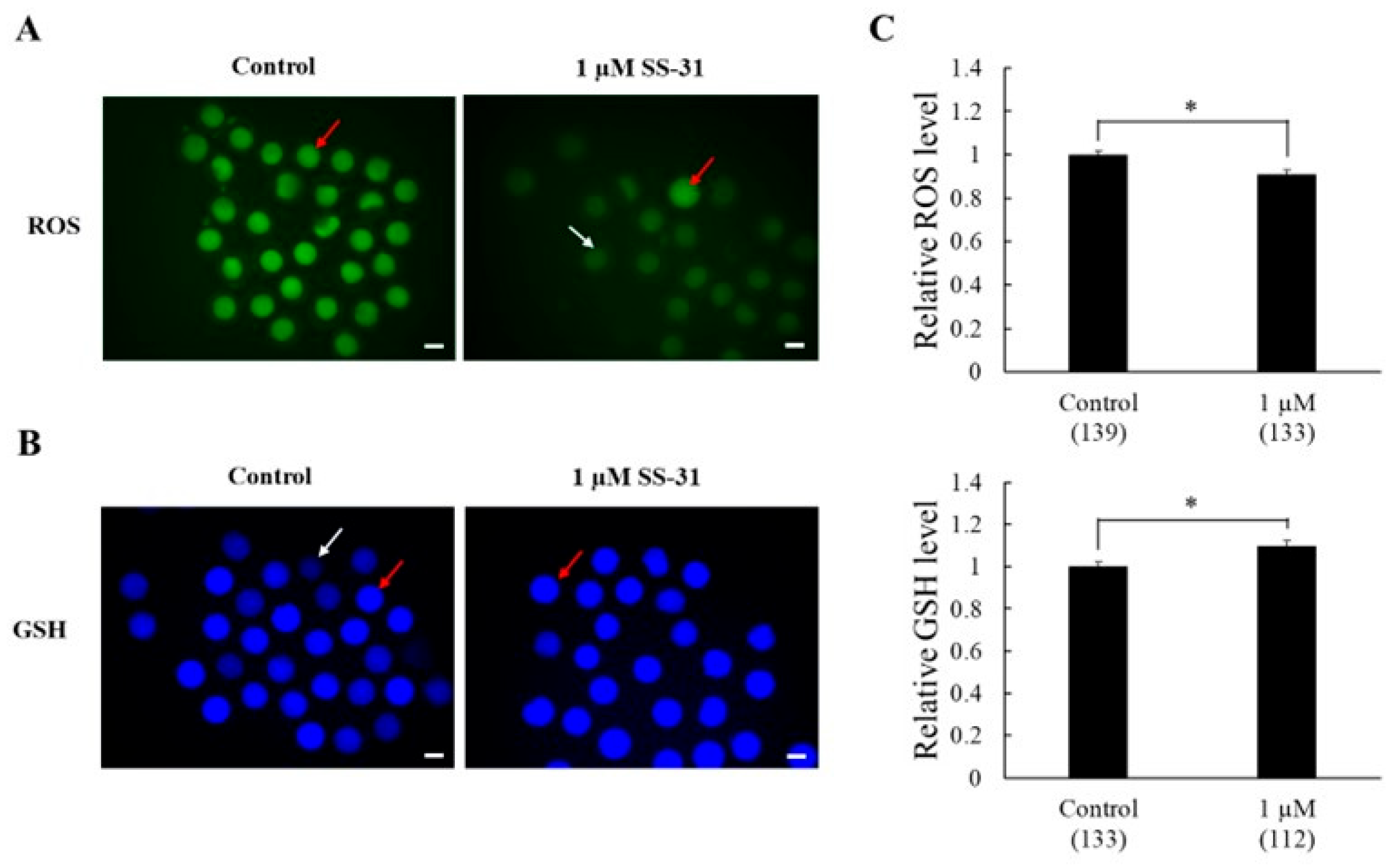
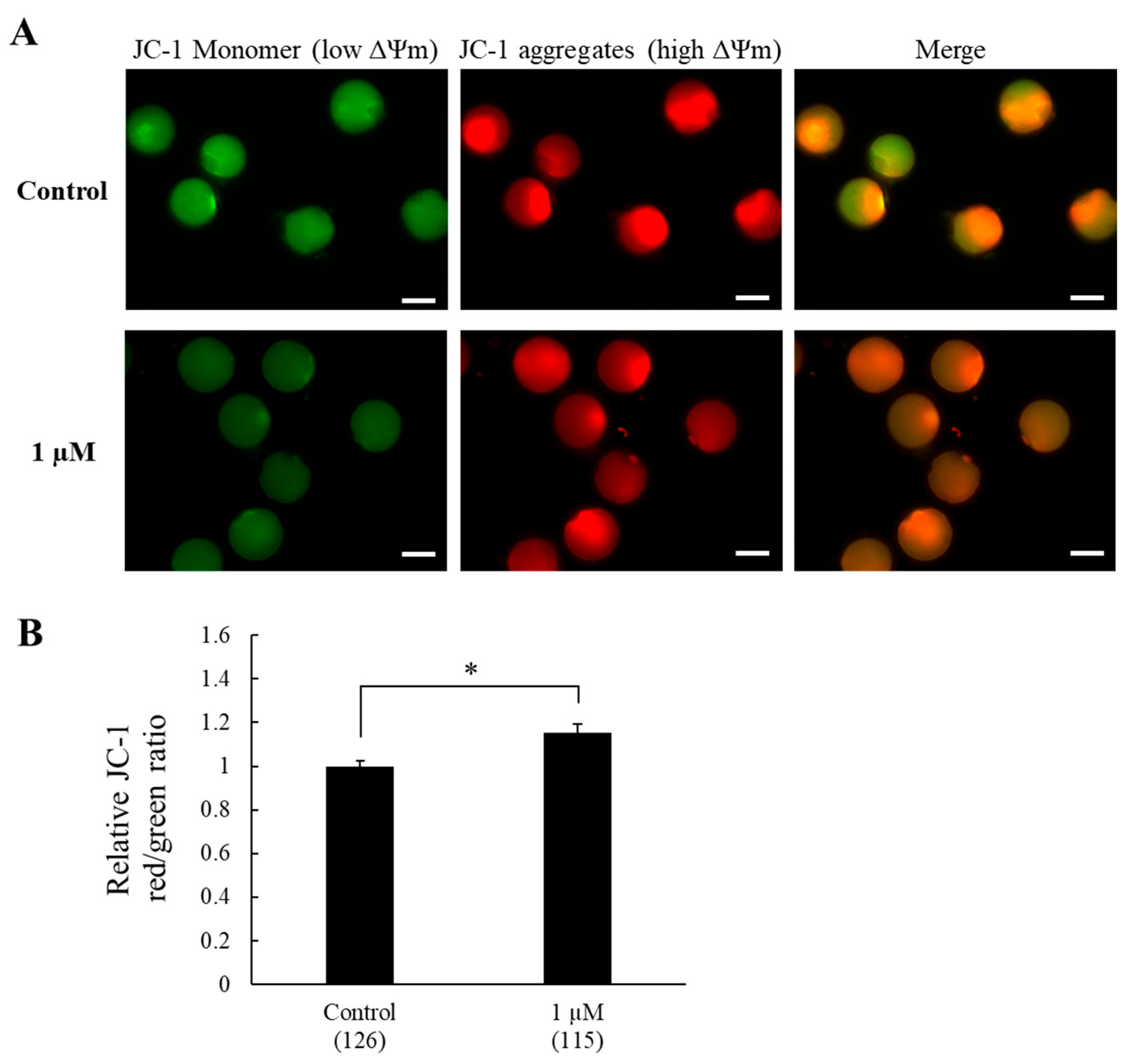
3.2. Experiment 2. Effects of SS-31 Supplementation on Antioxidant Capacity and Mitochondrial Membrane Potential After IVM
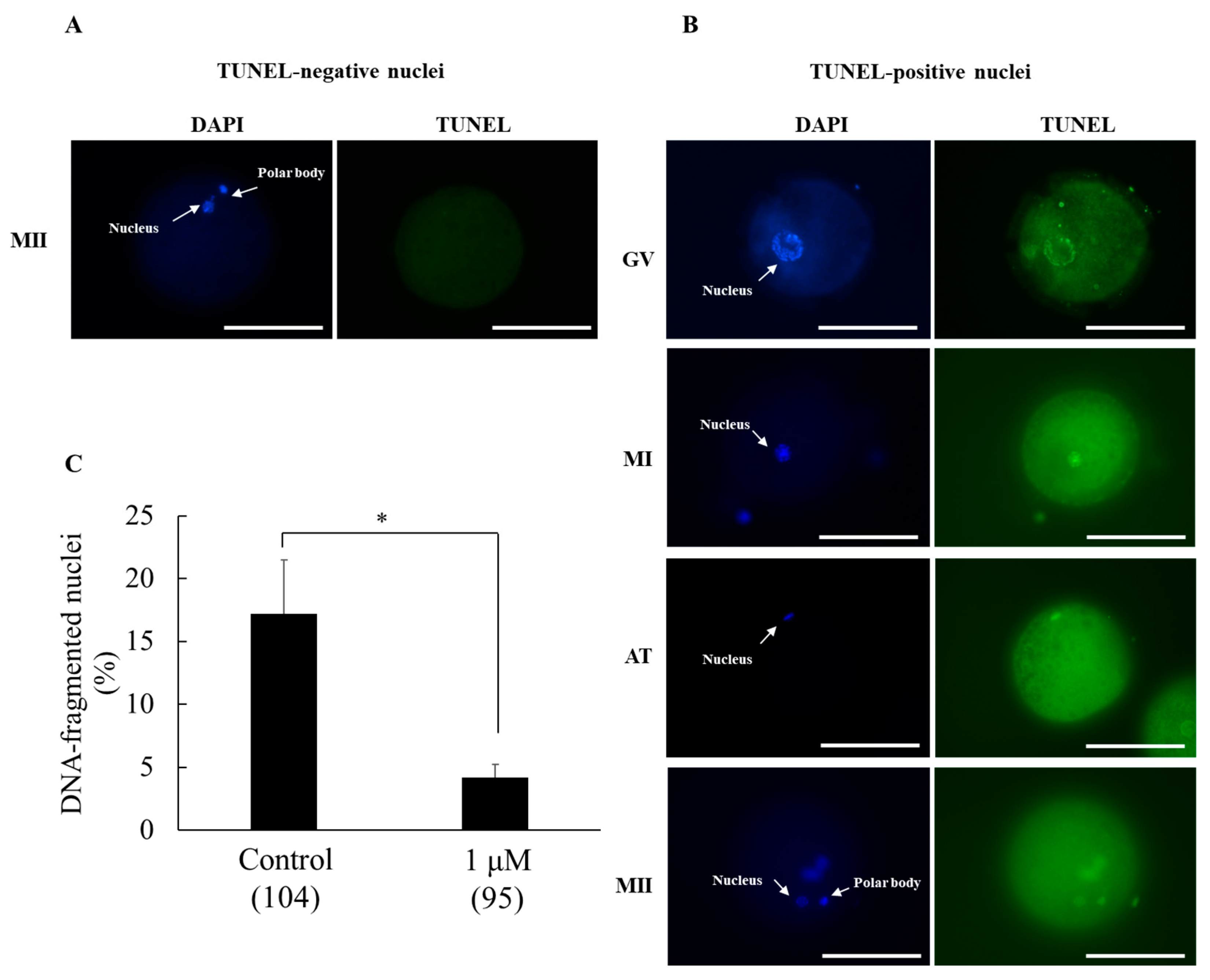
3.3. Experiment 3. Effect of SS-31 Supplementation on DNA-Fragmented Nuclei in Oocytes After IVM
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATP | adenosine-5-triphosphate |
| CL | cardiolipin |
| COC | cumulus–oocyte complex |
| DMSO | dimethyl sulfoxide |
| GSH | glutathione |
| IVM | in vitro maturation |
| OXPHOS | oxidative phosphorylation |
| PFM | porcine fertilization medium |
| ROS | reactive oxygen species |
| SS | Szeto–Schiller |
References
- Chen, W.; Zhao, H.; Li, Y. Mitochondrial dynamics in health and disease: Mechanisms and potential targets. Signal Transduct. Target. Ther. 2023, 8, 333. [Google Scholar] [CrossRef]
- Cha, D.; Choi, S.; Lee, Y.; Cho, J.; Lee, S. Mitoquinone improves porcine embryo development through modulating oxidative stress and mitochondrial function. Theriogenology 2025, 231, 90–100. [Google Scholar] [CrossRef]
- Jauslin, M.L.; Meier, T.; Smith, R.A.J.; Murphy, P.M. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003, 17, 1–10. [Google Scholar] [CrossRef]
- Dalton, C.M.; Szabadkai, G.; Carroll, J. Measurement of ATP in Single Oocytes: Impact of Maturation and Cumulus Cells on Levels and Consumption. J. Cell. Physiol. 2014, 229, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Kere, M.; Liu, P.-C.; Chen, Y.-K.; Chao, P.-C.; Tsai, L.-K.; Yeh, T.-Y.; Siriboon, C.; Intawicha, P.; Lo, N.-W.; Chiang, H.-I.; et al. Ultrastructural Characterization of Porcine Growing and In Vitro Matured Oocytes. Animals 2020, 10, 664. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Zheng, Y.; Deng, X.; Guo, J.; Xu, Z.; Scharffetter-Kochanek, K.; Dou, Y.; Jiang, M. Maintaining mitochondrial DNA copy number mitigates ROS-induced oocyte decline and female reproductive aging. Commun. Biol. 2024, 7, 1229. [Google Scholar] [CrossRef]
- Yildirim, R.M.; Seli, E. Mitochondria as therapeutic targets in assisted reproduction. Hum. Reprod. 2024, 39, 2147–2159. [Google Scholar] [CrossRef] [PubMed]
