LvSlc12A2 Is a Negative Growth Regulator in Whiteleg Shrimp, Litopenaeus vannamei
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of Materials
2.2. RNA Extraction and cDNA Synthesis
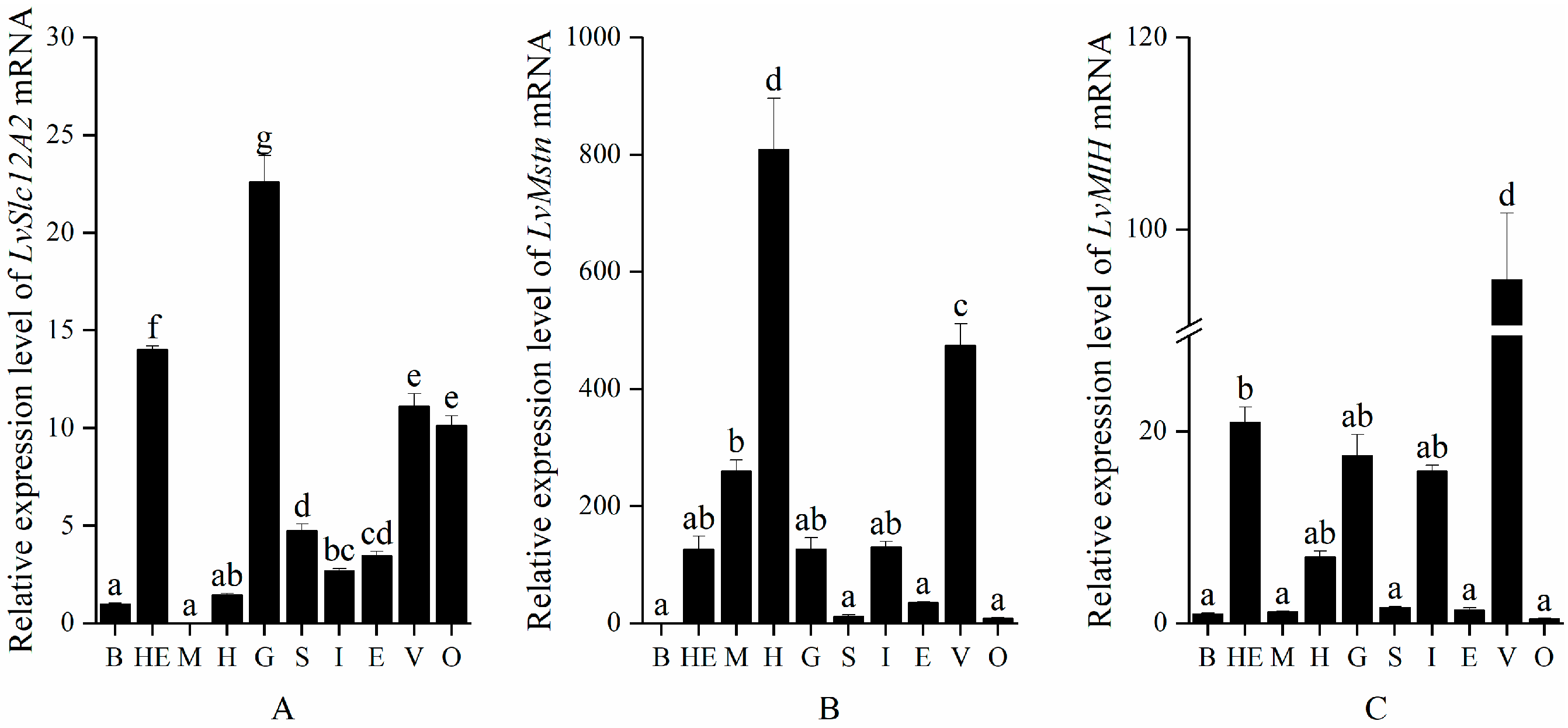
2.3. Analysis of LvSlc12A2, LvMstn, and LvMIH Expression Characteristics
2.4. LvSlc12A2 Gene Overexpression
2.5. RNA Interference
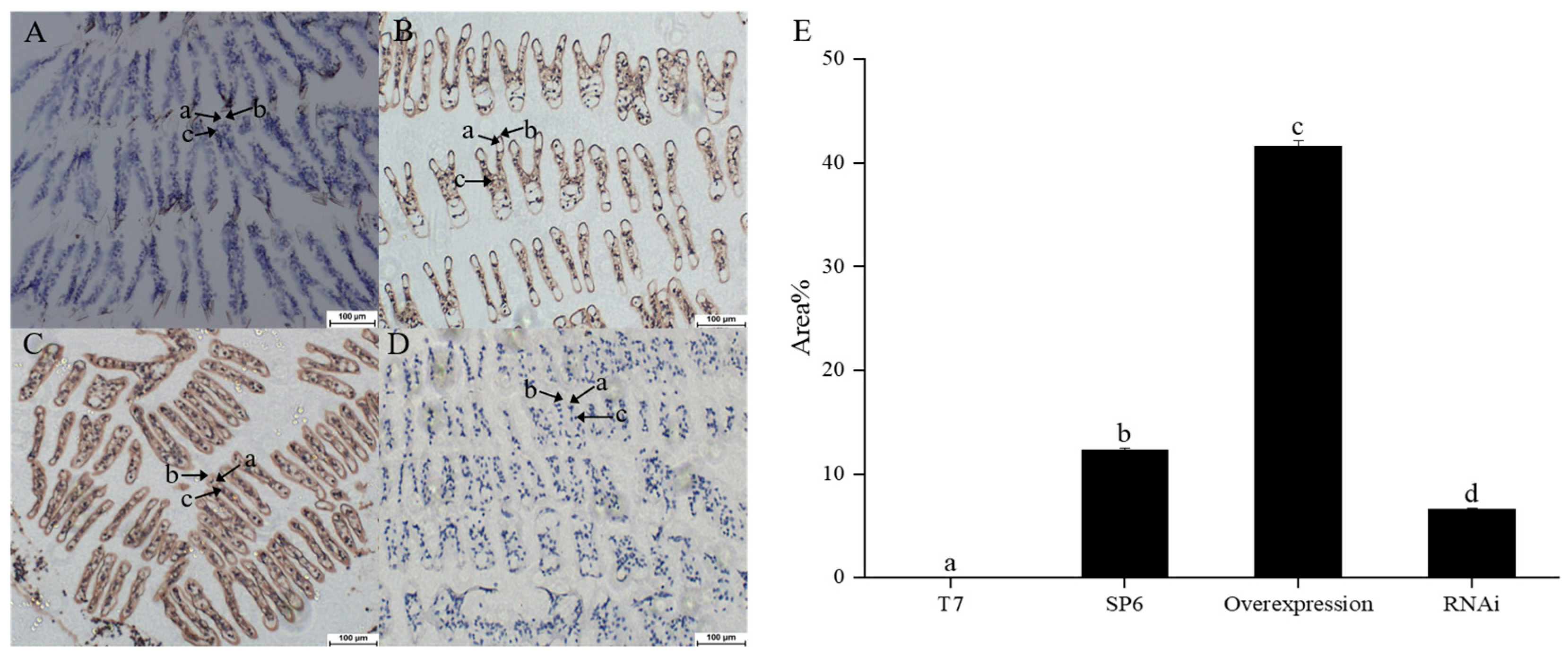
2.6. In Situ Hybridization
2.7. Data Analysis
3. Results
3.1. Expression Characteristics of LvSlc12A2, LvMstn, and LvMIH in Different Tissues of L. vannamei
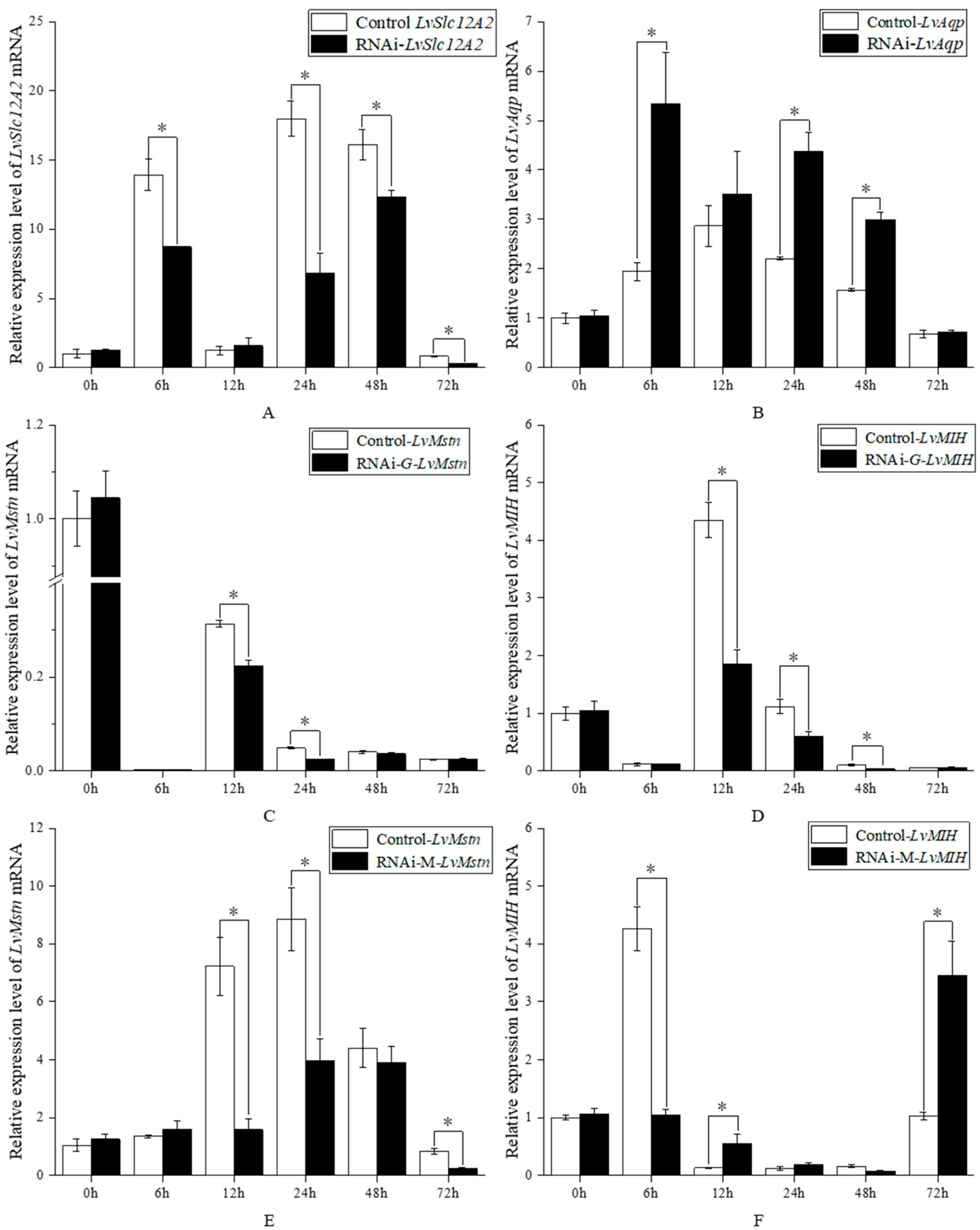
3.2. Effects of LvSlc12A2 Overexpression on the LvAqp, LvMstn, and LvMIH Genes
3.3. Effects of Knockdown LvSlc12A2 Genes on LvAqp, LvMstn, and LvMIH Genes
3.4. Localization of LvSlc12A2 mRNA in Gill Tissue
4. Discussion
4.1. Distribution Characteristics and Function of LvSlc12A2
4.2. Regulatory Links Between LvSlc12A2 and the Expression of LvMstn and LvMIH
4.3. LvSlc12A2 Biological Function in the Growth and Development of L. vannamei
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schwartz, A.L.; Dickerson, E.; Dagia, N.; Malgor, R.; McCall, K.D. TLR signaling inhibitor, phenylmethimazole, in combination with tamoxifen inhibits human breast cancer cell viability and migration. Oncotarget 2016, 8, 113295–113302. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.P.; Kahle, K.T.; Gamba, G. The SLC12 family of electroneutral cation-coupled chloride cotransporters. Mol. Asp. Med. 2013, 34, 288–298. [Google Scholar] [CrossRef]
- Hebert, S.C.; Mount, D.B.; Gamba, G. Molecular physiology of cation-coupled Cl− cotransport: The SLC12 family. Pflügers Arch. 2004, 447, 580–593. [Google Scholar] [CrossRef]
- Delpire, E.; Mount, D.B. Human and murine phenotypes associated with defects in cation-chloride cotransport. Annu. Rev. Physiol. 2002, 64, 803–843. [Google Scholar] [CrossRef]
- Gamba, G. Molecular physiology and pathophysiology of electroneutral cation-chloride cotransporters. Physiol. Rev. 2005, 85, 423–493. [Google Scholar] [CrossRef]
