A Low Dose of Berberine Is Metabolized in Weaned Piglets Without Major Changes to Gut Morphology or Gut Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Sample Collection
2.2. Intestinal Morphology
2.3. DNA Extraction from Intestinal Contents
2.4. 16S rRNA Gene Sequencing and Bioinformatics
2.5. SCFA Quantification
2.6. Berberine Quantification in Plasma and Small and Large Intestinal Contents
2.6.1. Plasma Sample Preparation Procedure
2.6.2. Intestinal Content Sample Preparation Procedure
2.6.3. Method Validation
2.7. Statistics
3. Results and Discussion
3.1. Low Dose of Berberine Does Not Affect Body Weight or Gut Morphology
3.2. Microbiome Composition Is Not Significantly Altered with a Low Dose of Berberine
3.3. Low Dose of Berberine Does Not Induce Changes in SCFA Production
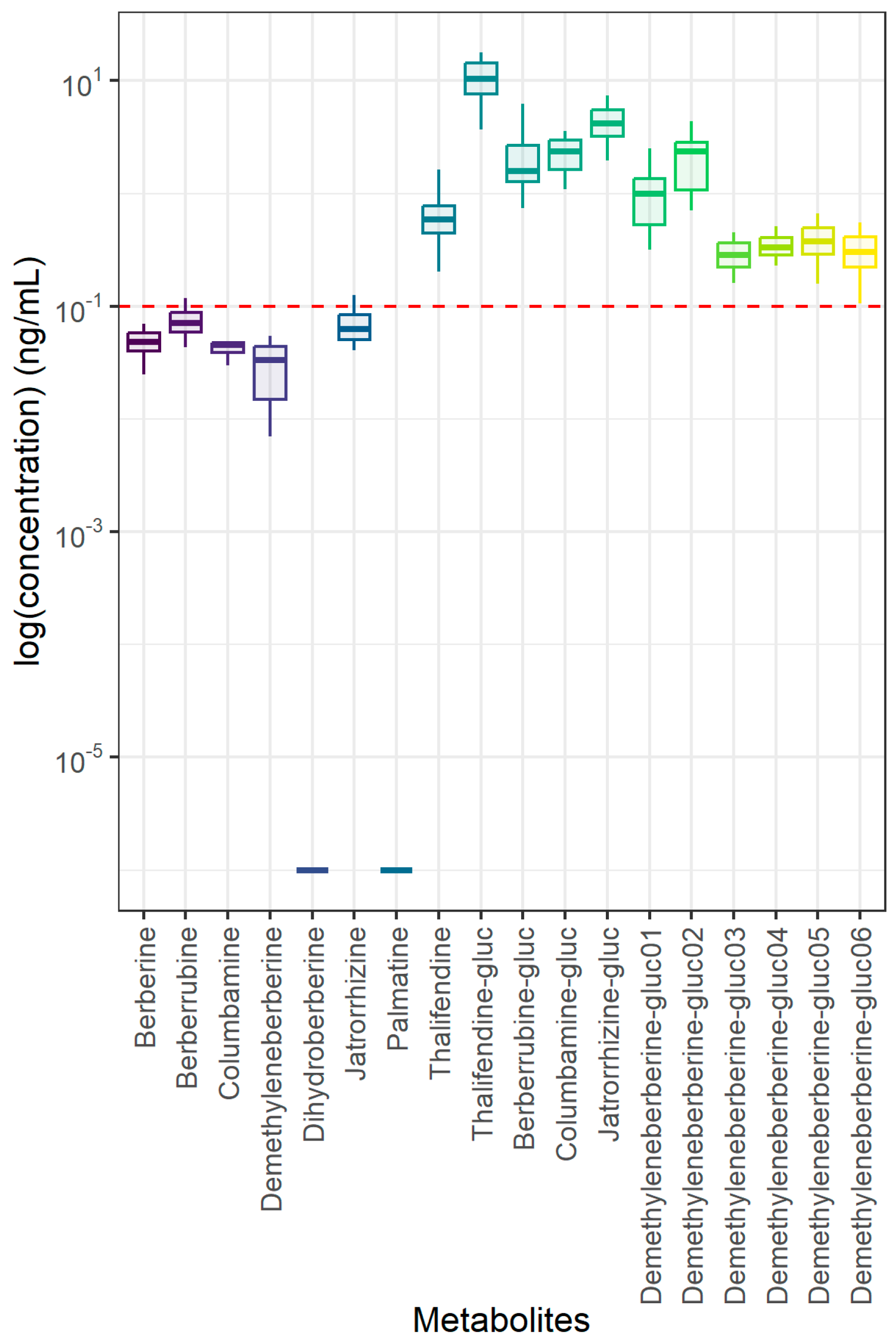
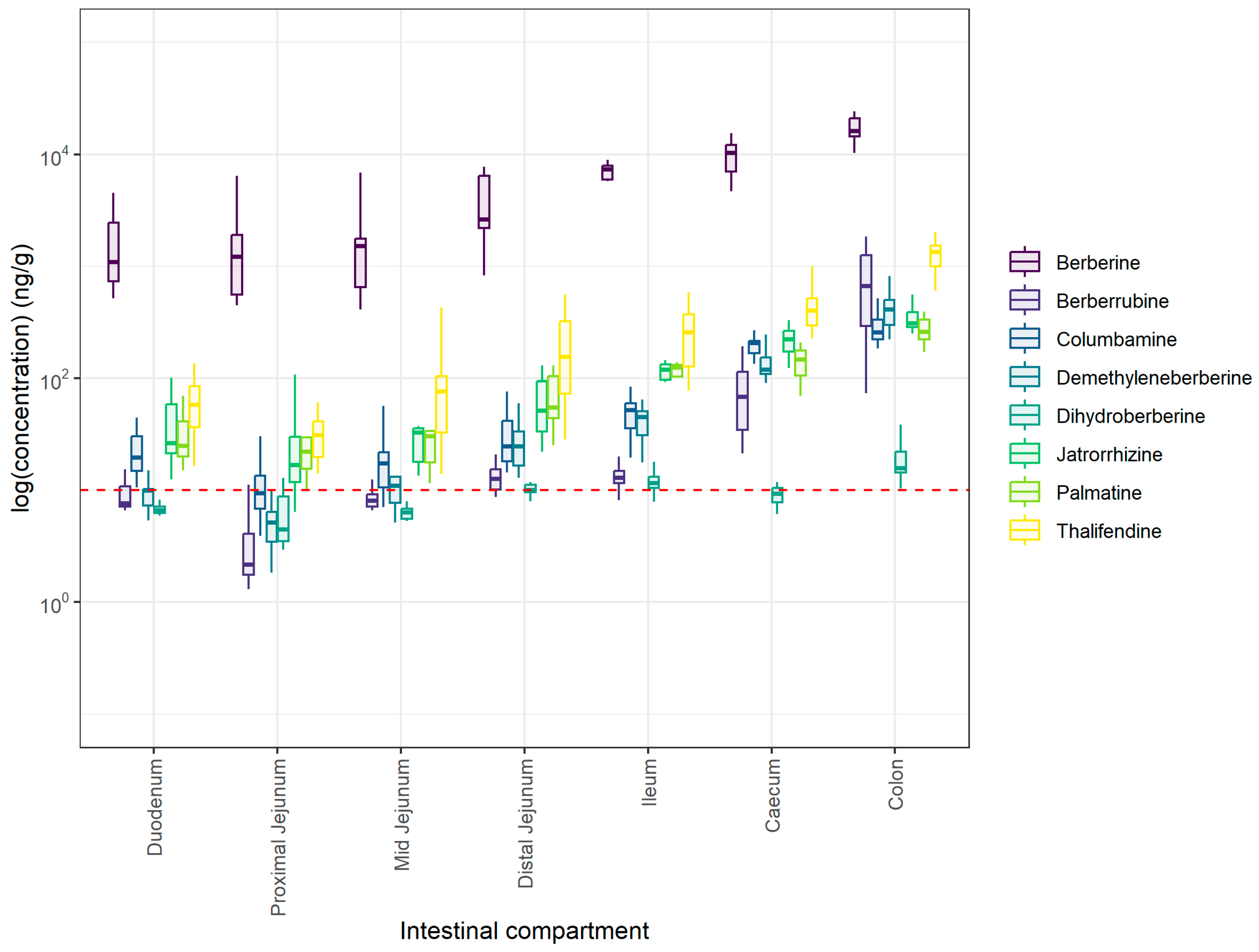
3.4. Berberine Quantification in Plasma and Intestinal Contents of Piglets
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCFAs | Short-chain fatty acids. |
| PWD | Post-weaning diarrhea. |
| BBR | Berberine. |
| LOQ | Limit of quantification. |
| UPLC-MS/MS | Ultra performance liquid chromatography-tandem mass spectrometry. |
| ROS | Reactive oxygen species. |
| CTR | Control. |
| ZnO | Zinc oxide. |
| ETEC | Enterotoxigenic Escherichia coli. |
| CTAB | Cetyltrimethylammonium bromide. |
| bCFA | Branched-Chain Fatty Acids. |
| GIT | Gastrointestinal tract. |
| •OHs | Hydroxyl radicals. |
| gEECs | Goat endometrial epithelial cells. |
References
- Wijtten, P.J.A.; Meulen, J.V.D.; Verstegen, M.W.A. Intestinal Barrier Function and Absorption in Pigs after Weaning: A Review. Br. J. Nutr. 2011, 105, 967–981. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Duarte, M.E.; Sevarolli Loftus, A.; Kim, S.W. Intestinal Health of Pigs Upon Weaning: Challenges and Nutritional Intervention. Front. Vet. Sci. 2021, 8, 628258. [Google Scholar] [CrossRef] [PubMed]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post Weaning Diarrhea in Pigs: Risk Factors and Non-Colistin-Based Control Strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, A.; Tugnoli, B.; Piva, A.; Grilli, E. Towards Zero Zinc Oxide: Feeding Strategies to Manage Post-Weaning Diarrhea in Piglets. Animals 2021, 11, 642. [Google Scholar] [CrossRef]
