Genetic Diversity and Distribution of Italian Cave Crickets (Dolichopoda): Toward a Better Understanding of Lineage Structure
Simple Summary
Abstract
1. Introduction
2. Material and Methods
2.1. Sampling
2.2. DNA Extraction, Amplification, and Sequencing
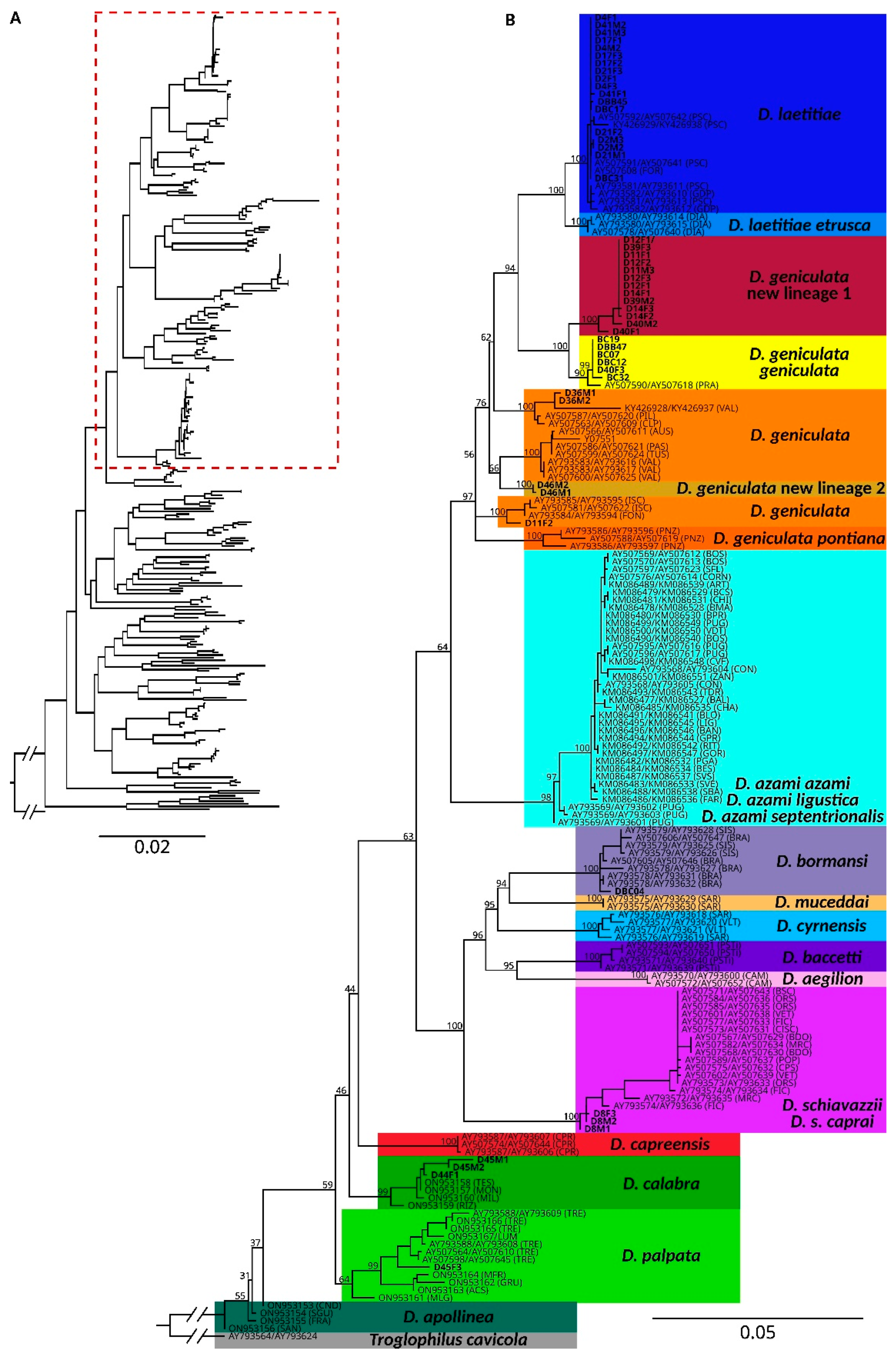
3. Result
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Allegrucci, G.; Sbordoni, V. Insights into the Molecular Phylogeny of Rhaphidophoridae, an Ancient, Worldwide Lineage of Orthoptera. Mol. Phylogenet. Evol. 2019, 138, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Di Russo, C.; Allegrucci, G.; Rampini, M. Molecular and Morphological Analyses Disclose the Existence of Three Species of Dolichopoda (Orthoptera: Rhaphidophoridae) in the Calabria Region (Italy). J. Nat. Hist. 2023, 57, 372–394. [Google Scholar] [CrossRef]
- Cigliano, M.M.; Braun, H.; Eades, D.C.; Otte, D. Orthoptera Species File. Version 5.0/5.0. Available online: http://orthoptera.speciesfile.org (accessed on 30 May 2025).