- Xu, J.C.; Lytle, C.; Zhu, T.T.; Payne, J.A.; Benz Jr, E.; Forbush, B., 3rd. Molecular cloning and functional expression of the bumetanide-sensitive Na-K-Cl cotransporter. Proc. Natl. Acad. Sci. USA 1994, 91, 2201–2205. [Google Scholar] [CrossRef]
- Park, J.H.; Saier, M.H., Jr. Phylogenetic, structural and functional characteristics of the Na-K-Cl cotransporter family. J. Membr. Biol. 1996, 149, 161–168. [Google Scholar] [CrossRef]
- Motoshima, T.; Nagashima, A.; Ota, C.; Oka, H.; Hosono, K.; Braasch, I.; Nishihara, H.; Kato, A. Na+/Cl− cotransporter 2 is not fish-specific and is widely found in amphibians, non-avian reptiles, and select mammals. Physiol. Genom. 2023, 55, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Virtanen, M.A.; Uvarov, P.; Hübner, C.A.; Kaila, K. NKCC1, an elusive molecular target in brain development: Making sense of the existing data. Cells 2020, 9, 2607. [Google Scholar] [CrossRef]
- Gagnon, É.; Forbush, B.; Caron, L.; Isenring, P. Functional comparison of renal Na-K-Cl cotransporters between distant species. Am. J. Physiol. Cell Physiol. 2003, 284, C365–C370. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Y.; Sun, W.; Chen, N.; Xu, C.; Wang, X.X.; Dong, K.; Zhang, B.X.; Zhang, J.; Hao, N.; Sun, A.; et al. Discovery of NKCC1 as a potential therapeutic target to inhibit hepatocellular carcinoma cell growth and metastasis. Oncotarget 2017, 8, 66328. [Google Scholar] [CrossRef]
- Chen, Y.G.; Yuan, K.; Zhang, Z.Z.; Yuan, F.H.; Wen, S.P.; Yue, H.T.; He, J.G.; Chen, Y.H. Identification and functional characterization of a solute carrier family 15, member 4 gene in Litopenaeus vannamei. Dev. Comp. Immunol. 2016, 57, 57–66. [Google Scholar] [CrossRef]
- Peng, M.; Zeng, D.G.; Zhu, W.L.; Chen, X.L.; Yang, C.L.; Liu, Q.Y.; Li, Q.Y.; Wang, H.W.; Liu, H.; Liang, J.Z.; et al. Construction of a high-density genetic map and identification of quantitative trait loci for nitrite tolerance in the Pacific white shrimp (Litopenaeus vannamei). Front. Genet. 2020, 11, 571880. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Chen, J.L.; Chen, Y.; Pan, C.K.; Lin, Z.Y.; Chen, J.M. WSV056-mediated downregulation of PvSMCT1 contributes to immune evasion of white spot syndrome virus in the infection of Penaeus vannamei. Aquaculture 2023, 563, 739010. [Google Scholar] [CrossRef]