- Yu, T.; Zhu, C.; Chen, S.; Gao, L.; Lv, H.; Feng, R.; Zhu, Q.; Xu, J.; Chen, Z.; Jiang, Z. Dietary High Zinc Oxide Modulates the Microbiome of Ileum and Colon in Weaned Piglets. Front. Microbiol. 2017, 8, 825. [Google Scholar] [CrossRef] [PubMed]
- Pieper, R.; Dadi, T.H.; Pieper, L.; Vahjen, W.; Franke, A.; Reinert, K.; Zentek, J. Concentration and Chemical Form of Dietary Zinc Shape the Porcine Colon Microbiome, Its Functional Capacity and Antibiotic Resistance Gene Repertoire. ISME J. 2020, 14, 2783–2793. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Garaguso, I.; Britti, M.S.; Mengheri, E. Zinc Oxide Protects Cultured Enterocytes from the Damage Induced by Escherichia Coli. J. Nutr. 2003, 133, 4077–4082. [Google Scholar] [CrossRef]
- Shurson, G.C.; Urriola, P.E.; Hung, Y.-T. Too Much of a Good Thing: Rethinking Feed Formulation and Feeding Practices for Zinc in Swine Diets to Achieve One Health and Environmental Sustainability. Animals 2022, 12, 3374. [Google Scholar] [CrossRef]
- Ciesinski, L.; Guenther, S.; Pieper, R.; Kalisch, M.; Bednorz, C.; Wieler, L.H. High Dietary Zinc Feeding Promotes Persistence of Multi-Resistant E. Coli in the Swine Gut. PLoS ONE 2018, 13, e0191660. [Google Scholar] [CrossRef]
- Habtemariam, S. Berberine Pharmacology and the Gut Microbiota: A Hidden Therapeutic Link. Pharmacol. Res. 2020, 155, 104722. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, J.; Tan, Y.; Feng, W.; Peng, C. Interactions between Gut Microbiota and Berberine, a Necessary Procedure to Understand the Mechanisms of Berberine. J. Pharm. Anal. 2022, 12, 541–555. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological Properties and Clinical Applications of Berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Du, M.; Liu, X.; Ji, X.; Wang, Y.; Liu, X.; Zhao, C.; Jin, E.; Gu, Y.; Wang, H.; Zhang, F. Berberine Alleviates Enterotoxigenic Escherichia Coli-Induced Intestinal Mucosal Barrier Function Damage in a Piglet Model by Modulation of the Intestinal Microbiome. Front. Nutr. 2025, 11, 1494348. [Google Scholar] [CrossRef]
