Early Feeding Strategies for the Larviculture of the Vermiculated Angelfish Chaetodontoplus mesoleucus: The Key Role of Copepods
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Collection
2.2. Live-Prey Organism Culture
2.3. Feeding Experiment
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Biondo, M.V.; Burki, R.P.; Aguayo, F.; Calado, R. An updated review of the marine ornamental fish trade in the European Union. Animals 2024, 14, 1761. [Google Scholar] [CrossRef] [PubMed]
- Biondo, M.V.; Burki, R.P. Monitoring the trade in marine ornamental fishes through the European Trade Control and Expert System TRACES: Challenges and possibilities. Mar. Policy 2019, 108, 103620. [Google Scholar] [CrossRef]
- Pouil, S.; Tlusty, M.F.; Rhyne, A.L.; Metian, M. Aquaculture of marine ornamental fish: Overview of the production trends and the role of academia in research progress. Rev. Aquac. 2020, 12, 1217–1230. [Google Scholar] [CrossRef]
- Livengood, E.; Chapman, F.A. The ornamental Fish Trade: An introduction with perspectives for responsible Aquarium Fish Ownership: FA124/FA124, 5/2007. Edis 2007, 2007. [Google Scholar] [CrossRef]
- Anil, M.; Rohini Krishna, M.; Gomathi, P.; Surya, S.; Gop, A.P.; Santhosh, B.; Siju, R.; Anand, V.; Krishnapriya, P.; Shalini, O. Recent advances in marine ornamental breeding and seed production at Vizhinjam Regional Centre of CMFRI India. Front. Mar. Sci. 2022, 9, 907568. [Google Scholar] [CrossRef]
- Hioki, S.; Suzuki, K. Spawning behavior, eggs, and larvae of the angelfish, Chaetodontoplus mesoleucus, in the aquarium. J. Sch. Mar. Sci. Technol. Tokai Univ. 1995, 39, 195–205. [Google Scholar]
- Moorhead, J.A.; Zeng, C. Development of captive breeding techniques for marine ornamental fish: A review. Rev. Fish. Sci. 2010, 18, 315–343. [Google Scholar] [CrossRef]
- Sun, Y.-H.; Lin, Y.-R.; Hsieh, H.-Y.; Meng, P.-J. Natural Spawning, Early Development, and First Successful Hatchery Production of the Vermiculated Angelfish (Chaetodontoplus mesoleucus), Exploring the Influence of Temperature and Salinity. Animals 2025, 15, 1657. [Google Scholar] [CrossRef]
- Muhamad Shaleh, S.R.; Ismail, R.; Mohd Faudzi, N.; Shapawi, R.; Fui, C.F. The Importance of Rotifer as Live Feed in Mariculture. In Essentials of Aquaculture Practices; Springer: Berlin/Heidelberg, Germany, 2024; pp. 41–59. [Google Scholar]