- Li, X.N.; Dai, X.L. Characterization and functional analysis of Litopenaeus vannamei Na+/K+/2Cl− cotransporter 1 under nitrite stress. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2024, 298, 111749. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, C.Y.; Wang, C.X.; Li, T.; Chang, L.R.; Si, L.J.; Yan, D.C. Enterocytozoon hepatopenaei (EHP) infection alters the metabolic processes and induces oxidative stress in Penaeus vannamei. Animals 2023, 13, 3661. [Google Scholar] [CrossRef]
- Hua, S.S.; Li, W.Y.; Zheng, D.W.; Zhou, X.Y.; Zhang, S.C.; Zhang, H.M.; Liu, X.; Baloch, W.A.; Yan, B.L.; Gao, H. The proliferating cell nuclear antigen gene (pcna) plays a key role in ovarian development in the ridgetail white prawn, Exopalaemon carinicauda. Aquac. Rep. 2024, 36, 102170. [Google Scholar] [CrossRef]
- Qian, Z.Y.; Mi, X.; Wang, X.Z.; He, S.L.; Liu, Y.J.; Hou, F.J.; Liu, Q.; Liu, X.L. cDNA cloning and expression analysis of myostatin/GDF11 in shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2013, 165, 30–39. [Google Scholar] [CrossRef]
- Zuo, H.L.; Yuan, J.; Niu, S.W.; Yang, L.W.; Weng, S.P.; He, J.G.; Xu, X.P. A molting-inhibiting hormone-like protein from Pacific white shrimp Litopenaeus vannamei is involved in immune responses. Fish Shellfish Immunol. 2018, 72, 544–551. [Google Scholar] [CrossRef]
- Xian, Y.F.; Duan, Y.F.; Wei, Z.K.; Zhu, X.Y.; Huang, J.H.; Zhang, J.S. Effects of nitrite and microplastic stress on immune, detoxification metabolism and osmoregulation-related indicators in gills of Litopenaeus vannamei. S. China Fish. Sci. 2023, 19, 70–77. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Cutler, C.P.; Cramb, G. Differential expression of absorptive intestinal and renal tissues of the cation-chloride-cotransporters in the European eel (Anguilla anguilla). Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2008, 149, 63–73. [Google Scholar] [CrossRef]
