The Effects of Inulin on the Growth, Oxidative Stress, and Immune Function of Weaned Kids
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Kids and Experimental Protocol
2.2. Growth Performance
2.3. Sample Collection
2.4. Intestinal Histomorphology
2.5. Determination of Serum Biochemical, Antioxidant, and Immune Indexes
2.6. The Effects of Intestinal Permeability, Jejunal Digestive Enzymes, and Colonic Volatile Fatty Acids
2.7. Intestinal Antioxidant Index and Immune Performance
2.8. qPCR Analysis
2.9. Statistical Analysis
3. Results
3.1. Effects of Inulin on Growth Performance of Weaned Kids GraphPad Prism
3.2. Effect of Inulin on Intestinal Histomorphology of Weaned Kids
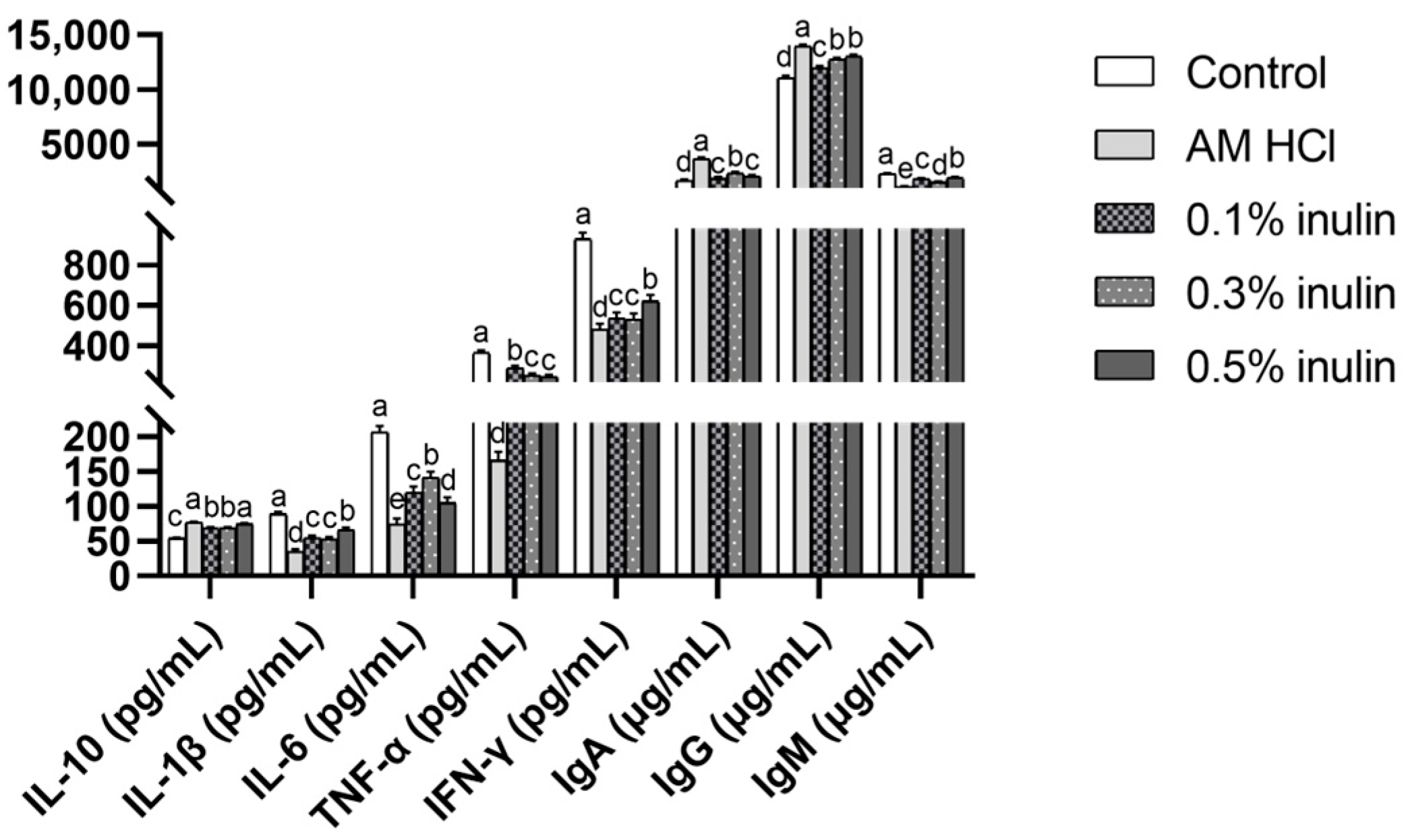
3.3. Effects of Inulin on Serum Biochemical Indicators, Antioxidant Indicators, Intestinal Permeability, and Immune Indicators of Weaned Kids
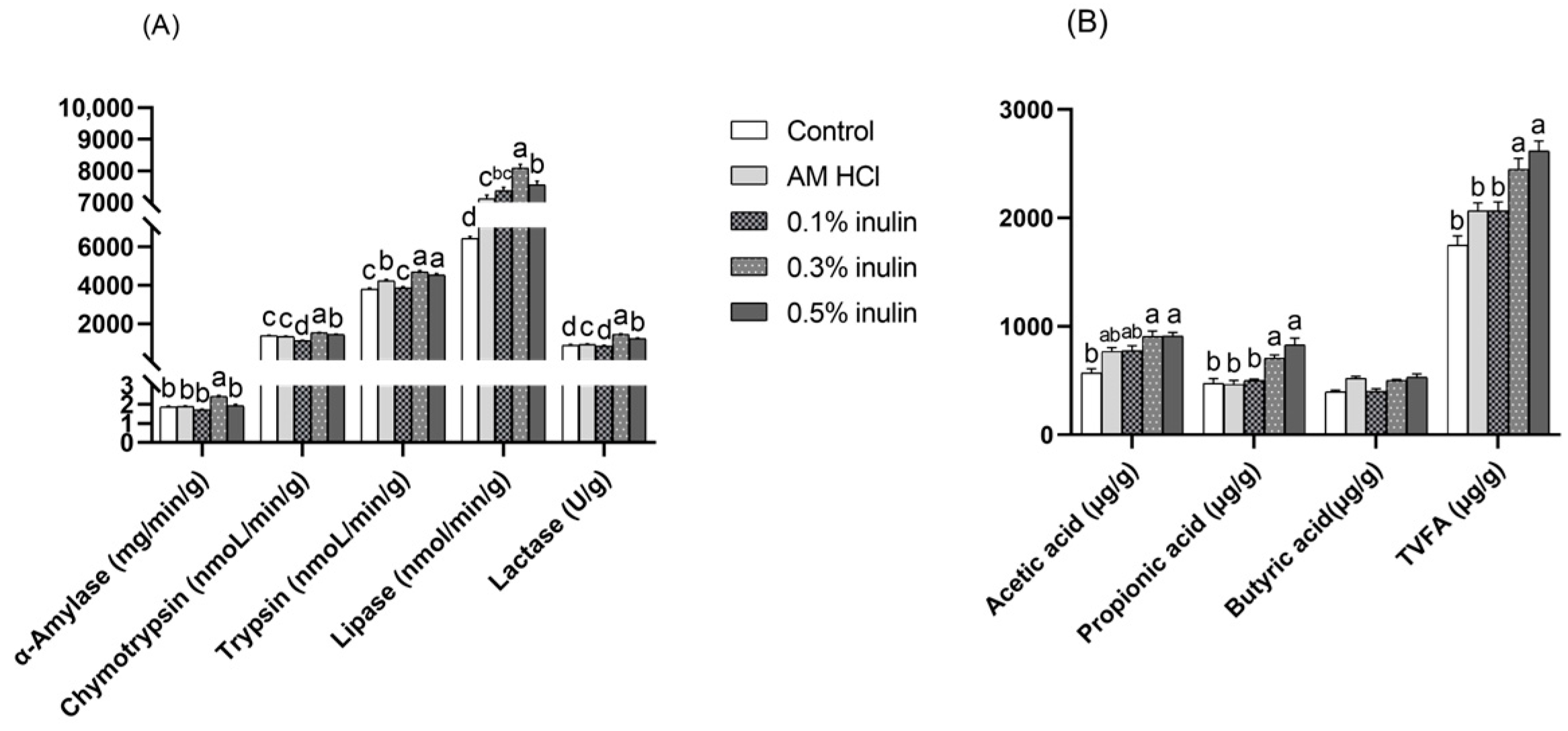
3.4. Effects of Inulin on Jejunal Digestive Enzymes and Cecal Volatile Fatty Acids in Weaned Kids
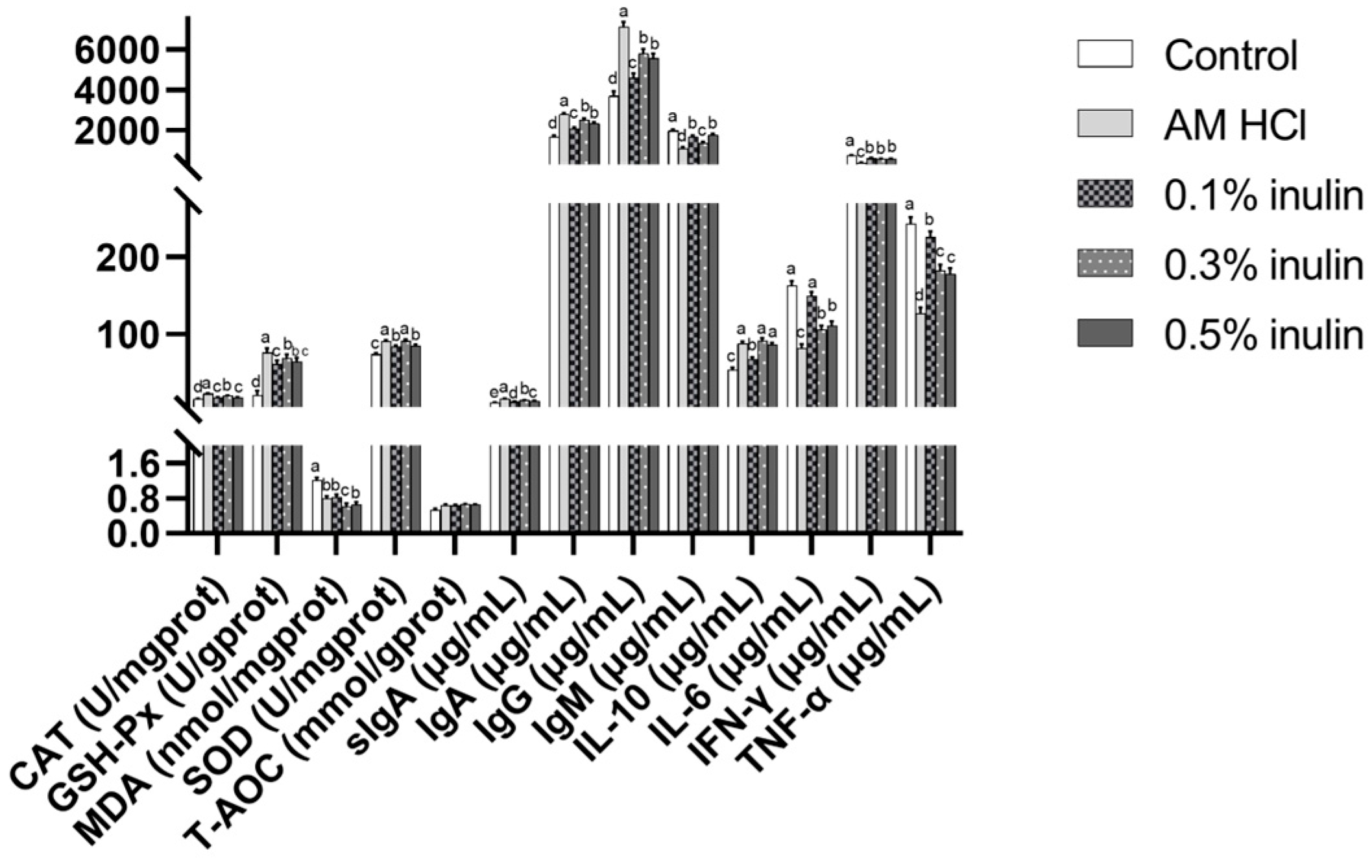
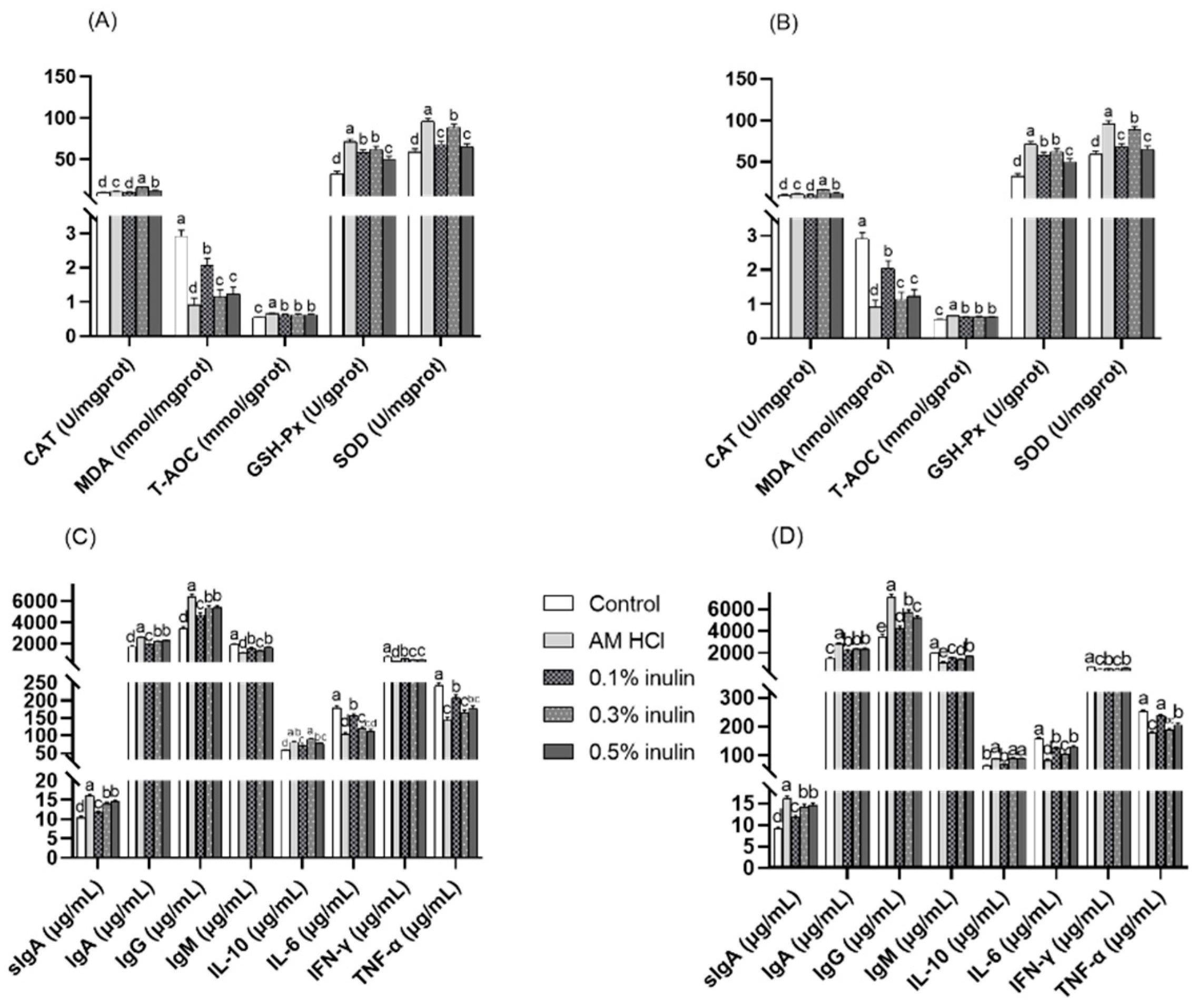
3.5. Effects of Inulin Intestinal Antioxidant Performance and Immune Performance of Weaned Kids
3.6. Effects of Inulin on the Expression of Intestinal Barrier-Related and Cytokine-Related Genes in the Jejunum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carrasco, S.; Panea, B.; Ripoll, G.; Sanz, A.; Joy, M. Influence of feeding systems on cortisol levels, fat colour and instrumental meat quality in light lambs. Meat Sci. 2009, 83, 50–56. [Google Scholar] [CrossRef]
- Mohapatra, A.; De, K.; Saxena, V.K.; Mallick, P.K.; Devi, I.; Singh, R. Behavioral and physiological adjustments by lambs in response to weaning stress. J. Vet. Behav. 2021, 41, 47–51. [Google Scholar] [CrossRef]
- Kazemi, S.; Hajimohammadi, A.; Mirzaei, A.; Nazifi, S. Effects of probiotic and yeast extract supplementation on oxidative stress, inflammatory response, and growth in weaning Saanen kids. Trop. Anim. Health Prod. 2023, 55, 282. [Google Scholar] [CrossRef] [PubMed]
- Riaz Rajoka, M.S.; Thirumdas, R.; Mehwish, H.M.; Umair, M.; Khurshid, M.; Hayat, H.F.; Phimolsiripol, Y.; Pallarés, N.; Martí-Quijal, F.J.; Barba, F.J. Role of food antioxidants in modulating gut microbial communities: Novel understandings in intestinal oxidative stress damage and their impact on host health. Antioxidants 2021, 10, 1563. [Google Scholar] [CrossRef]
