Effect of Epidermal Growth Factor and 6-Dimethylaminopurine on In Vitro Maturation and Artificial Activation of Spix’s Yellow-Toothed Cavy (Galea spixii Wagler, 1831) Oocytes
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Media
2.2. Bioethics and Animals
2.3. Collection, Transport of Ovaries, and Recovery of the Cumulus–Oocyte Complexes
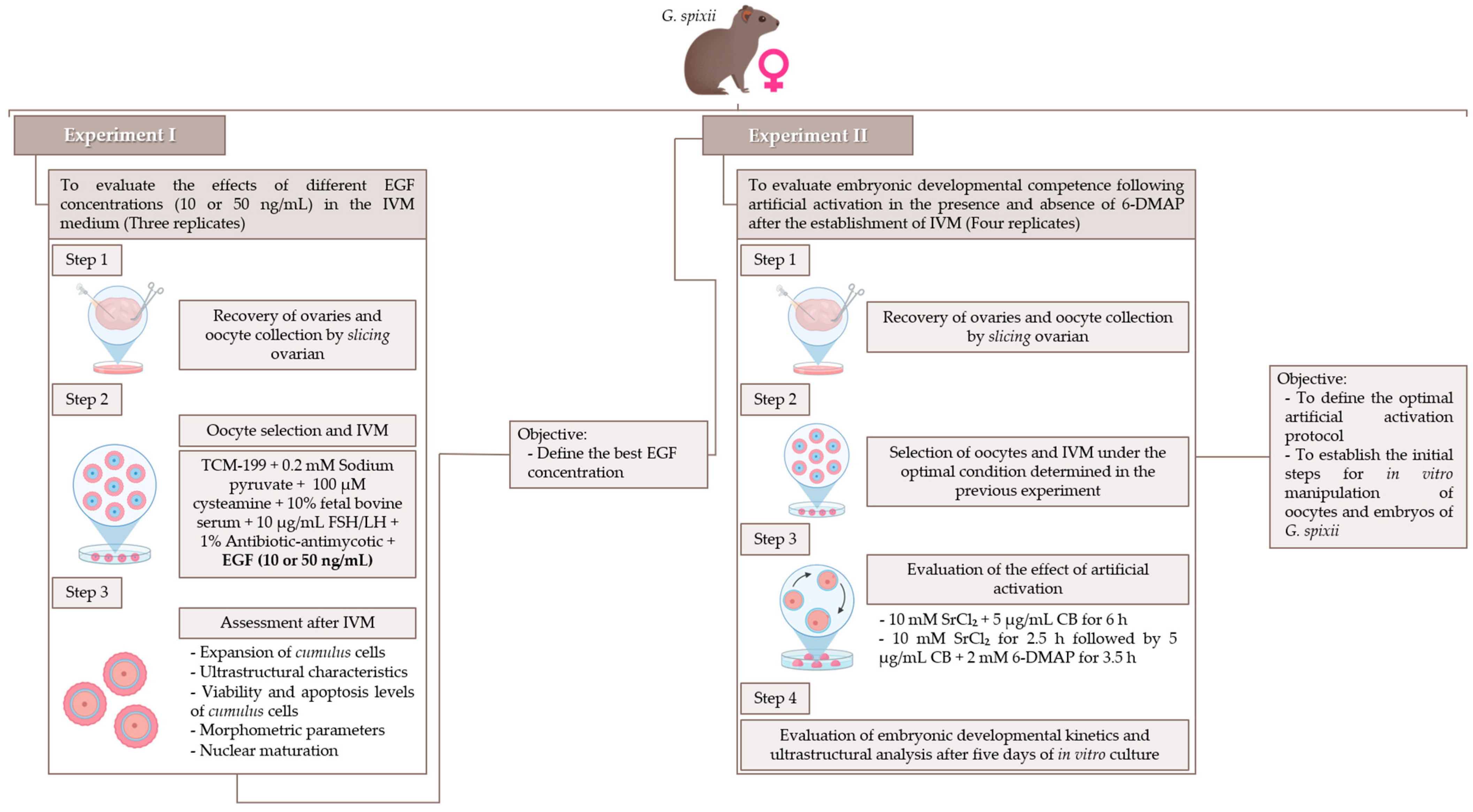
2.4. Experimental Design
2.5. In Vitro Maturation of Oocytes
2.6. Assessments of the Microenvironment and Oocyte Maturation
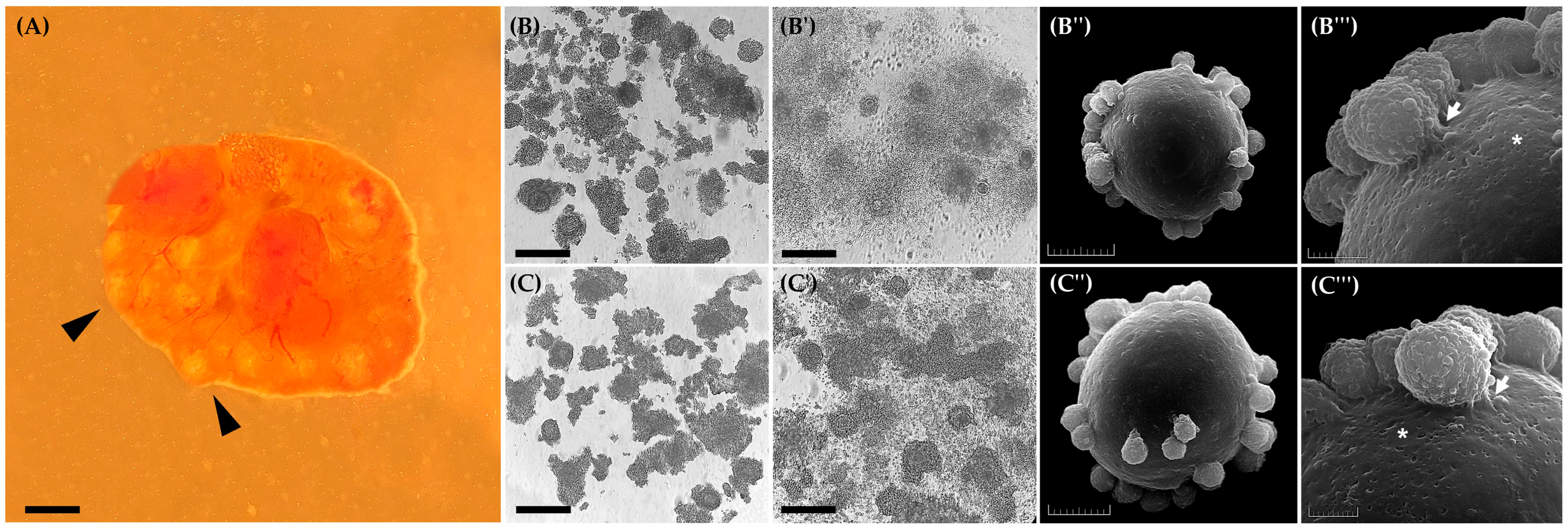
2.6.1. Expansion of Cumulus Cells
2.6.2. Ultrastructure Assessment by SEM
2.6.3. Viability and Apoptotic Levels of Cumulus Cells
2.6.4. Morphometric and 1PB Evaluation of Oocytes
2.6.5. Nuclear Stage Assessment
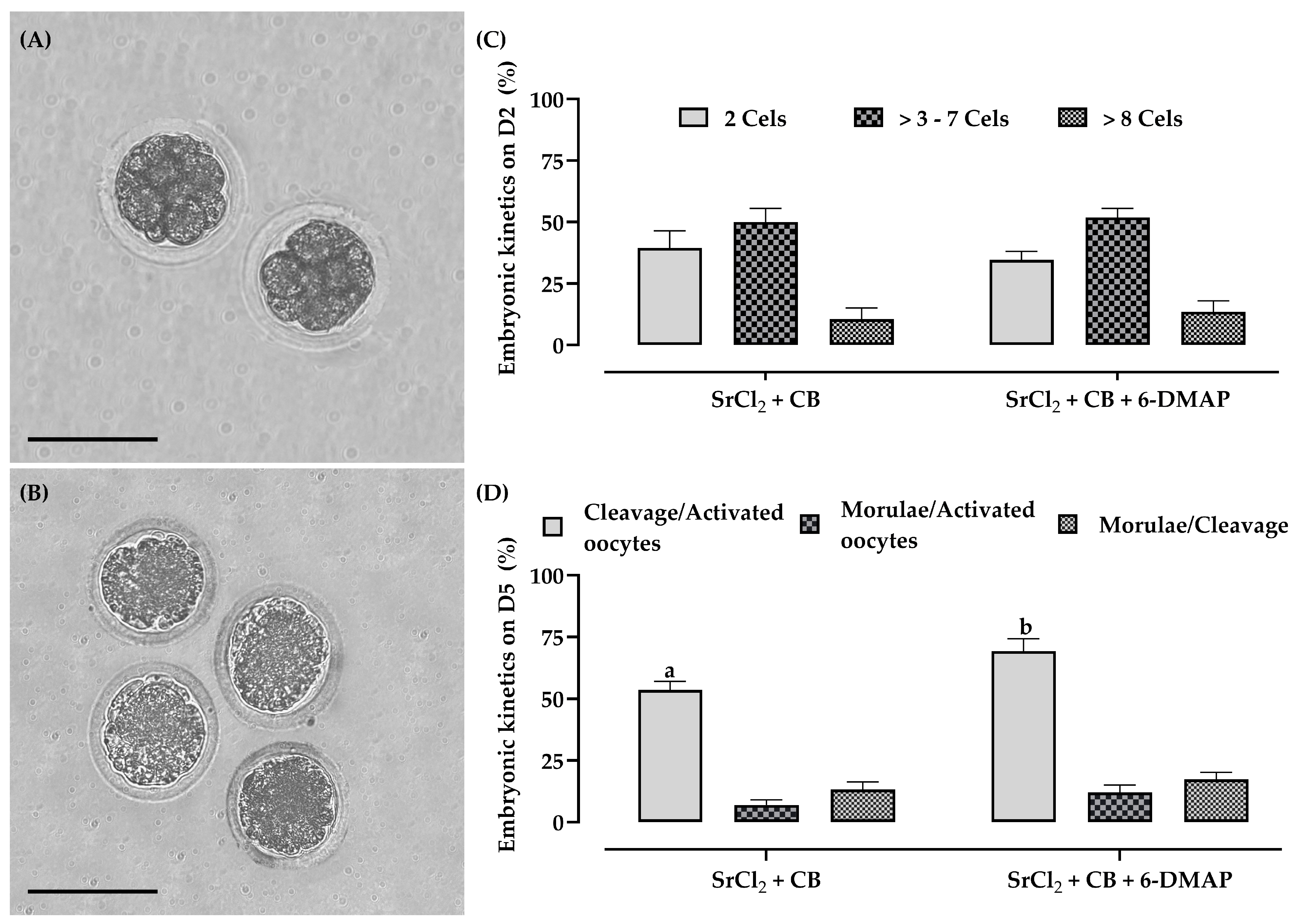
2.7. Artificial Activation, Ultrastructure Analysis, and Embryonic Kinetic Patterns
2.8. Statistical Analysis
3. Results
3.1. Experiment I: Effect of EGF on Cumulus Cells Expansion and Ultrastructural Visualization of Oocytes
3.2. Experiment I: EGF Effect on Viability and Apoptotic Levels of Cumulus Cells
3.3. Experiment I: Effect of EGF on Morphometric Parameters and Nuclear Maturation
3.4. Experiment II: Assessment of the Microenvironment and Quality of Oocytes After IVM
3.5. Experiment II: Effect of 6-DMAP on Activation and Kinetics of Embryonic Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1PB | First polar body |
| 6-DMAP | 6-dimethylaminopurine |
| ARTs | Assisted reproductive technologies |
| BSA | BSA bovine serum albumin |
| CB | Cytochalasin B |
| CEI | Cumulus expansion index |
| CEMAS | Centre of Multiplication of Wild Animals |
| CEUA | Animal Use Ethics Committee |
| COCs | Cumulus–oocyte complexes |
| EGF | Epidermal growth factor |
| GVBD | Oocyte in the germinal vesicle breakdown |
| hCG | Chorionic gonadotropin hormones |
| IOA | Internal oocyte area |
| IOD | Inner oocyte diameter |
| IVM | In vitro maturation |
| KSOM | Simple, optimized, and potassium-enriched medium |
| MPF | Meiosis-promoting factor |
| OA | Ooplasm area |
| OD | Ooplasm diameter |
| OOD | Outer oocyte diameter |
| ORM | Oocyte recovery medium |
| PBS | Phosphate buffer solutions |