- Allegrucci, G.; Rampini, M.; Chimenti, C.; Alexiou, S.; Di Russo, C. Dolichopoda Cave Crickets from Peloponnese (Orthoptera, Rhaphidophoridae): Molecular and Morphological Investigations Reveal Four New Species for Greece. Eur. Zool. J. 2021, 88, 505–524. [Google Scholar] [CrossRef]
- Iorio, C.; Scherini, R.; Fontana, P.; Buzzetti, F.; Kleukers, R.; Odé, B.; Massa, B. Grasshoppers & Crickets of Italy. A Photographic Field Guide to all the Species; World Biodiversity Association: Verona, Italy, 2019; Volume 10, pp. 464–467. [Google Scholar]
- Carchini, G.; Rampini, M.; Sbordoni, V. Life Cycle and Population Ecology of the Cave Cricket Dolichopoda Geniculata (Costa) from Valmarino Cave (Central Italy). Int. J. Speleol. 1994, 23, 203–218. [Google Scholar] [CrossRef]
- Allegrucci, G.; Trucchi, E.; Sbordoni, V. Tempo and Mode of Species Diversification in Dolichopoda Cave Crickets (Orthoptera, Rhaphidophoridae). Mol. Phylogenet. Evol. 2011, 60, 108–121. [Google Scholar] [CrossRef]
- Allegrucci, G.; Todisco, V.; Sbordoni, V. Molecular Phylogeography of Dolichopoda Cave Crickets (Orthoptera, Rhaphidophoridae): A Scenario Suggested by Mitochondrial DNA. Mol. Phylogenet. Evol. 2005, 37, 153–164. [Google Scholar] [CrossRef]
- Allegrucci, G.; Rampini, M.; Di Russo, C.; Lana, E.; Cocchi, S.; Sbordoni, V. Phylogeography and Systematics of the Westernmost Italian Dolichopoda Species (Orthoptera, Rhaphidophoridae). Zookeys 2014, 437, 1–23. [Google Scholar] [CrossRef]
- Massa, B.; Fontana, P. Endemism in Italian Orthoptera. Biodivers. J. 2020, 11, 405–434. [Google Scholar] [CrossRef]
- Allegrucci, G.; Minasi, M.G.; Sbordoni, V. Patterns of Gene Flow and Genetic Structure in Cave-Dwelling Crickets of the Tuscan Endemic, Dolichopoda Schiavazzii (Orthoptera, Rhaphidophoridae). Heredity 1997, 78, 665–673. [Google Scholar] [CrossRef]
- Menchetti, M.; Talavera, G.; Cini, A.; Salvati, V.; Dincă, V.; Platania, L.; Bonelli, S.; Balletto, E.; Vila, R.; Dapporto, L. Two Ways to Be Endemic. Alps and Apennines Are Different Functional Refugia during Climatic Cycles. Mol. Ecol. 2021, 30, 1297–1310. [Google Scholar] [CrossRef] [PubMed]
- Bogdanowicz, W.; Hulva, P.; Černá Bolfíková, B.; Buś, M.M.; Rychlicka, E.; Sztencel-Jabłonka, A.; Cistrone, L.; Russo, D. Cryptic Diversity of Italian Bats and the Role of the Apennine Refugium in the Phylogeography of the Western Palaearctic. Zool. J. Linn. Soc. 2015, 174, 635–648. [Google Scholar] [CrossRef]
- Canestrelli, D.; Sacco, F.; Nascetti, G. On Glacial Refugia, Genetic Diversity, and Microevolutionary Processes: Deep Phylogeographical Structure in the Endemic Newt Lissotriton Italicus. Biol. J. Linn. Soc. 2012, 105, 42–55. [Google Scholar] [CrossRef]
- Berrilli, E.; Biondi, M.; Garzia, M.; D’Alessandro, P.; Salvi, D. Apennine-Pyrenees Disjunct Distribution: An Unusual Biogeographic Pattern Revealed in Flea Beetles of the Longitarsus Candidulus Species-Group (Coleoptera, Chrysomelidae). Curr. Zool. 2023, 70, 668–677. [Google Scholar] [CrossRef]