- Dhont, J.; Dierckens, K.; Støttrup, J.; Van Stappen, G.; Wille, M.; Sorgeloos, P. Rotifers, Artemia and copepods as live feeds for fish larvae in aquaculture. In Advances in Aquaculture Hatchery Technology; Elsevier: Amsterdam, The Netherlands, 2013; pp. 157–202. [Google Scholar]
- Shao, L.; Zeng, C. Survival, growth, ingestion rate and foraging behavior of larval green mandarin fish (Synchiropus splendidus) fed copepods only versus co-fed copepods with rotifers. Aquaculture 2020, 520, 734958. [Google Scholar] [CrossRef]
- Amador-Marrero, U.; Cota-Taylor, R.; Contreras-Olguín, M.; Flores-Montijo, L.; Martínez-Díaz, S.; Cavallin, F.; García, G.; Rodriguez Montes de Oca, G.; Román-Reyes, J.C.; Dumas, S. Effects of prey size, microalgal density, and light intensity on survival of Pacific red snapper larvae. N. Am. J. Aquac. 2023, 85, 95–107. [Google Scholar] [CrossRef]
- Callan, C.K.; Burgess, A.I.; Rothe, C.R.; Touse, R. Development of improved feeding methods in the culture of yellow tang, Zebrasoma flavescens. J. World Aquac. Soc. 2018, 49, 493–503. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Dahms, H.-U.; Hwang, J.-S.; Souissi, S. Recent trends in live feeds for marine larviculture: A mini review. Front. Mar. Sci. 2022, 9, 864165. [Google Scholar] [CrossRef]
- Leu, M.-Y.; Hsu, Y.-C.; Tu, Y.-H.; Chiu, P.-S.; Yu, B.-H.; Wang, J.-B.; Tew, K.S.; Meng, P.-J. Natural spawning, early development and first successful hatchery production of the bluestreak cleaner wrasse, Labroides dimidiatus (Valenciennes, 1839), with application of an inorganic fertilization method in larviculture. Aquaculture 2022, 553, 738056. [Google Scholar] [CrossRef]
- Kendall, A.W., Jr.; Ahlstrom, E.H.; Moser, H.G. Early life history stages of fishes and their characters. In Ontogeny and Systematics of Fishes; Moser, H.G., Richards, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Jr., Richardson, S.L., Eds.; American Society of Ichthyologists and Herpetologists; Allen Press: Lawrence, KS, USA, 1984; pp. 11–22. [Google Scholar]
- Kritsanapuntu, S.; Chaitanawisuti, N.; Santhaweesuk, W.; Natsukari, Y. Effects of stocking density and water exchange regimes on growth and survival of juvenile spotted babylon, Babylona areolata (Link), cultured in experimental earthen ponds. Aquac. Res. 2009, 40, 337–343. [Google Scholar] [CrossRef]