- Zhu, C.; Le, M.; He, Z.; Bai, Y.; Yang, J.; Ye, J.; Chen, Z.; Jiang, Z. Dietary Berberine Supplementation Improves Growth Performance and Alleviates Gut Injury in Weaned Piglets by Modulating Ileal Microbiota and Metabolites. Food Funct. 2023, 14, 4143–4162. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Z.; Du, M.; Ji, X.; Liu, X.; Zhao, C.; Pang, X.; Jin, E.; Wen, A.; Li, S.; et al. Berberine Alleviates ETEC-Induced Intestinal Inflammation and Oxidative Stress Damage by Optimizing Intestinal Microbial Composition in a Weaned Piglet Model. Front. Immunol. 2024, 15, 1460127. [Google Scholar] [CrossRef] [PubMed]
- Nie, X.; Lu, Q.; Yin, Y.; He, Z.; Bai, Y.; Zhu, C. Microbiome and Metabolome Analyses Reveal Significant Alterations of Gut Microbiota and Bile Acid Metabolism in ETEC-Challenged Weaned Piglets by Dietary Berberine Supplementation. Front. Microbiol. 2024, 15, 1428287. [Google Scholar] [CrossRef] [PubMed]
- Dehau, T.; Cherlet, M.; Croubels, S.; Van Immerseel, F.; Goossens, E. A High Dose of Dietary Berberine Improves Gut Wall Morphology, Despite an Expansion of Enterobacteriaceae and a Reduction in Beneficial Microbiota in Broiler Chickens. mSystems 2023, 8, e01239-22. [Google Scholar] [CrossRef] [PubMed]
- Dehau, T.; Cherlet, M.; Croubels, S.; Van De Vliet, M.; Goossens, E.; Van Immerseel, F. Berberine-Microbiota Interplay: Orchestrating Gut Health through Modulation of the Gut Microbiota and Metabolic Transformation into Bioactive Metabolites. Front. Pharmacol. 2023, 14, 1281090. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Feng, X.; Chai, L.; Cao, S.; Qiu, F. The Metabolism of Berberine and Its Contribution to the Pharmacological Effects. Drug Metab. Rev. 2017, 49, 139–157. [Google Scholar] [CrossRef]
- Millet, S.; Aluwé, M.; De Boever, J.; De Witte, B.; Douidah, L.; Van Den Broeke, A.; Leen, F.; De Cuyper, C.; Ampe, B.; De Campeneere, S. The Effect of Crude Protein Reduction on Performance and Nitrogen Metabolism in Piglets (Four to Nine Weeks of Age) Fed Two Dietary Lysine Levels1. J. Anim. Sci. 2018, 96, 3824–3836. [Google Scholar] [CrossRef]