- Ponnampalam, E.; Kiani, A.; Santhiravel, S.; Holman, B.; Lauridsen, C.; Dunshea, F. The Importance of Dietary Antioxidants on Oxidative Stress, Meat and Milk Production, and Their Preservative Aspects in Farm Animals: Antioxidant Action, Animal Health, and Product Quality—Invited Review. Animals 2022, 12, 3279. [Google Scholar] [CrossRef]
- Rahman, M.R.T.; Fliss, I.; Biron, E. Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production. Antibiotics 2022, 11, 766. [Google Scholar] [CrossRef] [PubMed]
- Singer, R.S.; Finch, R.; Wegener, H.C.; Bywater, R.; Walters, J.; Lipsitch, M. Antibiotic resistance—The interplay between antibiotic use in animals and human beings. Lancet Infect. Dis. 2003, 3, 47–51. [Google Scholar] [CrossRef]
- Seukep, A.J.; Mbuntcha, H.G.; Kuete, V.; Chu, Y.; Fan, E.; Guo, M.-Q. What approaches to thwart bacterial efflux pumps-mediated resistance? Antibiotics 2022, 11, 1287. [Google Scholar] [CrossRef]
- Das, R.; Mehta, D.K. Microbial biofilm and quorum sensing inhibition: Endowment of medicinal plants to combat multidrug-resistant bacteria. Curr. Drug Targets 2018, 19, 1916–1932. [Google Scholar] [CrossRef] [PubMed]
- Redondo-Cuenca, A.; Herrera-Vázquez, S.E.; Condezo-Hoyos, L.; Gómez-Ordóñez, E.; Rupérez, P. Inulin extraction from common inulin-containing plant sources. Ind. Crops Prod. 2021, 170, 113726. [Google Scholar] [CrossRef]
- Matusek, A.; Merész, P.; Le, T.K.D.; Örsi, F. Effect of temperature and pH on the degradation of fructo-oligosaccharides. Eur. Food Res. Technol. 2009, 228, 355–365. [Google Scholar] [CrossRef]
- Yang, G.; Qiu, H.; Yu, R.; Xiong, L.; Yan, Q.; Wen, C.; Peng, M. Dietary supplementation of β-glucan, inulin and emodin modulates antioxidant response and suppresses intestinal inflammation of grass carp (Ctenopharyngodon idellus). Anim. Feed Sci. Technol. 2021, 272, 114789. [Google Scholar] [CrossRef]
- Pasqualetti, V.; Altomare, A.; Guarino, M.P.L.; Locato, V.; Cocca, S.; Cimini, S.; Palma, R.; Alloni, R.; De Gara, L.; Cicala, M. Antioxidant activity of inulin and its role in the prevention of human colonic muscle cell impairment induced by lipopolysaccharide mucosal exposure. PLoS ONE 2014, 9, e98031. [Google Scholar] [CrossRef]
- Man, S.; Liu, T.; Yao, Y.; Lu, Y.; Ma, L.; Lu, F. Friend or foe? The roles of inulin-type fructans. Carbohydr. Polym. 2021, 252, 117155. [Google Scholar] [CrossRef]