| PSA | Perivitelline space area |
| ROS | Reactive oxygen species |
| SCNT | Somatic cell nuclear transfer |
| SEM | Scanning electron microscopy |
| SPD | Space perivitelline diameter |
| SrCl2 | Strontium chloride |
| TALP | Tyrode’s albumin lactate pyruvate |
| UFERSA | Federal Rural University of Semi-Arid |
References
- Mendes, B.V. Plants and Animals for the Northeast, 1st ed.; Globo: Rio de Janeiro, Brazil, 1987. [Google Scholar]
- Silva, A.M.; Moreira, S.S.J.; Silva, A.R. The cavies. In Assisted Reproduction in Wild Mammals of South America (VitalSource Bookshelf), 1st ed.; Silva, A.R., Ed.; Taylor & Francis: São Paulo, Brazil, 2024; pp. 235–248. [Google Scholar] [CrossRef]
- Vale, A.M.; Oliveira, G.B.; Júnior, H.N.A.; Bezerra, F.V.F.; Sousa, A.C.F.C.; Diniz, J.A.R.A.; Lopes, I.R.G.; Oliveira, M.F. Gestational period and reproductive cycle in Spix’s yellow-toothed cavy (Galea spixii Wagler, 1831). Cienc. Anim. Bras. 2023, 24, e76803P. [Google Scholar] [CrossRef]
- Nowak, R.M. Walker’s Mammals of the World, 6th ed.; The Johns Hopkins University Press: Baltimore, MD, USA, 1999; Volume II. [Google Scholar]
- Rowe, D.L.; Honeycutt, R.L. Phylogenetic relationships, ecological correlates, and molecular evolution within the Cavioidea (Mammalia, Rodentia). Mol. Biol. Evol. 2002, 19, 263–277. [Google Scholar] [CrossRef]
- Fernandes, F.A.; Fernández-Stolz, G.P.; Lopes, C.M.; Freitas, T.R.O. The conservation status of the tucos-tucos, genus Ctenomys (Rodentia: Ctenomyidae), in southern Brazil. Braz. J. Biol. 2007, 67, 839–847. [Google Scholar] [CrossRef]
- Catzeflis, F.; Patton, J.; Percequillo, A.; Weksler, M. Galea spixii. The IUCN Red List of Threatened Species; International Union for Conservation of Nature: Gland, Switzerland, 2016. [CrossRef]
- Adams, L.; Liu, Y.; Polejaeva, I.A. Current status of interspecies somatic cell nuclear transfer and meta-analysis of the effects of phylogenetic distance on embryonic and fetal development. Mammal Rev. 2024, 54, 387–403. [Google Scholar] [CrossRef]