- Aguirre, M.; Vuorenmaa, J.; Valkonen, E.; Kettunen, H.; Callens, C.; Haesebrouck, F.; Ducatelle, R.; Van Immerseel, F.; Goossens, E. In-Feed Resin Acids Reduce Matrix Metalloproteinase Activity in the Ileal Mucosa of Healthy Broilers without Inducing Major Effects on the Gut Microbiota. Vet. Res. 2019, 50, 15. [Google Scholar] [CrossRef]
- Klindworth, A.; Pruesse, E.; Schweer, T.; Peplies, J.; Quast, C.; Horn, M.; Glöckner, F.O. Evaluation of General 16S Ribosomal RNA Gene PCR Primers for Classical and Next-Generation Sequencing-Based Diversity Studies. Nucleic Acids Res. 2013, 41, e1. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria. 2018. Available online: https://www.r-project.org/.
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glöckner, F.O. The SILVA Ribosomal RNA Gene Database Project: Improved Data Processing and Web-Based Tools. Nucleic Acids Res. 2012, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.S. DECIPHER: Harnessing Local Sequence Context to Improve Protein Multiple Sequence Alignment. BMC Bioinform. 2015, 16, 322. [Google Scholar] [CrossRef] [PubMed]
- Schliep, K.P. Phangorn: Phylogenetic Analysis in R. Bioinformatics 2011, 27, 592–593. [Google Scholar] [CrossRef] [PubMed]
- McMurdie, P.J.; Holmes, S. Phyloseq: An R Package for Reproducible Interactive Analysis and Graphics of Microbiome Census Data. PLoS ONE 2013, 8, e61217. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Shou, J.-W.; Zhao, Z.-X.; He, C.-Y.; Ma, C.; Huang, M.; Fu, J.; Tan, X.-S.; Li, X.-Y.; Wen, B.-Y.; et al. Transforming Berberine into Its Intestine-Absorbable Form by the Gut Microbiota. Sci. Rep. 2015, 5, 12155. [Google Scholar] [CrossRef] [PubMed]
- International Cooperation on Harmonisation of Technical Requirements for Registration of Veterinary Medicinal Products (VICH) Topic GL49: Studies to Evaluate the Metabolism and Residues Kinetics of Veterinary Drugs in Human Food-Producing Animals: Validation of Analytical Methods Used in Residue Depletion Studies -EMEA/CVMP/VICH/463202/2009, January 2016. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/vich-gl49-studies-evaluate-metabolism-and-residue-kinetics-veterinary-drugs-food-producing-animals-validation-analytical-methods-used-residue-depletion-studies_en.pdf (accessed on 1 January 2025).