- Yang, W.K.; Wu, Y.C.; Tang, C.H.; Lee, T.H. Microtubule-dependent changes in morphology and localization of chloride transport proteins in gill mitochondria-rich cells of the tilapia, Oreochromis mossambicus. J. Morphol. 2016, 277, 1113–1122. [Google Scholar] [CrossRef]
- Liu, H.T.; Wang, J.; Mao, Y.; Qiao, Y.; Zhong, S.P.; Su, Y.Q. Molecular cloning and expression anaysis of Na-K-2CI cotransporter from Marsupenaeus japonicus. J. Xiamen Univ. Nat. Sci. 2016, 55, 46–54. [Google Scholar] [CrossRef]
- Wang, W.H.; Dong, H.B.; Sun, Y.X.; Sun, C.Y.; Duan, Y.F.; Gu, Q.H.; Li, Y.; Xie, M.J.; Zhang, J.S. (Immune and physiological responses of juvenile Chinese sea bass (Lateolabrax maculatus) to eugenol and tricaine methanesulfonate (MS-222) in gills. Aquac. Rep. 2020, 18, 100554. [Google Scholar] [CrossRef]
- Freire, C.A.; Onken, H.; McNamara, J.C. A structure–function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 272–304. [Google Scholar] [CrossRef]
- Hediger, M.A.; Romero, M.F.; Peng, J.B.; Rolfs, A.; Takanaga, H.; Bruford, E.A. The ABCs of solute carriers: Physiological, pathological and therapeutic implications of human membrane transport proteins: Introduction. Pflügers Arch. 2004, 447, 465–468. [Google Scholar] [CrossRef]
- O’grady, S.M.; Palfrey, H.C.; Field, M. Characteristics and functions of Na-K-Cl cotransport in epithelial tissues. Am. J. Physiol. Cell Physiol. 1987, 253, C177–C192. [Google Scholar] [CrossRef]
- Payne, J.A.; Forbush III, B. Molecular characterization of the epithelial Na K Cl cotransporter isoforms. Curr. Opin. Cell Biol. 1995, 7, 493–503. [Google Scholar] [CrossRef]
- Palfrey, C.; O’Donnell, M.E. Characteristics and regulation of the Na/K/2CI cotransporter. Cell. Physiol. Biochem. 1992, 2, 293–307. [Google Scholar] [CrossRef]
- Pan, L.Q.; Liu, H.Y. Advances in the physiology of osmotic regulation in crustaceans. J. Fish. China 2005, 29, 109–114. [Google Scholar]