- Praxedes, E.A.; Santos, M.V.O.; Oliveira, L.R.M.; Aquino, L.V.C.; Oliveira, M.F.; Pereira, A.F. Synergistic effects of follicle-stimulating hormone and epidermal growth factor on in vitro maturation and parthenogenetic development of red-rumped agouti oocytes. Reprod. Domest. Anim. 2023, 58, 1368–1378. [Google Scholar] [CrossRef]
- Cowl, V.B.; Comizzoli, P.; Appeltant, R.; Bolton, R.L.; Browne, R.K.; Holt, W.V.; Penfold, L.M.; Swegen, A.; Walker, S.L.; Williams, S.A. Cloning for the Twenty-First Century and its place in endangered species conservation. Annu. Rev. Anim. Biosci. 2024, 12, 91–112. [Google Scholar] [CrossRef]
- Pereira, A.F.; Oliveira, L.R.M.; Aquino, L.V.C.; Viana, J.V.S.; Rodrigues, L.L.V. Strategies for the Establishment of Fibroblastic Lines for the Conservation of Wild Mammals. In Theriogenology-Recent Advances in the Field, 1st ed.; Silva, A.R., Pereira, A.F., Eds.; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Men, H. Evolution of media supporting the development of mammalian preimplantation embryos in vitro. Biology 2024, 13, 789. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Sun, Q.; Xia, S.; Cui, J.; Yang, L.; An, L.; Zhang, J.; Su, L.; Su, Y.; et al. Combined treatment with cysteamine and leukemia inhibitory factor promotes guinea pig oocyte meiosis in vitro. Am. J. Transl. Res. 2019, 15, 7479–7491. [Google Scholar]
- Yao, M.; Gong, Z.; Xu, W.; Shi, X.; Liu, X.; Tang, Y.; Xuan, S.; Su, Y.; Xu, X.; Luo, M.; et al. Establishment and optimization of an in vitro guinea pig oocyte maturation system. PLoS ONE 2023, 18, e0285016. [Google Scholar] [CrossRef]
- Downs, S.M.; Daniel, S.A.; Eppig, J.J. Induction of maturation in cumulus cell-enclosed mouse oocytes by follicle-stimulating hormone and epidermal growth factor: Evidence for a positive stimulus of somatic cell origin. J. Exp. Zool. 1988, 245, 86–96. [Google Scholar] [CrossRef]
- Downs, S.M.; Chen, J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol. Reprod. Dev. 2008, 75, 105–114. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Lane, M.; Thompson, J.G. Oocyte-secreted factors: Regulators of cumulus cell function and oocyte quality. Hum. Reprod. Update. 2008, 14, 159–177. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. The epidermal growth factor network: Role in oocyte growth, maturation and developmental competence. Hum. Reprod. Update. 2018, 24, 1–14. [Google Scholar] [CrossRef]