- International Council For Harmonisation. ICH Guideline M10 on Bioanalytical Method Validation and Study Sample Analysis EMA/CHMP/ICH/172948/2019; International Council For Harmonisation: Geneva, Switzerland, 2022. [Google Scholar]
- European Union. 1912002 Implementing Council Directive 96/23/EC Concerning the Performance of Analytical Methods and the Interpretation of Results, August 2002; European Union: Bruxelles/Brussels, Belgium, 2002. [Google Scholar]
- Liu, H.; Zeng, X.; Zhang, G.; Hou, C.; Li, N.; Yu, H.; Shang, L.; Zhang, X.; Trevisi, P.; Yang, F.; et al. Maternal Milk and Fecal Microbes Guide the Spatiotemporal Development of Mucosa-Associated Microbiota and Barrier Function in the Porcine Neonatal Gut. BMC Biol. 2019, 17, 106. [Google Scholar] [CrossRef] [PubMed]
- Tancredi, A.; Matthijs, T.; Cox, E.; Van Immerseel, F.; Goossens, E. From Mother to Piglet: The Lasting Influence of the Maternal Microbiome. Anim. Microbiome 2025, 7, 52. [Google Scholar] [CrossRef]
- Dixon, P. VEGAN, a Package of R Functions for Community Ecology. J. Veg. Sci. 2003, 14, 927–930. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.-A.; Cha, J.; Choi, S.; Kim, J.-H.; Kim, D. Early Colonization of the Intestinal Microbiome of Neonatal Piglets Is Influenced by the Maternal Microbiome. Animals 2023, 13, 3378. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A Potential Phytochemical with Multispectrum Therapeutic Activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef]
- Yu, H.-H.; Kim, K.-J.; Cha, J.-D.; Kim, H.-K.; Lee, Y.-E.; Choi, N.-Y.; You, Y.-O. Antimicrobial Activity of Berberine Alone and in Combination with Ampicillin or Oxacillin Against Methicillin-Resistant Staphylococcus Aureus. J. Med. Food 2005, 8, 454–461. [Google Scholar] [CrossRef]
- Guevarra, R.B.; Hong, S.H.; Cho, J.H.; Kim, B.-R.; Shin, J.; Lee, J.H.; Kang, B.N.; Kim, Y.H.; Wattanaphansak, S.; Isaacson, R.E.; et al. The Dynamics of the Piglet Gut Microbiome during the Weaning Transition in Association with Health and Nutrition. J. Anim. Sci. Biotechnol. 2018, 9, 54. [Google Scholar] [CrossRef]
- Yang, Q.; Huang, X.; Wang, P.; Yan, Z.; Sun, W.; Zhao, S.; Gun, S. Longitudinal Development of the Gut Microbiota in Healthy and Diarrheic Piglets Induced by Age-related Dietary Changes. Microbiol. Open 2019, 8, e923. [Google Scholar] [CrossRef] [PubMed]
- Sebastià, C.; Folch, J.M.; Ballester, M.; Estellé, J.; Passols, M.; Muñoz, M.; García-Casco, J.M.; Fernández, A.I.; Castelló, A.; Sánchez, A.; et al. Interrelation between Gut Microbiota, SCFA, and Fatty Acid Composition in Pigs. mSystems 2023, 9, e01049-23. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of Short Chain Fatty Acids by the Gut Microbiota and Their Impact on Human Metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The Role of Short-Chain Fatty Acids in Intestinal Barrier Function, Inflammation, Oxidative Stress, and Colonic Carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, M.; Inoue, R.; Tomonaga, S.; Fukuta, K.; Tsukahara, T. Production, Absorption, and Blood Flow Dynamics of Short-Chain Fatty Acids Produced by Fermentation in Piglet Hindgut during the Suckling–Weaning Period. Nutrients 2018, 10, 1220. [Google Scholar] [CrossRef]