- Herosimczyk, A.; Ożgo, M.; Lepczyński, A.; Cabała, S.; Barszcz, M.; Taciak, M.; Adaszyńska-Skwirzyńska, M. Diets enriched with chicory-derived native inulin can affect kidney and liver mineral content in nursery pigs. J. Anim. Feed Sci. 2025, 34, 55–60. [Google Scholar] [CrossRef]
- Jonova, S.; Ilgaza, A.; Zolovs, M. The impact of inulin and a novel synbiotic (yeast Saccharomyces cerevisiae strain 1026 and inulin) on the development and functional state of the gastrointestinal canal of calves. Vet. Med. Int. 2021, 2021, 8848441. [Google Scholar] [CrossRef] [PubMed]
- Suthama, N.; Mangisah, I.; Krismiyanto, L.; Yunianto, V.; Mulyono, M. Feeding dietary inclusion of inulin on immune status, protein metabolism, and growth performance of Kedu chicken. J. Indones. Trop. Anim. Agric. 2025, 50, 22–32. [Google Scholar] [CrossRef]
- Tóth, S. Effect of mannanoligosaccharide (MOS) and inulin supplementation on the performance of calves reared on milk replacer. Rev. Agric. Rural Dev. 2019, 8, 81–84. [Google Scholar] [CrossRef]
- Jonova, S.; Ilgaza, A.; Grinfelde, I.; Zolovs, M. Impact of the flour of Jerusalem artichoke on the production of methane and carbon dioxide and growth performance in calves. Vet. World 2018, 11, 1532–1538. [Google Scholar] [CrossRef]
- Feldman, A.T.; Wolfe, D. Tissue Processing and Hematoxylin and Eosin Staining. In Histopathology: Methods and Protocols; Day, C.E., Ed.; Springer: New York, NY, USA, 2014; pp. 31–43. [Google Scholar] [CrossRef]
- Kareem, K.Y.; Loh, T.C.; Foo, H.L.; Akit, H.; Samsudin, A.A. Effects of dietary postbiotic and inulin on growth performance, IGF1 and GHR mRNA expression, faecal microbiota and volatile fatty acids in broilers. BMC Vet. Res. 2016, 12, 163. [Google Scholar] [CrossRef]
- da Silva, C.I.; Schneider, C.R.; Hygino, B.; Duarte, V.; Teixeira, U.H.G.; Alcalde, C.R.; de Oliveira, A.J.B. Performance, carcass characteristics, and meat quality of goat kids supplemented with inulin. Livest. Sci. 2022, 265, 105094. [Google Scholar] [CrossRef]
- Uerlings, J.; Arevalo Sureda, E.; Schroyen, M.; Kroeske, K.; Tanghe, S.; De Vos, M.; Bruggeman, G.; Wavreille, J.; Bindelle, J.; Purcaro, G. Impact of citrus pulp or inulin on intestinal microbiota and metabolites, barrier, and immune function of weaned piglets. Front. Nutr. 2021, 8, 650211. [Google Scholar] [CrossRef]