- Fu, X.Y.; Sun, J.Q.; Lv, J.; Shi, R.L. Research progress on Na+-K+-2Cl-cotransporter 1 and its relationship to nervous system diseases. Chin. J. Contemp. Neurol. Neurosurg. 2021, 21, 223. [Google Scholar] [CrossRef]
- Plotkin, M.D.; Kaplan, M.R.; Peterson, L.N.; Gullans, S.R.; Hebert, S.C.; Delpire, E. Expression of the Na (+)-K (+)-2Cl-cotransporter BSC2 in the nervous system. Am. J. Physiol. Cell Physiol. 1997, 272, C173–C183. [Google Scholar] [CrossRef] [PubMed]
- De Santis, C.; Wade, N.M.; Jerry, D.R.; Preston, N.P.; Glencross, B.D.; Sellars, M.J. Growing backwards: An inverted role for the shrimp ortholog of vertebrate myostatin and GDF11. Am. J. Physiol. Cell Physiol. 2011, 214, 2671–2677. [Google Scholar] [CrossRef]
- Covi, J.A.; Kim, H.W.; Mykles, D.L. Expression of alternatively spliced transcripts for a myostatin-like protein in the blackback land crab, Gecarcinus lateralis. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 150, 423–430. [Google Scholar] [CrossRef]
- Kim, K.S.; Kim, Y.-J.; Jeon, J.M.; Kang, Y.S.; Kang, Y.S.; Oh, C.W.; Kim, H.-W. Molecular characterization of myostatin-like genes expressed highly in the muscle tissue from Morotoge shrimp, Pandalopsis japonica. Aquac. Res. 2010, 41, e862–e871. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Liu, Y.; Zhou, J.; Wang, J.X.; Jin, G.Y.; Wang, X.D. Breast cancer vaccine containing a novel toll-like receptor 7 agonist and an aluminum adjuvant exerts antitumor effects. Int. J. Mol. Sci. 2022, 23, 15130. [Google Scholar] [CrossRef]
- Engberink, A.O.; Meijer, J.H.; Michel, S. Chloride cotransporter KCC2 is essential for GABAergic inhibition in the SCN. Neuropharmacology 2018, 138, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Demian, W.L.; Persaud, A.; Jiang, C.; Coyaud, É.; Liu, S.X.; Kapus, A.; Kafri, R.; Raught, B.; Rotin, D. The ion transporter NKCC1 links cell volume to cell mass regulation by suppressing mTORC1. Cell Rep. 2019, 27, 1886–1896. [Google Scholar] [CrossRef]