- Cañón-Beltrán, K.; García-García, R.M.; Cajas, Y.N.; Fierro, N.; Lorenzo, P.I.; Arias-Álvares, M. Improvement of oocyte competence and in vitro oocyte maturation with EGF and IGF-I in guinea pig model. Theriogenology 2024, 15, 206–214. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Miao, D.; Han, Z.; Zhang, X.; Miao, Y.; Yanagimachi, R.; Tan, J. Parthenogenetic activation of mouse oocytes by strontium chloride: A search for the best conditions. Theriogenology 2005, 64, 1142–1157. [Google Scholar] [CrossRef]
- Bezerra, F.V.F.; Favaron, P.O.; Mess, A.M.; Júnior, H.N.A.; Oliveira, G.B.; Pereira, A.F.; Miglino, M.A.; Oliveira, M.F. Subplacental development in Galea spixii. Pesq. Vet. Bras. 2018, 38, 2175–2182. [Google Scholar] [CrossRef]
- Oliveira, L.R.M.; Aquino, L.V.C.; Pereira, A.B.M.; Rodrigues, A.L.R.; Rodrigues, L.L.V.; Bezerra, L.G.P.; Oliveira, M.F.; Silva, A.R.; Pereira, A.F. Comparative analysis of sperm selection methods and their impact on in vitro fertility in the red-rumped agouti (Dasyprocta leporina). Res. Vet. Sci. 2025, 193, 105756. [Google Scholar] [CrossRef]
- Yao, M.; Cheng, W.; Liu, L.; Zheng, H.; Gu, W.; Miao, F.; Zhang, J.; Wang, L.; Su, Y.; Liu, Y.; et al. Relationship between chromatin configuration and in vitro maturation ability in guinea pig oocytes. Vet. Med. Sci. 2021, 7, 2410–2417. [Google Scholar] [CrossRef]
- Zhao, H.; Ge, J.; Wei, J.; Liu, J.; Liu, C.; Ma, C.; Zhao, X.; Wei, Q.; Ma, B. Effect of FSH on E2/GPR30-mediated mouse oocyte maturation in vitro. Cell. Signal. 2020, 66, 109464. [Google Scholar] [CrossRef]
- Fagbohun, F.C.; Downs, S.M. Maturation of the mouse oocyte-cumulus cell complex: Stimulation by lectins. Biol. Reprod. 1990, 42, 413–423. [Google Scholar] [CrossRef]
- Li, C.; Wang, Z.; Zheng, Z.; Hu, L.; Zhong, S.; Lei, L. Number of blastomeres and distribution of microvilli in cloned mouse embryos during compaction. Zygote 2010, 19, 271–276. [Google Scholar] [CrossRef]