- de Campos, C.M.; Zanuzzo, F.S.; Gimbo, R.Y.; Favero, G.C.; Soares, M.P.; Pilarski, F.; Urbinati, E.C. Dietary inulin modulated the cortisol response and increased the protection against pathogens in juvenile pacu (Piaractus mesopotamicus). Aquac. Res. 2021, 53, 860–869. [Google Scholar] [CrossRef]
- Danyer, E.; Bilal, T.; Altiner, A.; Aytekin, I.; Atalay, H. The effect of vitamin E treatment on selected immune and oxidative parameters in Kivircik ewes suffering from transport stress. J. Anim. Physiol. Anim. Nutr. 2021, 105 (Suppl. 1), 34–41. [Google Scholar] [CrossRef]
- Soriano, V.S.; e Sá, J.; Junior, H.P.R.; Torbitz, V.D.; Moresco, R.N.; Stefani, L.M.; Da Silva, A.S. Postpartum nitric oxide, oxidants and antioxidants levels in ewes and their lambs. Small Rumin. Res. 2015, 123, 13–16. [Google Scholar] [CrossRef]
- Stoyanova, S.; Geuns, J.; Hideg, E.; Van Den Ende, W. The food additives inulin and stevioside counteract oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 207–214. [Google Scholar] [CrossRef]
- Melekoglu, E.; Cetinkaya, M.A.; Kepekci-Tekkeli, S.E.; Kul, O.; Samur, G. Effects of prebiotic oligofructose-enriched inulin on gut-derived uremic toxins and disease progression in rats with adenine-induced chronic kidney disease. PLoS ONE 2021, 16, e0258145. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Chin, Y.-W.; Chae, H.-S.; Yoon, K.D.; Kim, J. Anti-adipogenic constituents from Dioscorea opposita in 3T3-L1 cells. Biol. Pharm. Bull. 2014, 37, 1683–1688. [Google Scholar] [CrossRef]
- Yongqing, L.I.U.; Chen, L.I.U.; Zewei, F.; Kai, Z.; Qiang, L.I.U. Effects of Complex Probiotic Preparation and Inulin on Growth Performance, Nutrient Digestion, Rumen Fermentation and Blood Metabolism of Finishing Bulls. Chin. J. Anim. Nutr. 2022, 34, 2446–2456. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K. Intrauterine growth retardation affects intestinal health of suckling piglets via altering intestinal antioxidant capacity, glucose uptake, tight junction, and immune responses. Oxidative Med. Cell. Longev. 2022, 2022, 2644205. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Wang, S.; Gebeyew, K.; Yang, X.; Tang, S.; Zhou, C.; Khan, N.A.; Tan, Z.; Liu, Y. A low-carbon high inulin diet improves intestinal mucosal barrier function and immunity against infectious diseases in goats. Front. Vet. Sci. 2023, 9, 1098651. [Google Scholar] [CrossRef]