- Al-Zubaidi, U.; Adhikari, D.; Cinar, O.; Zhang, Q.H.; Yuen, W.S.; Murphy, M.P.; Rombauts, L.; Robker, R.L.; Carroll, J. Mitochondria-targeted therapeutics, MitoQ and BGP-15, reverse aging-associated meiotic spindle defects in mouse and human oocytes. Hum. Reprod. 2021, 36, 771–784. [Google Scholar] [CrossRef]
- Zhao, R.Z.; Jiang, S.; Zhang, L.; Yu, Z.B. Mitochondrial electron transport chain, ROS generation and uncoupling (Review). Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Okoye, C.N.; Koren, S.A.; Wojtovich, A.P. Mitochondrial complex I ROS production and redox signaling in hypoxia. Redox Biol. 2023, 67, 102926. [Google Scholar] [CrossRef]
- Quinlan, C.L.; Orr, A.L.; Perevoshchikova, I.V.; Treberg, J.R.; Ackrell, B.A.; Brand, M.D. Mitochondrial complex II can generate reactive oxygen species at high rates in both the forward and reverse reactions. J. Biol. Chem. 2012, 287, 27255–27264. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative stress in oocyte aging and female reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [PubMed]
- Combelles, C.M.; Gupta, S.; Agarwal, A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod. BioMed. Online 2009, 18, 864–880. [Google Scholar] [CrossRef] [PubMed]
- Soto-Heras, S.; Paramio, M.-T. Impact of oxidative stress on oocyte competence for in vitro embryo production programs. Res. Vet. Sci. 2020, 132, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Antonucci, S.; Di Lisa, F.; Kaludercic, N. Mitochondrial reactive oxygen species in physiology and disease. Cell Calcium 2021, 94, 102344. [Google Scholar] [CrossRef]
- Yapryntseva, M.A.; Zhivotovsky, B.; Gogvadze, V. Permeabilization of the outer mitochondrial membrane: Mechanisms and consequences. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167317. [Google Scholar] [CrossRef]
- Tyuryaeva, I.; Lyublinskaya, O. Expected and Unexpected Effects of Pharmacological Antioxidants. Int. J. Mol. Sci. 2023, 24, 9303. [Google Scholar] [CrossRef]