- Rodrigues, I.S.R.; Coelho, P.S.A.; Sousa, A.J.O.; Pantoja, L.C.; Nascimento, H.S.; Rodrigues, A.L.; Aleixo, L.C.; Miranda, M.S. Effects of hydroxychloroquine on in vitro maturation of bovine oocytes. In Proceedings of the 36th Annual Meeting of the Brazilian Embryo Technology Society (SBTE), Campinas, Brazil, 9–12 August 2023. [Google Scholar]
- Takahashi, A.; Matsumoto, H.; Yuki, K.; Yasumoto, J.; Kajiwara, M.; Aoki, Y.; Furusawa, K.; Ohnishi, T. High-LET radiation enhanced apoptosis but not necrosis regardless of p53 status. Int. J. Radiat. Oncol. Biol. Phys. 2004, 60, 591–597. [Google Scholar] [CrossRef]
- Aquino, L.V.C.; Olindo, S.L.; Silva, Y.L.F.; Oliveira, L.R.M.; Moura, Y.B.F.; Rodrigues, A.L.R.; Praxedes, E.A.; Oliveira, M.F.; Silva, A.R.; Pereira, A.F. Cryopreservation and passaging optimization for Galea spixii (Wagler, 1831) adult skin fibroblast lines: A step forward in species management and genetic studies. Acta Histochem. 2024, 126, 152185. [Google Scholar] [CrossRef]
- Borges, A.A.; Santos, M.V.O.; Nascimento, L.E.; Lira, G.P.O.; Praxedes, E.A.; Oliveira, M.F.; Silva, A.R.; Pereira, A.F. Production of collared peccary (Pecari tajacu Linnaeus, 1758) parthenogenic embryos following different oocyte chemical activation and in vitro maturation conditions. Theriogenology 2020, 142, 320–327. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Swelum, A.A.; Yaqoob, S.H.; Alowaimer, A.N. Morphometric assessment of in vitro matured dromedary camel oocytes determines the developmental competence after parthenogenetic activation. Theriogenology 2017, 95, 141–148. [Google Scholar] [CrossRef]
- Szollosi, M.S.; Kubiak, J.Z.; Debey, P.; Pennart, H.; Szolli, D.; Maro, B. Inhibition of protein kinases by 6-dimethylaminopurine accelerates the transition to interphase in activated mouse oocytes. J. Cell Sci. 1993, 104, 861–872. [Google Scholar] [CrossRef]
- Moses, R.M.; Masui, Y. Enhancement of mouse egg activation by the kinase inhibitor, 6-dimethylaminopurine (6-DMAP). J. Exp. Zool. 1994, 270, 211–218. [Google Scholar] [CrossRef]
- Praxedes, E.C.G.; Lima, G.L.; Silva, A.M.; Apolinário, C.A.C.; Bezarra, J.A.B.; Souza, A.L.P.; Oliveira, M.F.; Rodrigues, A.P.R.; Silva, A.R. Characterization and cryopreservation of the ovarian preantral follicle population from Spix’s yellow-toothed cavies (Galea spixii Wagler, 1831). Reprod. Fertil. Dev. 2017, 29, 594–602. [Google Scholar] [CrossRef]