- Giffard-Mena, I.; Boulo, V.; Aujoulat, F.; Flicoteaux, R.; Lorin-Nebel, C.; Charmantier, G. Aquaporin Molecular Characterization in the Sea-Bass (Dicentrarchus labrax): The Effect of Salinity on AQP1 and AQP3 Expression. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 148, 430–444. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.L.; Zhang, C.J.; Gao, Q.X.; Peng, S.M.; Shi, Z.H. New insights into ionocytes and ion channles. Mar. Fish. 2015, 37, 77–86. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhou, J.Y.; Zhu, L.L.; Cheng, Y.X.; Xia, D.M. Gene Cloning and Functional Analysis of Na+-K+-Cl- Contransporter from Eriocheir sinensis. J. Fudan Univ. (Nat. Sci.) 2021, 60, 84–92. [Google Scholar] [CrossRef]
- Yang, Z.G.; Zhang, L.; Chen, C.Y.; Cheng, Y.X.; Xia, D.M. Study on Cloning, Expression and RNA Interference of Aquaporin 1 Gene of Eriocheir sinensis. J. Fudan Univ. (Nat. Sci.) 2021, 60, 93–101. [Google Scholar] [CrossRef]
- Emerenciano, M.G.C.; Rombenso, A.N.; Vieira, F.D.N.; Martins, M.A.; Coman, G.J.; Truong, H.H.; Noble, T.H.; Simon, C.J. Intensification of penaeid shrimp culture: An applied review of advances in production systems, nutrition and breeding. Animals 2022, 12, 236. [Google Scholar] [CrossRef]
- Xue, B.; Zhang, B.; Li, Z.H.; Zhao, L.; Tai, X.F.; Xu, Y.; Lai, X.F.; Gao, H.; Yan, B.L. Expression Profiles of Myostatin(MSTN) Gene in Different Tissues and at Different Post-molt Times in Exopalaemon carinicadua. J. Huaihai Inst. Technol. (Nat. Sci. Ed.) 2016, 25, 66–70. [Google Scholar]
- Lee, J.H.; Momani, J.; Kim, Y.M.; Kang, C.K.; Choi, J.H.; Baek, H.J.; Kim, H.W. Effective RNA-silencing strategy of Lv-MSTN/GDF11 gene and its effects on the growth in shrimp, Litopenaeus vannamei. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 179, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Mykles, D.L. Crustacean muscle plasticity: Molecular mechanisms determining mass and contractile properties. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1997, 117, 367–378. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.Y. Isolation, Identification, Expression and Function Studies of Growth Performance Candidate Genes in Litopenaeus vannamei. Ph.D. Thesis, Northwest A&F University, Yangling, China, 2014. [Google Scholar]