- Tiwari, S.; Mohanty, T.K.; Bhakat, M.; Kumar, N.; Baithalu, R.K.; Nath, S.; Yadav, H.P.; Dewry, R.K. Comparative evidence support better antioxidant efficacy of mitochondrial-targeted (Mitoquinone) than cytosolic (Resveratrol) antioxidant in improving in-vitro sperm functions of cryopreserved buffalo (Bubalus bubalis) semen. Cryobiology 2021, 101, 125–134. [Google Scholar] [CrossRef]
- Maharjan, S.; Oku, M.; Tsuda, M.; Hoseki, J.; Sakai, Y. Mitochondrial impairment triggers cytosolic oxidative stress and cell death following proteasome inhibition. Sci. Rep. 2014, 4, 5896. [Google Scholar] [CrossRef]
- Szeto, H.H.; Birk, A.V. Serendipity and the Discovery of Novel Compounds That Restore Mitochondrial Plasticity. Clin. Pharmacol. Ther. 2014, 96, 672–683. [Google Scholar] [CrossRef]
- Zhao, K.; Zhao, G.-M.; Wu, D.; Soong, Y.; Birk, A.V.; Schiller, P.W.; Szeto, H.H. Cell-permeable Peptide Antioxidants Targeted to Inner Mitochondrial Membrane inhibit Mitochondrial Swelling, Oxidative Cell Death, and Reperfusion Injury*. J. Biol. Chem. 2004, 279, 34682–34690. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.; Varzideh, F.; Farroni, E.; Mone, P.; Kansakar, U.; Jankauskas, S.S.; Santulli, G. Elamipretide: A Review of Its Structure, Mechanism of Action, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.M.; Wang, Y.T.; Wei, R.M.; Li, X.Y.; Luo, B.L.; Zhang, J.Y.; Zhang, K.X.; Fang, S.K.; Liu, X.C.; Chen, G.H. Mitochondrial antioxidant elamipretide improves learning and memory impairment induced by chronic sleep deprivation in mice. Brain Behav. 2024, 14, e3508. [Google Scholar] [CrossRef]
- Nie, Y.; Li, J.; Zhai, X.; Wang, Z.; Wang, J.; Wu, Y.; Zhao, P.; Yan, G. Elamipretide(SS-31) Attenuates Idiopathic Pulmonary Fibrosis by Inhibiting the Nrf2-Dependent NLRP3 Inflammasome in Macrophages. Antioxidants 2023, 12, 2022. [Google Scholar] [CrossRef] [PubMed]
- Whitson, J.A.; Martín-Pérez, M.; Zhang, T.; Gaffrey, M.J.; Merrihew, G.E.; Huang, E.; White, C.C.; Kavanagh, T.J.; Qian, W.J.; Campbell, M.D.; et al. Elamipretide (SS-31) treatment attenuates age-associated post-translational modifications of heart proteins. Geroscience 2021, 43, 2395–2412. [Google Scholar] [CrossRef]