- Santos, E.A.A.; Lima, G.L.; Praxedes, E.C.G.; Silva, A.M.; Maia, K.M.; Oliveira, M.F.; Rodrigues, A.P.R.; Silva, A.R. Estimation, morphometry and ultrastructure of ovarian preantral follicle population in agouti (Dasyprocta leporina). Pesq. Vet. Bras. 2018, 38, 175–182. [Google Scholar] [CrossRef]
- Kim, K.; Lee, H.; Threadgill, D.W.; Lee, D. Epiregulin-dependent amphiregulin expression and ERBB2 signaling are involved in luteinizing hormone-induced paracrine signaling pathways in mouse ovary. Biochem. Biophys. Res. Commun. 2011, 405, 319–324. [Google Scholar] [CrossRef]
- Chen, J.; Torcia, S.; Xie, F.; Lin, C.; Cakmak, H.; Franciosi, F.; Horner, K.; Onodera, C.; Sonda, J.S.; Cedars, M.I.; et al. Somatic cells regulate maternal mRNA translation and developmental competence of mouse oocytes. Nat. Cell Biol. 2013, 15, 1415–1423. [Google Scholar] [CrossRef]
- Zhang, M.; Su, Y.Q.; Sugiura, K.; Xia, G.; Eppig, J.J. Granulosa cell ligand NPPC and its receptor NPR2 maintain meiotic arrest in mouse oocytes. Science 2010, 330, 366–369. [Google Scholar] [CrossRef]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Richani, D.; Sutton-McDowall, M.L.; Frank, L.A.; Gilchrist, R.B.; Thompson, J.G. Effect of epidermal growth factor-like peptides on the metabolism of in vitro-matured mouse oocytes and cumulus cells. Biol. Reprod. 2014, 90, 49. [Google Scholar] [CrossRef]
- Richani, D.; Wang, X.; Zeng, H.T.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol. Reprod. Dev. 2014, 81, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Pantaleon, M.; Tan, H.Y.; Kafer, G.R.; Kaye, P.L. Toxic effects of hyperglycemia are mediated by the hexosamine signaling pathway and o-linked glycosylation in early mouse embryos. Biol. Reprod. 2010, 82, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Frank, L.A.; Sutton-McDowall, M.L.; Brown, H.M.; Russel, D.L.; Gilchrist, R.B.; Thompson, T.G. Hyperglycaemic conditions perturb mouse oocyte in vitro developmental competence via beta-O-linked glycosylation of heat shock protein 90. Hum. Reprod. 2014, 29, 1292–1303. [Google Scholar] [CrossRef]