- Clark, J.A.; May, R.M. Taxonomic Bias in Conservation Research. Science 2002, 297, 191–192. [Google Scholar] [CrossRef]
- Fattorini, S. Biotope Prioritisation in the Central Apennines (Italy): Species Rarity and Cross-Taxon Congruence. Biodivers. Conserv. 2010, 19, 3413–3429. [Google Scholar] [CrossRef]
- Sanbrook, J.; Fritsch, E.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Habour Laboratory: Ithaca, NY, USA, 1989; Volume 11, p. 31. [Google Scholar]
- Salvi, D.; D’Alessandro, P.; Biondi, M. Host Plant Associations in Western Palaearctic Longitarsus Flea Beetles (Chrysomelidae, Galerucinae, Alticini): A Preliminary Phylogenetic Assessment. ZooKeys 2019, 856, 101–114. [Google Scholar] [CrossRef]
- Lunt, D.; Zhang, D.-X.; Szymura, J.M.; Hewltt, O. The Insect Cytochrome Oxidase I Gene: Evolutionary Patterns and Conserved Primers for Phylogenetic Studies. Insect Mol. Biol. 1996, 5, 153–165. [Google Scholar] [CrossRef]
- Palumbi, S.; Martin, A.; Romano, S.; McMillan, W.; Stice, L.; Grabowski, G. The Simple Fool’s Guide to PCR, Version 2.0; University of Hawaii: Honolulu, HI, USA, 1991; Volume 45. [Google Scholar]
- Salvi, D.; Berrilli, E.; D’Alessandro, P.; Biondi, M. Sharpening the DNA Barcoding Tool through a Posteriori Taxonomic Validation: The Case of Longitarsus Flea Beetles (Coleoptera: Chrysomelidae). PLoS ONE 2020, 15, e0233573. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A Novel Method for Rapid Multiple Sequence Alignment Based on Fast Fourier Transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; Von Haeseler, A.; Minh, B.Q. IQ-TREE: A Fast and Effective Stochastic Algorithm for Estimating Maximum-Likelihood Phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.-T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A Fast Online Phylogenetic Tool for Maximum Likelihood Analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Allegrucci, G.; Rampini, M.; Gratton, P.; Todisco, V.; Sbordoni, V. Testing Phylogenetic Hypotheses for Reconstructing the Evolutionary History of Dolichopoda Cave Crickets in the Eastern Mediterranean. J. Biogeogr. 2009, 36, 1785–1797. [Google Scholar] [CrossRef]
- Allegrucci, G.; Ketmaier, V.; Di Russo, C.; Rampini, M.; Sbordoni, V.; Cobolli, M. Molecular Phylogeography of Troglophilus Cave Crickets (Orthoptera, Rhaphidophoridae): A Combination of Vicariance and Dispersal Drove Diversification in the East Mediterranean Region. J. Zool. Syst. Evol. Res. 2017, 55, 310–325. [Google Scholar] [CrossRef]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.F.; von Haeseler, A.; Jermiin, L.S. ModelFinder: Fast Model Selection for Accurate Phylogenetic Estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast Approximation for Phylogenetic Bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Hoang, D.T.; Chernomor, O.; Von Haeseler, A.; Minh, B.Q.; Vinh, L.S. UFBoot2: Improving the Ultrafast Bootstrap Approximation. Mol. Biol. Evol. 2018, 35, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Rambaut, A.; Drummond, A. FigTree. Version 1.3. 1 [Computer Program]. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 21 October 2009).