- Tang, Z.; Yang, Y.; Wu, Z.; Ji, Y. Heat stress-induced intestinal barrier impairment: Current insights into the aspects of oxidative stress and endoplasmic reticulum stress. J. Agric. Food Chem. 2023, 71, 5438–5449. [Google Scholar] [CrossRef]
- Jiang, J.; Qi, L.; Lv, Z.; Jin, S.; Wei, X.; Shi, F. Dietary stevioside supplementation alleviates lipopolysaccharide-induced intestinal mucosal damage through anti-inflammatory and antioxidant effects in broiler chickens. Antioxidants 2019, 8, 575. [Google Scholar] [CrossRef]
- Larocca, M.; Perna, A.M.; Simonetti, A.; Gambacorta, E.; Iannuzzi, A.; Perucatti, A.; Rossano, R. Antioxidant and anti-inflammatory effects of cauliflower leaf powder-enriched diet against LPS induced toxicity in rabbits. Food Funct. 2017, 8, 3288–3296. [Google Scholar] [CrossRef]
- Zhang, S.; Zhu, C.; Xie, H.; Wang, L.; Hu, J. Effect of Gan Cao (Glycyrrhiza uralensis Fisch) polysaccharide on growth performance, immune function, and gut microflora of broiler chickens. Poult. Sci. 2022, 101, 102068. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Han, Q.; Wang, K.; Xu, Y.; Lan, J.; Cao, G. Astragalus and ginseng polysaccharides improve developmental, intestinal morphological, and immune functional characters of weaned piglets. Front. Physiol. 2019, 10, 418. [Google Scholar] [CrossRef] [PubMed]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, M.; Liu, H.; Yang, G. In vitro fermentation profiles of different soybean oligosaccharides and their effects on skatole production and cecal microbiota of broilers. Anim. Biosci. 2022, 35, 1195. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Jung, T.-H.; Park, J.H.; Jeon, W.-M.; Han, K.-S. Butyrate modulates bacterial adherence on LS174T human colorectal cells by stimulating mucin secretion and MAPK signaling pathway. Nutr. Res. Pract. 2015, 9, 343–349. [Google Scholar] [CrossRef]
- Louis, P.; Hold, G.L.; Flint, H.J. The gut microbiota, bacterial metabolites and colorectal cancer. Nat. Rev. Microbiol. 2014, 12, 661–672. [Google Scholar] [CrossRef]
- Tang, X.; Xiong, K.; Fang, R.; Li, M. Weaning stress and intestinal health of piglets: A review. Front. Immunol. 2022, 13, 1042778. [Google Scholar] [CrossRef]
- Wang, J.; Li, K.; Wang, L.; Xu, Q. Effects of different levels of stevioside on growth performance, digestive enzyme activity, antioxidant capacity and gene expression of juvenile mirror carp (Cyprinus carpio). Aquaculture 2021, 543, 737019. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Nan, X.; Jiang, L.; Wang, Y.; Liu, J.; Yao, J.; Rahman, M.T.; Xiong, B. Responses of Lactation, Rumen Fermentation and Blood Biochemical Parameters with Increasing Dietary Inulin Supplementation in Mid-Lactation Dairy Cows. Agriculture 2022, 12, 521. [Google Scholar] [CrossRef]