- Barros, F.F.P.C.; Teixeira, P.P.M.; Padilha-Nakaghi, L.C.; Uscategui, R.A.R.; Lima, M.R.; Santos, V.J.C.; Rossy, K.C.; Borges, L.P.B.; Machado, M.R.F.; Vicente, W.R.R. Ovum pick-up and in vitro maturation in spotted paca (Cuniculus paca Linnaeus, 1766). Reprod. Domest. Anim. 2020, 55, 442–447. [Google Scholar] [CrossRef]
- Ferraz, M.S.; Carvalho, M.A.M.; Moraes Júnior, F.J.; Feitosa, M.L.T.; Bertolini, M.; Almeida, H.M.; Bezerra, D.O.; Pessoa, G.T.; Pires, L.C.; Albuquerque, D.M.N. In vitro maturation of agoutis (Dasyprocta prymnolopha) oocytes followed by in vitro fertilization and parthenogenetic activation. Arq. Bras. Med. Vet. Zootec. 2020, 72, 443–451. [Google Scholar] [CrossRef]
- Saadeldin, I.M.; Ehab, S.; Alshammari, M.E.F.; Abdezalim, A.M.; Assiri, A.M. The mammalian oocyte: A central hub for cellular reprogramming and stemness. Stem. Cells Cloning Adv. Appl. 2025, 18, 15–34. [Google Scholar] [CrossRef]
- Diaz, F.J.; Wigglesworth, K.; Eppig, J.J. Oocytes are required for the preantral granulosa cell to cumulus cell transition in mice. Dev. Biol. 2007, 305, 300–311. [Google Scholar] [CrossRef]
- Ritter, I.J.; Sugimura, S.; Gilchrist, R.B. Oocyte induction of EGF responsiveness in somatic cells is associated with the acquisition of porcine oocyte developmental competence. Endocrinology 2015, 156, 2299–2312. [Google Scholar] [CrossRef] [PubMed]
- Sugimura, S.; Ritter, L.J.; Rose, R.D.; Thompson, J.G.; Smitz, J.; Mottershead, D.G.; Gilchrist, R.B. Promotion of EGF receptor signaling improves the quality of low developmental competence oocytes. Dev. Biol. 2015, 403, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Kline, D. Activation of the mouse egg. Theriogenology 1996, 45, 81–90. [Google Scholar] [CrossRef]
- Bos-Mikich, A.; Swann, K.; Whittingham, D.G. Calcium oscillations and protein synthesis inhibition synergistically activate mouse oocytes. Mol. Reprod. Dev. 1995, 41, 84–90. [Google Scholar] [CrossRef]
- Liu, L.; Trimarchi, J.; Keefe, D.I. Haploidy but not parthenogenetic activation leads to increased incidence of apoptosis in mouse embryos. Biol. Reprod. 2002, 66, 204–210. [Google Scholar] [CrossRef]