- Chen, J.Y.; Zeng, C. The effects of live prey and greenwater on the early larval rearing of orchid dottyback Pseudochromis fridmani. Aquaculture 2021, 543, 737008. [Google Scholar] [CrossRef]
- Chen, M.; Kim, D.; Liu, H.; Kang, C.-K. Variability in copepod trophic levels and feeding selectivity based on stable isotope analysis in Gwangyang Bay of the southern coast of the Korean Peninsula. Biogeosciences 2018, 15, 2055–2073. [Google Scholar] [CrossRef]
- Moon, S.Y.; Lee, W.; Soh, H.Y. A new species of Bestiolina (Crustacea: Copepoda: Calanoida) from the Yellow Sea, with notes on the zoogeography of the genus. Proc. Biol. Soc. Wash. 2010, 123, 32–46. [Google Scholar] [CrossRef]
- Gatesoupe, F.J.; Luquet, P. Practical diet for mass culture of the rotifer Brachionus plicatilis: Application to larval rearing of sea bass, Dicentrarchus labrax. Aquaculture 1981, 22, 149–163. [Google Scholar] [CrossRef]
- Huang, Y.-H.; Tao, H.-H.; Gong, G.-C.; Hsieh, C.-H. Importance of prey size on investigating prey availability of larval fishes. PLoS ONE 2021, 16, e0251344. [Google Scholar] [CrossRef]
- Riley, K.L.; Binion, S.M.; Overton, A.S. Estimating the food requirements and prey size spectra of larval American shad. Mar. Coast. Fish. 2012, 4, 228–238. [Google Scholar] [CrossRef]
- Munk, P. Prey size spectra and prey availability of larval and small juvenile cod. J. Fish Biol. 1997, 51, 340–351. [Google Scholar] [CrossRef]
- Nagano, N.; Iwatsuki, Y.; Kamiyama, T.; Nakata, H. Effects of marine ciliates on survivability of the first-feeding larval surgeonfish, Paracanthurus hepatus: Laboratory rearing experiments. Hydrobiologia 2000, 432, 149–157. [Google Scholar] [CrossRef]
- Nagano, N.; Iwatsuki, Y.; Kamiyama, T.; Shimizu, H.; Nakata, H. Ciliated protozoans as food for first-feeding larval grouper, Epinephelus septemfasciatus: Laboratory experiment. Plankton Biol. Ecol. 2000, 47, 93–99. [Google Scholar]
- Leu, M.-Y.; Tai, K.-Y.; Meng, P.-J.; Tang, C.-H.; Wang, P.-H.; Tew, K.S. Embryonic, larval and juvenile development of the longfin batfish, Platax teira (Forsskål, 1775) under controlled conditions with special regard to mitigate cannibalism for larviculture. Aquaculture 2018, 493, 204–213. [Google Scholar] [CrossRef]