- Campbell, M.D.; Martín-Pérez, M.; Egertson, J.D.; Gaffrey, M.J.; Wang, L.; Bammler, T.; Rabinovitch, P.S.; MacCoss, M.; Qian, W.J.; Villen, J.; et al. Elamipretide effects on the skeletal muscle phosphoproteome in aged female mice. Geroscience 2022, 44, 2913–2924. [Google Scholar] [CrossRef]
- Pharaoh, G.; Kamat, V.; Kannan, S.; Stuppard, R.S.; Whitson, J.; Martín-Pérez, M.; Qian, W.J.; MacCoss, M.J.; Villén, J.; Rabinovitch, P.; et al. The mitochondrially targeted peptide elamipretide (SS-31) improves ADP sensitivity in aged mitochondria by increasing uptake through the adenine nucleotide translocator (ANT). Geroscience 2023, 45, 3529–3548. [Google Scholar] [CrossRef] [PubMed]
- Piao, M.J.; Kang, K.A.; Fernando, P.; Herath, H.; Hyun, J.W. Silver nanoparticle-induced cell damage via impaired mtROS-JNK/MnSOD signaling pathway. Toxicol. Mech. Methods 2024, 34, 803–812. [Google Scholar] [CrossRef]
- Romanova, N.; Sule, K.; Issler, T.; Hebrok, D.; Persicke, M.; Thévenod, F.; Prenner, E.J.; Lee, W.K. Cadmium-cardiolipin disruption of respirasome assembly and redox balance through mitochondrial membrane rigidification. J. Lipid. Res. 2025, 66, 100750. [Google Scholar] [CrossRef]
- Chiao, Y.A.; Zhang, H.; Sweetwyne, M.; Whitson, J.; Ting, Y.S.; Basisty, N.; Pino, L.K.; Quarles, E.; Nguyen, N.-H.; Campbell, M.D.; et al. Late-life restoration of mitochondrial function reverses cardiac dysfunction in old mice. eLife 2020, 9, e55513. [Google Scholar] [CrossRef]
- Lin, Q.; Le, Q.A.; Takebayashi, K.; Hirata, M.; Tanihara, F.; Sawamoto, O.; Kikuchi, T.; Otoi, T. Short-term preservation of porcine zygotes at ambient temperature using a chemically defined medium. Anim. Sci. J. 2022, 93, e13711. [Google Scholar] [CrossRef]
- Yousefian, I.; Zare-Shahneh, A.; Goodarzi, A.; Baghshahi, H.; Fouladi-Nashta, A.A. The effect of Tempo and MitoTEMPO on oocyte maturation and subsequent embryo development in bovine model. Theriogenology 2021, 176, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shim, J.; Ko, N.; Kim, H.-J.; Kim, J.