- José Lahm Cardoso, M.; Fagnani, R.; Zaghi Cavalcante, C.; de Souza Zanutto, M.; Júnior, A.Z.; Holsback da Silveira Fertonani, L.; Calesso, J.R.; Melussi, M.; Pinheiro Costa, H.; Yudi Hashizume, E. Blood pressure, serum glucose, cholesterol, and triglycerides in dogs with different body scores. Vet. Med. Int. 2016, 2016, 8675283. [Google Scholar] [CrossRef]
- Idowu, M.; Taiwo, G.; Sidney, T.; Treon, E.; Leal, Y.; Ologunagba, D.; Eichie, F.; Pech-Cervantes, A.; Ogunade, I.M. Effects of rumen-bypass protein supplement on growth performance, hepatic mitochondrial protein complexes, and hepatic immune gene expression of beef steers with divergent residual feed intake. PLoS ONE 2024, 19, e0293718. [Google Scholar] [CrossRef]
- Makris, K.; Mousa, C.; Cavalier, E. Alkaline phosphatases: Biochemistry, functions, and measurement. Calcif. Tissue Int. 2023, 112, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Leenen, C.H.; Dieleman, L.A. Inulin and oligofructose in chronic inflammatory bowel disease. J. Nutr. 2007, 137, 2572S–2575S. [Google Scholar] [CrossRef]
- Dowlatshahi, E.A.; van der Voort, E.A.; Arends, L.R.; Nijsten, T. Markers of systemic inflammation in psoriasis: A systematic review and meta-analysis. Br. J. Dermatol. 2013, 169, 266–282. [Google Scholar] [CrossRef] [PubMed]
- Fagarasan, S.; Kawamoto, S.; Kanagawa, O.; Suzuki, K. Adaptive immune regulation in the gut: T cell–dependent and T cell–independent IgA synthesis. Annu. Rev. Immunol. 2010, 28, 243–273. [Google Scholar] [CrossRef] [PubMed]
- Seifert, S.; Watzl, B. Inulin and Oligofructose: Review of Experimental Data on Immune Modulation. J. Nutr. 2007, 137, 2563S–2567S. [Google Scholar] [CrossRef]
- Liang, G.H.; Weber, C.R. Molecular aspects of tight junction barrier function. Curr. Opin. Pharmacol. 2014, 19, 84–89. [Google Scholar] [CrossRef]
- Lépine, A.F.; Konstanti, P.; Borewicz, K.; Resink, J.-W.; de Wit, N.J.; Vos, P.d.; Smidt, H.; Mes, J.J. Combined dietary supplementation of long chain inulin and Lactobacillus acidophilus W37 supports oral vaccination efficacy against Salmonella Typhimurium in piglets. Sci. Rep. 2019, 9, 18017. [Google Scholar] [CrossRef] [PubMed]