- Pieper, R.; Villodre Tudela, C.; Taciak, M.; Bindelle, J.; Pérez, J.F.; Zentek, J. Health Relevance of Intestinal Protein Fermentation in Young Pigs. Anim. Health Res. Rev. 2016, 17, 137–147. [Google Scholar] [CrossRef]
- Hu, H.; Xu, K.; Wang, K.; Zhang, F.; Bai, X. Dissecting the Effect of Berberine on the Intestinal Microbiome in the Weaned Piglets by Metagenomic Sequencing. Front. Microbiol. 2022, 13, 862882. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Yu, Z.; Li, Z.; Liu, H.; Li, W.; Zhao, J.; Ren, Y.; Ma, L. Dietary Berberine and Ellagic Acid Supplementation Improve Growth Performance and Intestinal Damage by Regulating the Structural Function of Gut Microbiota and SCFAs in Weaned Piglets. Microorganisms 2023, 11, 1254. [Google Scholar] [CrossRef]
- Hua, W.; Ding, L.; Chen, Y.; Gong, B.; He, J.; Xu, G. Determination of Berberine in Human Plasma by Liquid Chromatography–Electrospray Ionization–Mass Spectrometry. J. Pharm. Biomed. Anal. 2007, 44, 931–937. [Google Scholar] [CrossRef]
- Chen, W.; Miao, Y.-Q.; Fan, D.-J.; Yang, S.-S.; Lin, X.; Meng, L.-K.; Tang, X. Bioavailability Study of Berberine and the Enhancing Effects of TPGS on Intestinal Absorption in Rats. AAPS PharmSciTech 2011, 12, 705–711. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-T.; Hao, H.-P.; Xie, H.-G.; Lai, L.; Wang, Q.; Liu, C.-X.; Wang, G.-J. Extensive Intestinal First-Pass Elimination and Predominant Hepatic Distribution of Berberine Explain Its Low Plasma Levels in Rats. Drug Metab. Dispos. 2010, 38, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Wang, K.; Cao, S.; Ding, L.; Qiu, F. Pharmacokinetics and Excretion of Berberine and Its Nine Metabolites in Rats. Front. Pharmacol. 2021, 11, 594852. [Google Scholar] [CrossRef]
- Helke, K.L.; Nelson, K.N.; Sargeant, A.M.; Jacob, B.; McKeag, S.; Haruna, J.; Vemireddi, V.; Greeley, M.; Brocksmith, D.; Navratil, N.; et al. Pigs in Toxicology: Breed Differences in Metabolism and Background Findings. Toxicol. Pathol. 2016, 44, 575–590. [Google Scholar] [CrossRef] [PubMed]
- Yan, D.; Jin, C.; Xiao, X.-H.; Dong, X.-P. Antimicrobial Properties of Berberines Alkaloids in Coptis Chinensis Franch by Microcalorimetry. J. Biochem. Biophys. Methods 2008, 70, 845–849. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Zhang, M.; Luo, H. Identification and Antimicrobial Activity of Two Alkaloids from Traditional Chinese Medicinal Plant Tinospora capillipes. Ind. Crops Prod. 2012, 37, 298–302. [Google Scholar] [CrossRef]
- Hao, Y.; Xing, M.; Gu, X. Research Progress on Oxidative Stress and Its Nutritional Regulation Strategies in Pigs. Animals 2021, 11, 1384. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, Y.; Zhang, X.; Lu, Y.; Chen, H. New Insights in Intestinal Oxidative Stress Damage and the Health Intervention Effects of Nutrients: A Review. J. Funct. Foods 2020, 75, 104248. [Google Scholar] [CrossRef]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 761264. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.H.; Kim, H.Y.; Kang, K.S.; Yokozawa, T.; Park, J.H. Hydroxyl Radical Scavenging Activities of Isoquinoline Alkaloids Isolated from Coptis Chinensis. Arch. Pharm. Res. 2009, 32, 341–345. [Google Scholar] [CrossRef]