-H.; Kim, H.; Choi, K. Docosahexaenoic acid supplementation during porcine oocyte in vitro maturation improves oocyte quality and embryonic development by enhancing the homeostasis of energy metabolism. Theriogenology 2024, 227, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Le, Q.A.; Takebayashi, K.; Hirata, M.; Tanihara, F.; Thongkittidilok, C.; Sawamoto, O.; Kikuchi, T.; Otoi, T. Viability and developmental potential of porcine blastocysts preserved for short term in a chemically defined medium at ambient temperature. Reprod. Domest. Anim. 2022, 57, 556–563. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Lu, Q.; Wu, Y.; Liu, J.; Liu, N.; Huang, X.; Xu, C. Effect of Elamipretide on the Vitrification of Mouse Ovarian Tissue by Freezing. Biopreserv. Biobank 2024, 22, 600–608. [Google Scholar] [CrossRef]
- Bai, H.; Zhang, Y.; Tian, S.; Hu, R.; Liang, Y.; Gao, J.; Wang, Y.; Wu, B. Elamipretide as a potential candidate for relieving cryodamage to human spermatozoa during cryopreservation. Cryobiology 2020, 95, 138–142. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Czerniawska Piątkowska, E. Antioxidant effect of Elamipretide on bull’s sperm cells during freezing/thawing process. Andrology 2021, 9, 1275–1281. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Cao, J.; Xing, X.; Liang, Y.; Zhang, Y.; Tang, X.; Lin, S.; Wu, Z.; Li, Z.; et al. Supplementation of SkQ1 Increases Mouse In Vitro Oocyte Maturation and Subsequent Embryonic Development by Reducing Oxidative Stress. Pharmaceuticals 2024, 17, 455. [Google Scholar] [CrossRef]
- Yang, S.G.; Park, H.J.; Kim, J.W.; Jung, J.M.; Kim, M.J.; Jegal, H.G.; Kim, I.S.; Kang, M.J.; Wee, G.; Yang, H.Y.; et al. Mito-TEMPO improves development competence by reducing superoxide in preimplantation porcine embryos. Sci. Rep. 2018, 8, 10130. [Google Scholar] [CrossRef]
- Murphy, M.P.; Smith, R.A. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 629–656. [Google Scholar] [CrossRef] [PubMed]
- Lai, F.N.; Ma, J.Y.; Liu, J.C.; Wang, J.J.; Cheng, S.F.; Sun, X.F.; Li, L.; Li, B.; Nyachoti, C.M.; Shen, W. The influence of N-acetyl-l-cysteine on damage of porcine oocyte exposed to zearalenone in vitro. Toxicol. Appl. Pharmacol. 2015, 289, 341–348. [Google Scholar] [CrossRef]
- Jiao, Y.; Wang, Y.; Jiang, T.; Wen, K.; Cong, P.; Chen, Y.; He, Z. Quercetin protects porcine oocytes from in vitro aging by reducing oxidative stress and maintaining the mitochondrial functions. Front. Cell Dev. Biol. 2022, 10, 915898. [Google Scholar] [CrossRef] [PubMed]