- Wang, J.; Li, J.; Ge, Q.; Li, J. A potential negative regulation of myostatin in muscle growth during the intermolt stage in Exopalaemon carinicauda. Gen. Comp. Endocrinol. 2021, 314, 113902. [Google Scholar] [CrossRef]
- Zhuo, R.Q.; Zhou, T.T.; Yang, S.P.; Chan, S.F. Characterization of a Molt-Related Myostatin Gene (FmMstn) from the Banana Shrimp Fenneropenaeus merguiensis. Gen. Comp. Endocrinol. 2017, 248, 55–68. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Lu, X.; Kong, J.; Meng, X.; Luan, S.; Dai, P.; Chen, B.; Cao, B.; Luo, K. Molecular Characterization of Myostatin and Its Inhibitory Function on Myogenesis and Muscle Growth in Chinese Shrimp, Fenneropenaeus chinensis. Gene 2020, 758, 144986. [Google Scholar] [CrossRef]
- Chen, H.Y.; Toullec, J.Y.; Lee, C.Y. The crustacean hyperglycemic hormone superfamily: Progress made in the past decade. Front. Endocrinol. 2020, 11, 578958. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.K.; Kim, K.S.; Oh, C.W.; Mykles, D.L.; Lee, S.G.; Kim, H.J.; Kim, H.W. Twelve actin-encoding cDNAs from the American lobster, Homarus americanus: Cloning and tissue expression of eight skeletal muscle, one heart, and three cytoplasmic isoforms. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2009, 153, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.A.; Hong, S.H.; Kim, J.W.; Jang, I.S. Possible involvement of DNA methylation in NKCC1 gene expression during postnatal development and in response to ischemia. J. Neurochem. 2010, 114, 520–529. [Google Scholar] [CrossRef] [PubMed]

| Primer Name | Sequences | Genbank Accession No. | Length (bp) | Efficiency (%) | Purpose |
|---|---|---|---|---|---|
| 18S-F | TATACGCTAGTGGAGCTGGAA | EU920969 | 147 | 92.1 | Internal reference |
| 18S-R | GGGGAGGTAGTGACGAAAAAT | ||||
| LvMstn-F | GGGACTTCATTGTTGCTC | JQ045427.1 | 120 | 97.62 | Real-time fluorescent |
| LvMstn-R | CGCTGGTGCTATTCATCT | quantitative | |||
| LvMIH-F | AGCAGTTCAACAGGTGGATCAG | MF358695.1 | 123 | 108.82 | |