- Letterio, J.J.; Roberts, A.B. Regulation of immune responses by TGF-β. Annu. Rev. Immunol. 1998, 16, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, K.; Dawson, H.D.; Wasmuth, E.V.; Roneker, C.A.; Chen, C.; Urban, J.F.; Welch, R.M.; Miller, D.D.; Lei, X.G. Supplemental dietary inulin influences expression of iron and inflammation related genes in young pigs. J. Nutr. 2009, 139, 2018–2023. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.I.; Lee, F.; Miyajima, A.; Miyatake, S.; Arai, N.; Yokota, T. Cytokines: Coordinators of immune and inflammatory responses. Annu. Rev. Biochem. 1990, 59, 783–836. [Google Scholar] [CrossRef]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef]


| Items | Content |
|---|---|
| Ingredients (%) | |
| Pennisetum | 50.00 |
| Corn | 29.00 |
| Soybean meal | 10.00 |
| Bran | 7.50 |
| Premix 1 | 2.00 |
| CaHPO4 | 0.50 |
| NaHCO3 | 0.50 |
| NaCl | 0.50 |
| Total | 100.00 |
| Nutrient levels | |
| ME/(MJ/kg) 2 | 10.81 |
| Crude protein | 17.20 |
| Crude fat | 2.90 |
| Crude ash | 6.30 |
| NDF | 30.33 |
| ADF | 16.58 |
| Ca | 0.97 |
| p | 0.53 |
| Gene Name | Primer Sequence (5’-3’) | GenBank Accession No. | Product Length (bp) |
|---|---|---|---|
| TJP1(zo-1) | F1: TTCCTAAGACAGCAGGGGGA | XM_018066116.1 | 121 |
| R1: AACTTGGTCTGGACTGGCTG | |||
| CLDN-3 | F1: CACCATCATCCGGGACTTCT | XM_018041071.1 | 179 |
| R1: GCGCCGAGTAGACGATCTTG | |||
| OCLN | F1: CTGCTGCCGACGAGTACAAT | XM_018065680.1 | 135 |
| R1: TCCGTCGGTCGTAATCTCCA | |||
| TGFB-1 | F1: ACAATTCCTGGCGCTACCTC | NM_001314142.1 | 199 |
| R1: CCGGAACTGAACCCGTTGAT | |||
| IL-6 | F1: CTTCACAAGCGCCTTCAGTC | NM_001285640.1 | 126 |
| R1: AGTAGTCTGCTTGGGGTGGT | |||
| IL-10 | F1: CATGGGCCTGACATCAAGGA | XM_005690416.3 | 113 |
| R1: GCCTTGCTCTTGTTTTCGCA | |||
| GAPDH | F1: ACGTGTCCGTTGTGGATCTG | XM_005680968.3 | 142 |
| R1: AAGTCGCAGGAGACAACCTG |
| Items | Control | AM HCl | 0.1% Inulin | 0.3% Inulin | 0.5% Inulin | SEM 1 | p-Value |
|---|---|---|---|---|---|---|---|
| Days 1 to 25 | |||||||
| Initial BW (kg) | 9.03 | 9.00 | 9.00 | 9.02 | 8.97 | 0.21 | 1.000 |
| Final BW (kg) | 10.37 | 10.70 | 10.41 | 10.63 | 10.53 | 0.14 | 0.831 |
| ADG (g/d) | 53.60 | 68.00 | 56.43 | 64.33 | 62.5 | 5.15 | 0.704 |
| ADFI (g/d) | 517.66 b | 501.10 b | 537.75 b | 553.45 a | 527.50 b | 5.78 | 0.039 |
| F/G | 9.66 a | 7.37 c | 9.52 a | 8.60 b | 8.44 b | 0.14 | <0.001 |