- Suntres, Z.E. Liposomal Antioxidants for Protection against Oxidant-Induced Damage. J. Toxicol. 2011, 2011, 152474. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, K.; Abe, J.; Hyodo, M.; Haga, S.; Ozaki, M.; Harashima, H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control. Release 2015, 213, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Do, L.T.; Shibata, Y.; Taniguchi, M.; Nii, M.; Nguyen, T.V.; Tanihara, F.; Takagi, M.; Otoi, T. Melatonin Supplementation During In Vitro Maturation and Development Supports the Development of Porcine Embryos. Reprod. Domest. Anim. 2015, 50, 1054–1058. [Google Scholar] [CrossRef]
- Thongkittidilok, C.; Le, Q.A.; Lin, Q.; Takebayashi, K.; Do, T.K.L.; Namula, Z.; Hirata, M.; Tanihara, F.; Otoi, T. Effects of individual or in-combination antioxidant supplementation during in vitro maturation culture on the developmental competence and quality of porcine embryos. Reprod. Domest. Anim. 2022, 57, 314–320. [Google Scholar] [CrossRef]
- Mitchell, W.; Ng, E.A.; Tamucci, J.D.; Boyd, K.J.; Sathappa, M.; Coscia, A.; Pan, M.; Han, X.; Eddy, N.A.; May, E.R.; et al. The mitochondria-targeted peptide SS-31 binds lipid bilayers and modulates surface electrostatics as a key component of its mechanism of action. J. Biol. Chem. 2020, 295, 7452–7469. [Google Scholar] [CrossRef]
- Liu, N.K.; Deng, L.X.; Wang, M.; Lu, Q.B.; Wang, C.; Wu, X.; Wu, W.; Wang, Y.; Qu, W.; Han, Q.; et al. Restoring mitochondrial cardiolipin homeostasis reduces cell death and promotes recovery after spinal cord injury. Cell Death Dis. 2022, 13, 1058. [Google Scholar] [CrossRef]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef]
- Campbell, M.D.; Duan, J.; Samuelson, A.T.; Gaffrey, M.J.; Merrihew, G.E.; Egertson, J.D.; Wang, L.; Bammler, T.K.; Moore, R.J.; White, C.C.; et al. Improving mitochondrial function with SS-31 reverses age-related redox stress and improves exercise tolerance in aged mice. Free Radic. Biol. Med. 2019, 134, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Ravenscraft, B.; Lee, D.-H.; Dai, H.; Watson, A.L.; Aparicio, G.I.; Han, X.; Deng, L.