- Kraul, S. Live Food for Marine Fish Larvae. In Avances en Nutrición Acuícola VIII. VIII Simposium Internacional de Nutrición Acuícola; Cruz Suárez, L.E.R.M.D., Tapia Salazar, M., Nieto López, M.G., Villarreal Cavazos, D.A., Puello Cruz, A.C., García Ortega, A., Eds.; Universidad Autónoma de Nuevo León: Monterrey, Mexico, 2006; pp. 55–61. [Google Scholar]
- de Freitas Côrtes, G.; Tsuzuki, M.Y.; Melo, E.M.C. Monoculture of the ciliate protozoan Euplotes sp.(Ciliophora; Hypotrichia) fed with different diets. Acta Scientiarum. Biol. Sci. 2013, 35, 15–19. [Google Scholar]
- Decamp, O.; Moss, S.; Nagano, N. Live protozoa: Suitable live food for larval fish and shrimp. Glob. Aquac. Advocate 2001, 4, 28–29. [Google Scholar]
- Zingel, P.; Agasild, H.; Karus, K.; Buholce, L.; Nõges, T. Importance of ciliates as food for fish larvae in a shallow sea bay and a large shallow lake. Eur. J. Protistol. 2019, 67, 59–70. [Google Scholar] [CrossRef]
- Rasdi, N.W.; Qin, J.G. Copepod supplementation as a live food improved growth and survival of Asian seabass Lates calcarifer larvae. Aquac. Res. 2018, 49, 3606–3613. [Google Scholar] [CrossRef]
- Blaxter, J. The effect of temperature on larval fishes. Neth. J. Zool. 1992, 42, 336–357. [Google Scholar] [CrossRef]
- Daw, A.L.; Sarkisian, B.L.; Blaylock, R.B.; Saillant, E.A. Removal of Free-Living Ciliates from Stock Cultures of Two Calanoid Copepods with Sodium Hypochlorite. N. Am. J. Aquac. 2021, 83, 381–389. [Google Scholar] [CrossRef]
- Melaku, S.; Geremew, A.; Getahun, A.; Mengestou, S.; Belay, A. Challenges and prospects of using live feed substitutes for larval fish. Fish. Aquat. Sci. 2024, 27, 475–487. [Google Scholar] [CrossRef]
- Støttrup, J. The elusive copepods: Their production and suitability in marine aquaculture. Aquac. Res. 2000, 31, 703–711. [Google Scholar] [CrossRef]
- Davis, D.A.; Derbes, T.J., II; Head, M.E. Culture of Small Zooplankton for the Feeding of Larval Fish; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2018. [Google Scholar]
- Graeb, B.D.; Dettmers, J.M.; Wahl, D.H.; Cáceres, C.E. Fish size and prey availability affect growth, survival, prey selection, and foraging behavior of larval yellow perch. Trans. Am. Fish. Soc. 2004, 133, 504–514. [Google Scholar] [CrossRef]