| Days 26 to 40 | |||||||
| Final BW (kg) | 11.55 | 12.07 | 11.62 | 12.05 | 11.83 | 0.13 | 0.521 |
| ADG (g/d) | 78.67 c | 91.11 a | 80.68 c | 94.67 a | 86.94 b | 7.26 | 0.038 |
| ADFI (g/d) | 587.13 | 548.34 | 588.28 | 592.89 | 561.01 | 4.23 | 0.097 |
| F/G | 7.46 a | 6.01 d | 6.83 b | 6.26 c | 6.45 c | 0.28 | <0.001 |
| Days 1 to 40 | |||||||
| ADG (g/d) | 63.06 c | 76.77 a | 65.55 b | 75.73 a | 71.67 ab | 4.87 | 0.022 |
| ADFI (g/d) | 552.41 a | 524.74 b | 563.02 a | 560.21 a | 544.26 ab | 6.44 | 0.002 |
| F/G | 8.76 a | 6.83 c | 8.58 a | 7.40 b | 7.59 b | 0.16 | <0.001 |
| Items | Control | AM HCl | 0.1% Inulin | 0.3% Inulin | 0.5% Inulin | SEM 1 | p-Value |
|---|---|---|---|---|---|---|---|
| Duodenum | |||||||
| VH (μm) | 523.93 d | 736.50 b | 846.53 a | 888.80 a | 656.40 c | 35.35 | <0.001 |
| CD (μm) | 393.83 | 368.23 | 434.60 | 395.93 | 347.37 | 14.04 | 0.394 |
| VCR | 1.37 b | 2.00 a | 2.00 a | 2.26 a | 1.90 a | 0.10 | 0.022 |
| Jejunum | |||||||
| VH (μm) | 706.43 b | 698.13 b | 693.53 b | 808.33 a | 885.70 a | 22.61 | 0.001 |
| CD (μm) | 393.07 b | 351.70 b | 374.50 b | 400.13 b | 465.83 a | 11.80 | 0.004 |
| VCR | 1.80 | 1.99 | 1.85 | 2.04 | 1.90 | 0.04 | 0.290 |
| Ileum | |||||||
| VH (μm) | 365.03 c | 425.93 b | 428.87 b | 480.60 a | 476.70 a | 11.41 | <0.001 |
| CD (μm) | 320.57 c | 232.00 d | 332.20 c | 320.40 c | 372.60 a | 13.08 | <0.001 |
| VCR | 1.14 c | 1.84 a | 1.29 c | 1.50 b | 1.28 c | 0.07 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Du, C.; Wu, P.; Ma, J.; Gan, S.; Wang, Z.; Yin, F. The Effects of Inulin on the Growth, Oxidative Stress, and Immune Function of Weaned Kids. Animals 2025, 15, 2455. https://doi.org/10.3390/ani15162455
Zhou Z, Du C, Wu P, Ma J, Gan S, Wang Z, Yin F. The Effects of Inulin on the Growth, Oxidative Stress, and Immune Function of Weaned Kids. Animals. 2025; 15(16):2455. https://doi.org/10.3390/ani15162455
Chicago/Turabian StyleZhou, Zhiling, Chunmei Du, Pengxin Wu, Jian Ma, Shangquan Gan, Zhijing Wang, and Fuquan Yin. 2025. "The Effects of Inulin on the Growth, Oxidative Stress, and Immune Function of Weaned Kids" Animals 15, no. 16: 2455. https://doi.org/10.3390/ani15162455
APA StyleZhou, Z., Du, C., Wu, P., Ma, J., Gan, S., Wang, Z., & Yin, F. (2025). The Effects of Inulin on the Growth, Oxidative Stress, and Immune Function of Weaned Kids. Animals, 15(16), 2455. https://doi.org/10.3390/ani15162455