-X.; Liu, N.-K. Mitochondrial Cardiolipin-Targeted Tetrapeptide, SS-31, Exerts Neuroprotective Effects Within In Vitro and In Vivo Models of Spinal Cord Injury. Int. J. Mol. Sci. 2025, 26, 3327. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Lu, T.; Meng, L.; Luo, Y.; Fu, X.; Hou, Y. Mitochondrial Ca(2+) Overload Leads to Mitochondrial Oxidative Stress and Delayed Meiotic Resumption in Mouse Oocytes. Front. Cell Dev. Biol. 2020, 8, 580876. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Huang, Z.; Dong, S.; Ding, M.; Li, J.; Wang, M.; Zeng, X.; Zhang, X.; Sun, X. Calcium signaling in oocyte quality and functionality and its application. Front. Endocrinol. 2024, 15, 1411000. [Google Scholar] [CrossRef]
- Zhang, H.; Alder, N.N.; Wang, W.; Szeto, H.; Marcinek, D.J.; Rabinovitch, P.S. Reduction of elevated proton leak rejuvenates mitochondria in the aged cardiomyocyte. eLife 2020, 9, e60827. [Google Scholar] [CrossRef]
- Sivandzade, F.; Bhalerao, A.; Cucullo, L. Analysis of the Mitochondrial Membrane Potential Using the Cationic JC-1 Dye as a Sensitive Fluorescent Probe. Bio Protoc. 2019, 9, e3128. [Google Scholar] [CrossRef]
- Dai, J.; Wu, C.; Muneri, C.W.; Niu, Y.; Zhang, S.; Rui, R.; Zhang, D. Changes in mitochondrial function in porcine vitrified MII-stage oocytes and their impacts on apoptosis and developmental ability. Cryobiology 2015, 71, 291–298. [Google Scholar] [CrossRef]
- Siegel, M.P.; Kruse, S.E.; Percival, J.M.; Goh, J.; White, C.C.; Hopkins, H.C.; Kavanagh, T.J.; Szeto, H.H.; Rabinovitch, P.S.; Marcinek, D.J. Mitochondrial-targeted peptide rapidly improves mitochondrial energetics and skeletal muscle performance in aged mice. Aging Cell 2013, 12, 763–771. [Google Scholar] [CrossRef]
| SS-31 Concentration (µM) * | In Vitro Maturation | In Vitro Culture | ||||
|---|---|---|---|---|---|---|
| No. of Examined Oocytes | No. (%) of Oocytes at MII Stage ** (%) | No. of Examined Oocytes | No. (%) of Cleaved Embryos *** | No. (%) of Embryos Developed to Blastocysts **** | Total Cell Number in Blastocysts | |
| Control | 87 | 50 (55.2 ± 4.1) ab | 177 | 131 (73.5 ± 3.9) a | 5 (2.8 ± 1.8) a | 47.4 ± 8.5 |