- Karlsen, Ø.; van der Meeren, T.; Rønnestad, I.; Mangor-Jensen, A.; Galloway, T.F.; Kjørsvik, E.; Hamre, K. Copepods enhance nutritional status, growth and development in Atlantic cod (Gadus morhua L.) larvae—Can we identify the underlying factors? PeerJ 2015, 3, e902. [Google Scholar] [CrossRef]
- van der Meeren, T.; Olsen, R.E.; Hamre, K.; Fyhn, H.J. Biochemical composition of copepods for evaluation of feed quality in production of juvenile marine fish. Aquaculture 2008, 274, 375–397. [Google Scholar] [CrossRef]
- Zeng, C.; Shao, L.; Ricketts, A.; Moorhead, J. The importance of copepods as live feed for larval rearing of the green mandarin fish Synchiropus splendidus. Aquaculture 2018, 491, 65–71. [Google Scholar] [CrossRef]
- Burgess, A.I.; Callan, C.K.; Touse, R.; Delos Santos, M. Increasing survival and growth in larval leopard coral grouper (Plectropomus leopardus) using intensively cultured Parvocalanus crassirostris nauplii. J. World Aquac. Soc. 2020, 51, 171–182. [Google Scholar] [CrossRef]
- Olivotto, I.; Capriotti, F.; Buttino, I.; Avella, A.; Vitiello, V.; Maradonna, F.; Carnevali, O. The use of harpacticoid copepods as live prey for Amphiprion clarkii larviculture: Effects on larval survival and growth. Aquaculture 2008, 274, 347–352. [Google Scholar] [CrossRef]
- Malzahn, A.M.; Ribičić, D.; Hansen, B.H.; Sarno, A.; Kjørsvik, E.; Aase, A.S.N.; Musialak, L.A.; García-Calvo, L.; Hagemann, A. First feed matters: The first diet of larval fish programmes growth, survival, and metabolism of larval ballan wrasse (Labrus bergylta). Aquaculture 2022, 561, 738586. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Souissi, S.; Jepsen, P.M. Live feed for early ontogenetic development in marine fish larvae. Front. Mar. Sci. 2022, 9, 1115275. [Google Scholar] [CrossRef]
- Blaxter, J.H.; Hempel, G. The influence of egg size on herring larvae (Clupea harengus L.). ICES J. Mar. Sci. 1963, 28, 211–240. [Google Scholar] [CrossRef]
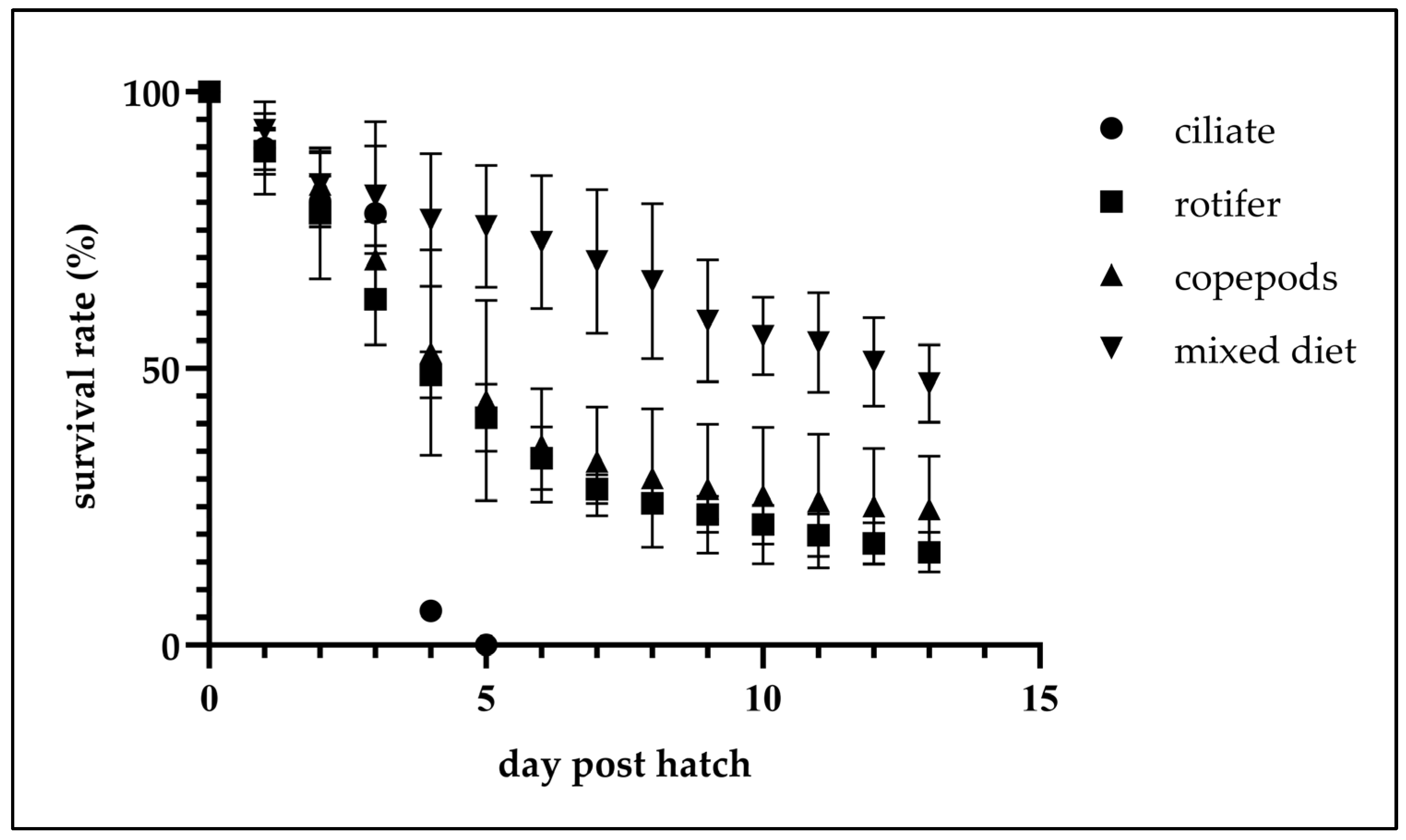
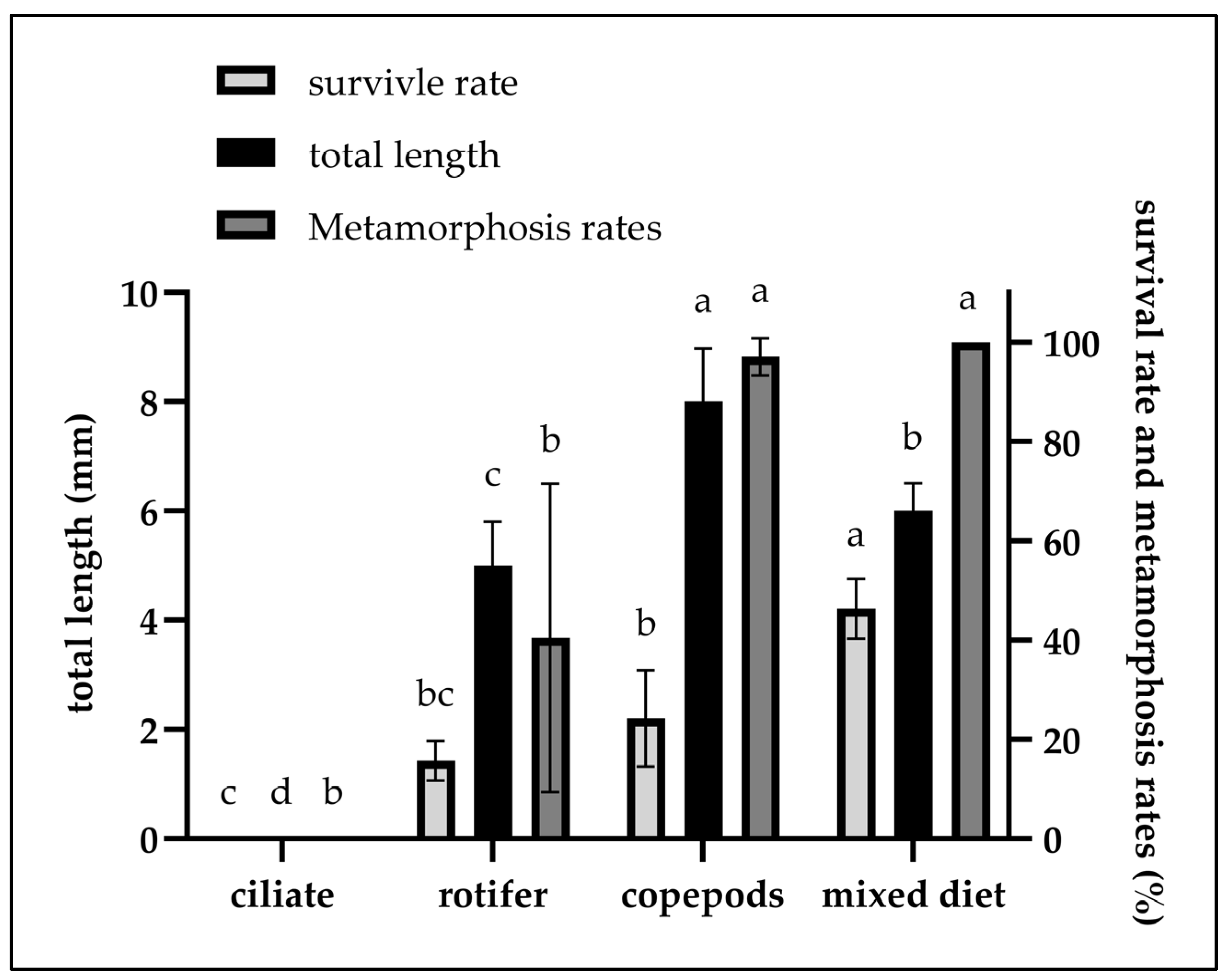
| Treatment | 2–7 dph | 8–10 dph | 11–14 dph |
|---|---|---|---|
| A | E (15–20 ind/mL) | E (15–20 ind/mL) | E (15–20 ind/mL) |