- Meier, G.; Scimè, P.; Kistler, P. Prima segnalazione di Dolichopoda geniculata (O.G. Costa, 1836) in Svizzera (Orthoptera, Rhaphidophoridae). Boll. Della Soc. Ticin. Di Sci. Nat. 2013, 101, 109–112. [Google Scholar]
- Berrilli, E.; Biondi, M.; D’Alessandro, P.; Salvi, D. Cryptic, Sibling or Neither of the Two? Integrative Species Delimitation of Psylliodes Flea Beetles with Overlapping Ranges. Zool. Scr. 2023, 52, 235–248. [Google Scholar] [CrossRef]
- Martinsen, L.; Venanzetti, F.; Bachmann, L. Phylogeography and Mitochondrial DNA Divergence in Dolichopoda Cave Crickets (Orthoptera, Rhahidophoridae). Hereditas 2009, 146, 33–45. [Google Scholar] [CrossRef]
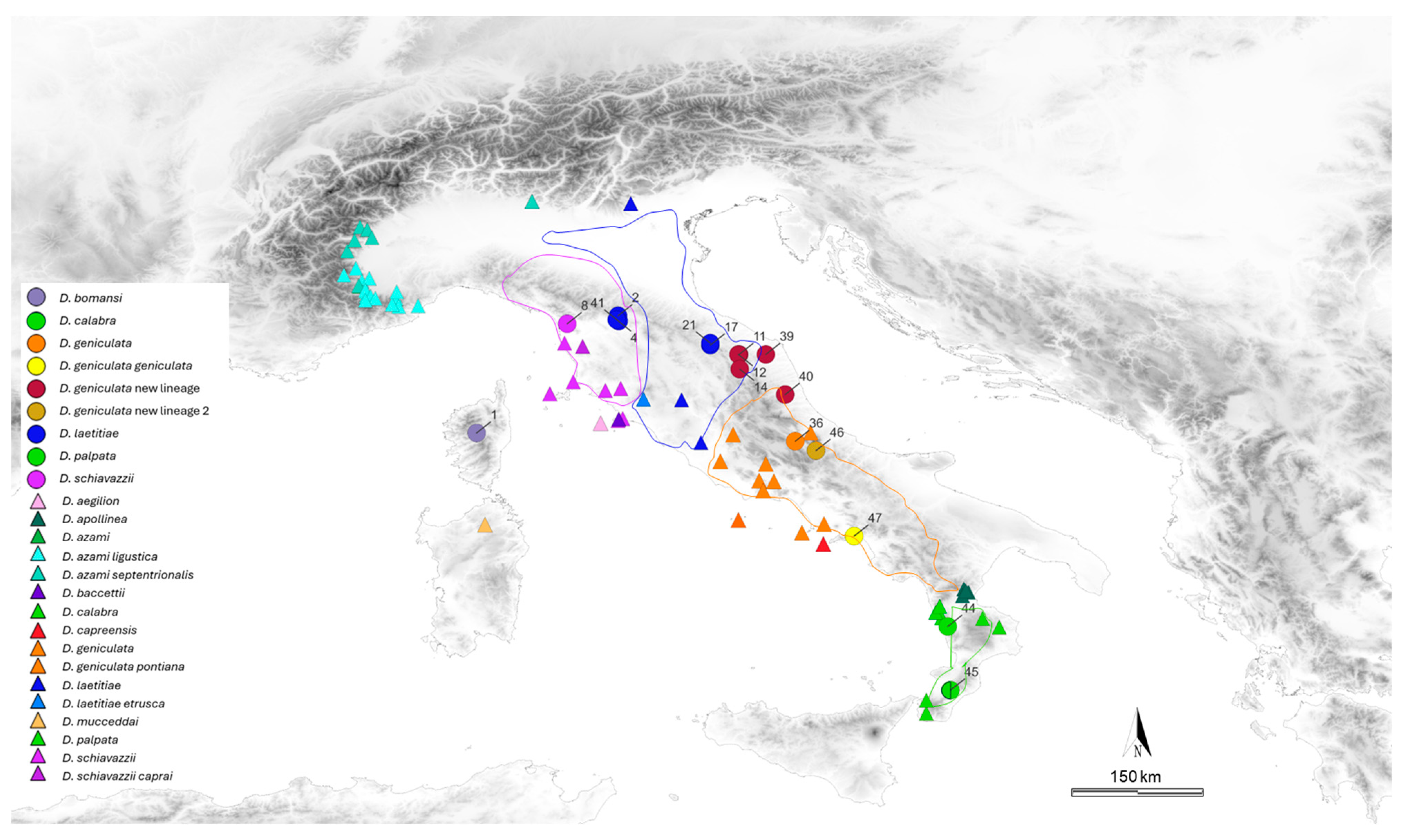
| Locality ID | Locality | Species | N | Lat (°) | Lon (°) |
|---|---|---|---|---|---|
| 1 | Restonica | D. bormansi | 1 | 42.22 | 9.05 |
| 2 | Tasso | D. laetitiae | 3 | 43.98 | 11.16 |
| 4 | Forra | D. laetitiae | 3 | 43.92 | 11.14 |
| 8 | Maggiano | D. schiavazzii | 3 | 43.86 | 10.4 |
| 11 | Solstizio | D. geniculata | 3 | 43.4 | 12.97 |
| 12 | Grotta Leonardo | D. geniculata | 4 | 43.4 | 12.96 |
| 14 | Vurgacci | D. geniculata | 3 | 43.18 | 12.98 |
| 17 | Grotta Nottole | D. laetitiae | 3 | 43.54 | 12.54 |
| 21 | Grotta Borghetto | D. laetitiae | 3 | 43.57 | 12.54 |
| 36 | Uccole | D. geniculata | 3 | 42.1 | 13.81 |
| 39 | Grotta Bella | D. geniculata | 3 | 43.4 | 13.37 |
| 40 | Pozzo Cambiano | D. geniculata | 3 | 42.8 | 13.66 |