| LvMIH-R | AAGGAGCAGCAGGAGGAGAG | ||||
| LvAqp-F | GCAGCCATCTTGAAGGGAGTGAC | XM_070140003.1 | 125 | 95.96 | |
| LvAqp-R | ACGAGGACGAAGGTGATGAGGAG | ||||
| LvSlc12A2-F | GACTCTCCTGCTGCCTTACATCC | PQ073211 | 122 | 91.38 | |
| LvSlc12A2-R | GGTTTGCCATGCTTCTCTGTTCC | ||||
| S-A1 | GATCACTAATACGACTCACTATAGGGCAAACGTAATCAGTCTGCTGGTCAGTT | 25 | RNA interference | ||
| S-A2 | AACTGACCAGCAGACTGATTACGTTTGCCCTATAGTGAGTCGTATTAGTGATC | ||||
| S-B1 | GATCACTAATACGACTCACTATAGGGCTGA CCAGCAGACTGATTACGTTTGTT | ||||
| S-B2 | AACAAACGTAATCAGTCTGCTGGTCA GCCCTATAGTGAGTCGTATTAGTGATC | ||||
| Pspt18-SF | GAGACCGGAATTCGAGCTCGGAACTCTACGGACCTCTTAGAGAACC | 1056 | Homologous recombination | ||
| Pspt18-SR | GAATACAAGCTTGCATGCCTGCCGGTCACACTTAGCCCAATTTG | ||||
| SP6-LvSlc12A2 | ATTTAGGTGACACTATAGAATACAAGCTTGCATGCCTGCCGGTCACACTTAGC | 1073 | In situ hybridization | ||
| T7--LvSlc12A2 | TAATACGACTCACTATAGGGAGACCGGAATTCGAGCTCGGAACTCTACGGACC | 1075 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, P.; Jiang, S.; Liu, M.; Chen, S.; Kong, J.; Luan, S.; Meng, X.; Xing, Q.; Zeng, Q.; Luo, K.; et al. LvSlc12A2 Is a Negative Growth Regulator in Whiteleg Shrimp, Litopenaeus vannamei. Animals 2025, 15, 2467. https://doi.org/10.3390/ani15172467
Niu P, Jiang S, Liu M, Chen S, Kong J, Luan S, Meng X, Xing Q, Zeng Q, Luo K, et al. LvSlc12A2 Is a Negative Growth Regulator in Whiteleg Shrimp, Litopenaeus vannamei. Animals. 2025; 15(17):2467. https://doi.org/10.3390/ani15172467
Chicago/Turabian StyleNiu, Panpan, Shanshan Jiang, Mianyu Liu, Siyu Chen, Jie Kong, Sheng Luan, Xianhong Meng, Qun Xing, Qifan Zeng, Kun Luo, and et al. 2025. "LvSlc12A2 Is a Negative Growth Regulator in Whiteleg Shrimp, Litopenaeus vannamei" Animals 15, no. 17: 2467. https://doi.org/10.3390/ani15172467
APA StyleNiu, P., Jiang, S., Liu, M., Chen, S., Kong, J., Luan, S., Meng, X., Xing, Q., Zeng, Q., Luo, K., & Gao, H. (2025). LvSlc12A2 Is a Negative Growth Regulator in Whiteleg Shrimp, Litopenaeus vannamei. Animals, 15(17), 2467. https://doi.org/10.3390/ani15172467