- Yan, B.; Wang, D.; Dong, S.; Cheng, Z.; Na, L.; Sang, M.; Yang, H.; Yang, Z.; Zhang, S.; Yan, Z. Palmatine Inhibits TRIF-Dependent NF-κB Pathway against Inflammation Induced by LPS in Goat Endometrial Epithelial Cells. Int. Immunopharmacol. 2017, 45, 194–200. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-T.; Xu, Y.-F.; Huang, Y.-F.; Qu, C.; Xu, L.-Q.; Su, Z.-R.; Zeng, H.-F.; Zheng, L.; Yi, T.-G.; Li, H.-L.; et al. Berberrubine Attenuates Mucosal Lesions and Inflammation in Dextran Sodium Sulfate-Induced Colitis in Mice. PLoS ONE 2018, 13, e0194069. [Google Scholar] [CrossRef]
- Li, C.; Dong, N.; Wu, B.; Mo, Z.; Xie, J.; Lu, Q. Dihydroberberine, an Isoquinoline Alkaloid, Exhibits Protective Effect against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice. Phytomedicine 2021, 90, 153631. [Google Scholar] [CrossRef]
- Liu, C.; Liang, X.; Wei, X.; Chen, F.; Tang, Q.; Tan, X. Comparative Metabolism of the Eight Main Bioactive Ingredients of Gegen Qinlian Decoction by the Intestinal Flora of Diarrhoeal and Healthy Piglets. Biomed. Chromatogr. 2019, 33, e4421. [Google Scholar] [CrossRef]
- De Groot, N.; Fariñas, F.; Cabrera-Gómez, C.G.; Pallares, F.J.; Ramis, G. Weaning Causes a Prolonged but Transient Change in Immune Gene Expression in the Intestine of Piglets. J. Anim. Sci. 2021, 99, skab065. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Song, M.; Liu, Y.; Ji, P. Enterotoxigenic Escherichia Coli Infection of Weaned Pigs: Intestinal Challenges and Nutritional Intervention to Enhance Disease Resistance. Front. Immunol. 2022, 13, 885253. [Google Scholar] [CrossRef] [PubMed]
- Establishment by the European Community of Maximum Residue Limits (MRLs) for Residues of Veterinary Medicinal Products in Foodstuffs of Animal Origin. The Rules Governing Medicinal Products in the European Community; Commission of the European Communities: Brussels, Luxembourg, 1991; Volume VI.
- European Commission. Rules governing medicinal products in the EU: Notice to Applicants and Note for Guidance: Establishment of maximum residue limits (MRLs) for residues of veterinary medicinal products in foodstuffs of animal origin, June 2003. Available online: https://www.ema.europa.eu/en/veterinary-regulatory-overview/research-development-veterinary-medicines/maximum-residue-limits-mrl (accessed on 1 January 2025).
| Ingredients | % |
|---|---|
| Barley | 26.677 |
| Wheat | 25 |
| Soybeans | 13.083 |
| Corn | 10 |
| Premix weaner | 9 |
| Soybean meal | 6 |
| Beet molasses | 3 |
| Potato protein | 2 |
| Wheat gluten | 1.571 |
| Monocalciumphosphate | 0.923 |
| Limestone | 0.747 |
| L-Lysine HCL | 0.604 |
| Salt | 0.492 |
| L-threonine | 0.259 |
| DL-methionine | 0.243 |
| L-Valine | 0.142 |
| L-Tryptophan | 0.079 |
| Ronozyme (500 FYT) | 0.05 |
| Ronozyme (1000 FYT) | 0.05 |
| Leucine valine 90/10 | 0.049 |
| Isoleucine valine 50/50 | 0.033 |
| Parameters | Control | BBR | p Value |
|---|---|---|---|
| Duodenum | |||
| Villus height (μm) | 408.92 ± 43.99 | 410.43 ± 89.79 | 0.347 |
| Crypt depth (μm) | 368 ± 122.5 | 437.41 ± 65.59 | 0.0045 |
| Villus/Crypt ratio | 1.19 ± 0.32 | 0.98 ± 0.37 | 0.088 |