| 41 | Torri | D. laetitiae | 3 | 43.9 | 11.17 |
| 44 | San Fili | D. calabra | 1 | 39.33 | 16.09 |
| 45 | Calcari | D. calabra | 3 | 38.38 | 16.13 |
| 45 | Calcari | D. palpata | 1 | 38.38 | 16.13 |
| 46 | Cava de Tirreni | D. geniculata | 1 | 40.68 | 14.69 |
| 46 | Maiella | D. geniculata | 2 | 41.96 | 14.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garzia, M.; Berrilli, E.; Lunghi, E.; Coppari, L.; Delcour, N.; Salvi, D. Genetic Diversity and Distribution of Italian Cave Crickets (Dolichopoda): Toward a Better Understanding of Lineage Structure. Animals 2025, 15, 2429. https://doi.org/10.3390/ani15162429
Garzia M, Berrilli E, Lunghi E, Coppari L, Delcour N, Salvi D. Genetic Diversity and Distribution of Italian Cave Crickets (Dolichopoda): Toward a Better Understanding of Lineage Structure. Animals. 2025; 15(16):2429. https://doi.org/10.3390/ani15162429
Chicago/Turabian StyleGarzia, Matteo, Emanuele Berrilli, Enrico Lunghi, Luca Coppari, Nathan Delcour, and Daniele Salvi. 2025. "Genetic Diversity and Distribution of Italian Cave Crickets (Dolichopoda): Toward a Better Understanding of Lineage Structure" Animals 15, no. 16: 2429. https://doi.org/10.3390/ani15162429
APA StyleGarzia, M., Berrilli, E., Lunghi, E., Coppari, L., Delcour, N., & Salvi, D. (2025). Genetic Diversity and Distribution of Italian Cave Crickets (Dolichopoda): Toward a Better Understanding of Lineage Structure. Animals, 15(16), 2429. https://doi.org/10.3390/ani15162429