| DMSO | 86 | 48 (55.6 ± 4.2) ab | 201 | 148 (74.2 ± 2.9) a | 6 (3.0 ± 0.4) a | 32.7 ± 3.0 |
| 0.001 | 87 | 54 (60.5 ± 7.9) ab | 179 | 147 (81.8 ± 3.2) a | 9 (4.9 ± 1.5) ab | 40.0 ± 8.3 |
| 0.01 | 79 | 51 (62.7 ± 5.4) ab | 195 | 160 (81.4 ± 3.5) a | 7 (3.6 ± 0.6) ab | 46.3 ± 9.5 |
| 0.1 | 80 | 55 (67.2 ± 5.4) ac | 185 | 145 (77.8 ± 3.5) ab | 8 (4.0 ± 1.9) ab | 42.1 ± 10.1 |
| 0.5 | 91 | 63 (69.6± 5.4) ac | 205 | 160 (78.0 ± 3.3) ab | 10 (4.9 ± 1.7) ab | 51.2 ± 7.7 |
| 1 | 81 | 63 (78.3 ± 3.8) c | 190 | 156 (81.7 ± 3.1) a | 14 (7.6 ± 1.6) b | 43.3 ± 5.8 |
| 1.5 | 92 | 56 (60.9 ± 2.9) ab | 202 | 158 (78.2 ± 3.5) ab | 9 (4.5 ± 1.4) ab | 43.2 ± 3.6 |
| 2.5 | 96 | 61 (63.4 ± 2.5) ab | 194 | 136 (70.0 ± 4.9) bc | 13 (6.4 ± 1.8) ab | 40.7 ± 3.2 |
| 5 | 95 | 49 (50.7 ± 8.5) b | 199 | 128 (64.6 ± 1.4) c | 5 (2.4 ± 1.3) a | 51.8 ± 11.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, S.T.; Otoi, T.; Namula, Z.; Widodo, O.S.; Tharasanit, T.; Chatdarong, K.; Nakayama, Y.; Nagahara, M.; Nakai, A.; Hirata, M.; et al. Mitochondrial-Targeted Protective Potential of Elamipretide for the In Vitro Production of Porcine Embryos. Animals 2025, 15, 2497. https://doi.org/10.3390/ani15172497
Nguyen ST, Otoi T, Namula Z, Widodo OS, Tharasanit T, Chatdarong K, Nakayama Y, Nagahara M, Nakai A, Hirata M, et al. Mitochondrial-Targeted Protective Potential of Elamipretide for the In Vitro Production of Porcine Embryos. Animals. 2025; 15(17):2497. https://doi.org/10.3390/ani15172497
Chicago/Turabian StyleNguyen, Suong T., Takeshige Otoi, Zhao Namula, Oky Setyo Widodo, Theerawat Tharasanit, Kaywalee Chatdarong, Yuichiro Nakayama, Megumi Nagahara, Aya Nakai, Maki Hirata, and et al. 2025. "Mitochondrial-Targeted Protective Potential of Elamipretide for the In Vitro Production of Porcine Embryos" Animals 15, no. 17: 2497. https://doi.org/10.3390/ani15172497
APA StyleNguyen, S. T., Otoi, T., Namula, Z., Widodo, O. S., Tharasanit, T., Chatdarong, K., Nakayama, Y., Nagahara, M., Nakai, A., Hirata, M., & Tanihara, F. (2025). Mitochondrial-Targeted Protective Potential of Elamipretide for the In Vitro Production of Porcine Embryos. Animals, 15(17), 2497. https://doi.org/10.3390/ani15172497