| Proximal Jejunum | |||
| Villus height (μm) | 401.79 ± 97.41 | 320.31 ± 94.17 | 0.061 |
| Crypt depth (μm) | 300.22 ± 86.68 | 342.93 ± 93.25 | 0.283 |
| Villus/Crypt ratio | 1.44 ± 0.49 | 1.02 ± 0.46 | 0.0484 |
| Middle Jejunum | |||
| Villus height (μm) | 369.57 ± 61.32 | 330.73 ± 87.75 | 0.464 |
| Crypt depth (μm) | 281.49 ± 76.31 | 330.02 ± 83.75 | 0.183 |
| Villus/Crypt ratio | 1.43 ± 0.49 | 1.12 ± 0.56 | 0.188 |
| Distal Jejunum | |||
| Villus height (μm) | 291.9 ± 53.83 | 305.34 ± 96.93 | 0.689 |
| Crypt depth (μm) | 256.52 ± 89.74 | 288.4 ± 72.17 | 0.357 |
| Villus/Crypt ratio | 1.27 ± 0.46 | 1.18 ± 0.62 | 0.727 |
| Ileum | |||
| Villus height (μm) | 295.34 ± 56.34 | 299.79 ± 69.03 | 0.873 |
| Crypt depth (μm) | 224.9 ± 74.70 | 280.83 ± 78.92 | 0.043 |
| Villus/Crypt ratio | 1.45 ± 0.51 | 1.17 ± 0.46 | 0.128 |
| Body weight (6 weeks) | 11.2 kg ± 1.1 | 11.3 kg ± 1.4 | 0.803 |
| Chao1 Index | |||
|---|---|---|---|
| Intestinal segment | BBR | Control | p Value |
| Mid Jejunum | 115.72 ± 102.39 | 122.73 ± 107.41 | 0.87 |
| Cecum | 381.8 ± 92.3 | 331.6 ± 70.7 | 0.16 |
| Colon | 573.84 ± 119.9 | 526.79 ± 92.49 | 0.27 |
| SCFA (µmol/g) | Cecum | p Value | Colon | p Value | ||
|---|---|---|---|---|---|---|
| BBR | Control | BBR | Control | |||
| Acetic Acid | 71.28 | 69.46 | 0.740 | 78.91 | 74.32 | 0.299 |
| Propionic Acid | 41.75 | 42.23 | 0.918 | 33.84 | 32.48 | 0.659 |
| Butyric Acid | 15.43 | 15.61 | 0.954 | 16.51 | 15.93 | 0.776 |
| Valeric Acid | 2.02 | 3.12 | 0.272 | 3.09 | 3.91 | 0.138 |
| Caproic Acid | 0.14 | 0.20 | 0.146 | 0.27 | 0.28 | 0.916 |
| Isobutyric Acid | 0.28 | 0.37 | 0.065 | 1.36 | 1.48 | 0.59 |
| Isovaleric Acid | 0.31 | 0.38 | 0.270 | 1.72 | 1.91 | 0.557 |
| Isocaproic Acid | 0.13 | 0.10 | 0.291 | 0.10 | 0.09 | 0.206 |
| Total bCFAs | 0.72 | 0.85 | 0.291 | 3.18 | 3.48 | 0.562 |
| Total SCFAs | 131.34 | 131.47 | 0.992 | 135.80 | 130.40 | 0.512 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mouchtoglou, C.; Cherlet, M.; Dehau, T.; Aluwe, M.; Ducatelle, R.; Goossens, E.; Croubels, S.; Van Immerseel, F. A Low Dose of Berberine Is Metabolized in Weaned Piglets Without Major Changes to Gut Morphology or Gut Microbiota. Animals 2025, 15, 2450. https://doi.org/10.3390/ani15162450
Mouchtoglou C, Cherlet M, Dehau T, Aluwe M, Ducatelle R, Goossens E, Croubels S, Van Immerseel F. A Low Dose of Berberine Is Metabolized in Weaned Piglets Without Major Changes to Gut Morphology or Gut Microbiota. Animals. 2025; 15(16):2450. https://doi.org/10.3390/ani15162450
Chicago/Turabian StyleMouchtoglou, Christina, Marc Cherlet, Tessa Dehau, Marijke Aluwe, Richard Ducatelle, Evy Goossens, Siska Croubels, and Filip Van Immerseel. 2025. "A Low Dose of Berberine Is Metabolized in Weaned Piglets Without Major Changes to Gut Morphology or Gut Microbiota" Animals 15, no. 16: 2450. https://doi.org/10.3390/ani15162450
APA StyleMouchtoglou, C., Cherlet, M., Dehau, T., Aluwe, M., Ducatelle, R., Goossens, E., Croubels, S., & Van Immerseel, F. (2025). A Low Dose of Berberine Is Metabolized in Weaned Piglets Without Major Changes to Gut Morphology or Gut Microbiota. Animals, 15(16), 2450. https://doi.org/10.3390/ani15162450