| B | R (15–20 ind/mL) | R (15–20 ind/mL) | R (15–20 ind/mL) |
| C | Cn (15–20 ind/mL) | Cn + Cc (15–20 ind/mL) | Cc (15–20 ind/mL) |
| D | E (5–7 ind/mL) + R (5–7 ind/mL) + Cn (5–7 ind/mL) | R (8–10 ind/mL) + Cn + Cc (8–10 ind/mL) | R (8–10 ind/mL) + Cc (8–10 ind/mL) |
| Treatment | 0 dph | 1 dph | 2 dph | 3 dph | 4 dph | 5 dph | 6 dph | 7 dph | 8 dph | 9 dph | 10 dph | 11 dph | 12 dph | 13 dph | 14 dph |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 100.0 a | 89.9 ± 8.4 a | 80.2 ± 4.6 a | 78.0 ± 16.6 a | 6.2 ± 1.6 b | 0.0 c | |||||||||
| B | 100.0 a | 89.3 ± 4.2 a | 78.0 ± 11.9 a | 62.5 ± 8.3 a | 48.9 ± 4.2 a | 41.1 ± 6.1 b | 33.8 ± 5.7 b | 28.2 ± 2.6 b | 25.6 ± 1.6 b | 23.6 ± 3.3 b | 21.8 ± 3.5 b | 19.9 ± 3.9 b | 18.4 ± 3.8 b | 16.8 ± 3.6 b | 15.7 ± 4.0 b |
| C | 100.0 a | 89.5 ± 3.6 a | 83.3 ± 5.8 a | 69.8 ± 6.8 a | 52.9 ± 18.6 a | 44.2 ± 18.1 b | 36.1 ± 10.3 b | 33.2 ± 9.8 b | 30.2 ± 12.5 b | 28.3 ± 11.9 b | 27.0 ± 12.7 b | 26.0 ± 12.1 b | 25.1 ± 10.4 b | 24.6 ± 9.5 b | 24.2 ± 9.7 b |
| D | 100.0 a | 93.1 ± 3.4 a | 83.3 ± 6.9 a | 81.2 ± 9.9 a | 76.9 ± 12.7 a | 75.7 ± 11.3 a | 72.9 ± 12.6 a | 69.4 ± 11.0 a | 65.8 ± 14.2 a | 58.6 ± 11.3 a | 55.9 ± 7.7 a | 54.7 ± 9.0 a | 51.2 ± 8.9 a | 47.3 ± 7.8 a | 46.3 ± 6.5 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.-H.; Lin, Y.-R.; Hsieh, H.-Y.; Meng, P.-J. Early Feeding Strategies for the Larviculture of the Vermiculated Angelfish Chaetodontoplus mesoleucus: The Key Role of Copepods. Animals 2025, 15, 2437. https://doi.org/10.3390/ani15162437
Sun Y-H, Lin Y-R, Hsieh H-Y, Meng P-J. Early Feeding Strategies for the Larviculture of the Vermiculated Angelfish Chaetodontoplus mesoleucus: The Key Role of Copepods. Animals. 2025; 15(16):2437. https://doi.org/10.3390/ani15162437
Chicago/Turabian StyleSun, Yu-Hsuan, Yu-Ru Lin, Hung-Yen Hsieh, and Pei-Jie Meng. 2025. "Early Feeding Strategies for the Larviculture of the Vermiculated Angelfish Chaetodontoplus mesoleucus: The Key Role of Copepods" Animals 15, no. 16: 2437. https://doi.org/10.3390/ani15162437
APA StyleSun, Y.-H., Lin, Y.-R., Hsieh, H.-Y., & Meng, P.-J. (2025). Early Feeding Strategies for the Larviculture of the Vermiculated Angelfish Chaetodontoplus mesoleucus: The Key Role of Copepods. Animals, 15(16), 2437. https://doi.org/10.3390/ani15162437