| Experiment | No. Ovaries | Classification of Immature Oocytes | Oocytes Type A/No. Ovaries | Oocytes Type B/No. Ovaries | Oocytes Type C/No. Ovaries | Total Oocytes/Ovaries | ||
|---|---|---|---|---|---|---|---|---|
| Type A (%) | Type B (%) | Type C (%) | ||||||
| I | 14 | 52.7 ± 5.5 (78/148) | 18.9 ± 3.7 (28/148) | 28.4 ± 2.1 (42/148) | 5.6 ± 1.6 | 2.0 ± 0.0 | 3.0 ± 0.5 | 10.7 ± 2.0 |
| II | 24 | 44.6 ± 5.0 (107/240) | 50.0 ± 2.3 (120/240) | 5.4 ± 3.2 (13/240) | 4.5 ± 0.2 | 5.0 ± 0.8 | 0.5 ± 0.3 | 10.0 ± 0.4 |
| Groups | No. Oocytes | Grade of Cumulus Cell Expansion (%) | CEI | ||||
|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | Score 4 | |||
| EGF10 | 54 | - | 1.8 ± 1.5 (1/54) | 13.0 ± 1.2 (7/54) | 29.6 ± 4.2 (16/54) | 55.6 ± 2.6 (30/54) | 3.4 ± 0.1 |
| EGF50 | 52 | - | 9.6 ± 0.3 (5/52) | 11.5 ± 3.5 (6/52) | 34.6 ± 9.0 (18/52) | 44.2 ± 7.5 (23/52) | 3.1 ± 0.1 |
| P | - | - | 0.1093 | 1.000 | 0.6784 | 0.3314 | 0.120 |
| Groups | 1PB | No. Oocytes | OOD (μm) | IOD (μm) | ZPT (μm) | OD (μm) | SPD (μm) | IOA (μm2) | OA (μm2) | PSA (μm2) |
|---|---|---|---|---|---|---|---|---|---|---|
| EGF10 | − | 19 | 92.8 ± 4.5 a | 76.4 ± 2.9 ab | 9.3 ± 0.5 ab | 69.2 ± 2.4 a | 7.2 ± 2.3 a | 14,431 ± 1087 ab | 11,833 ± 805 ab | 2598 ± 840 a |
| + | 35 | 95.7 ± 6.9 a | 78.8 ± 5.2 a | 9.4 ± 0.8 a | 69.6 ± 4.2 a | 9.2 ± 3.4 a | 15,502 ± 2466 a | 12,058 ± 1630 a | 3443 ± 1437 a | |
| EGF50 | − | 23 | 93.9 ± 0.2 a | 76.7 ± 2.6 ab | 8.9 ± 0.8 ab | 69.0 ± 3.3 a | 7.7 ± 2.7 a | 14,564 ± 1021 ab | 11,813 ± 1101 ab | 2751 ± 941 a |
| + | 29 | 94.3 ± 1.7 a | 74.9 ± 2.6 b | 8.5 ± 0.9 b | 64.8 ± 2.8 b | 10.1 ± 2.7 a | 13,877 ± 957 b | 10,412 ± 901 b | 3464 ± 926 a |
| Group | No. Oocytes | Grade of Cumulus Cell Expansion (%) | CEI | ||||
|---|---|---|---|---|---|---|---|
| Score 0 | Score 1 | Score 2 | Score 3 | Score 4 | |||
| EGF10 | 54 | 1.3 ± 0.9 (3/227) | 6.6 ± 1.2 (15/227) | 21.1 ± 3.9 (48/227) | 27.1 ± 3.4 (66/227) | 41.9 ± 2.9 (95/227) | 3.0 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aquino, L.V.C.; Olindo, S.L.; Silva, Y.L.F.; Silva, V.D.; Oliveira, L.R.M.; Oliveira, M.F.; Pereira, A.F. Effect of Epidermal Growth Factor and 6-Dimethylaminopurine on In Vitro Maturation and Artificial Activation of Spix’s Yellow-Toothed Cavy (Galea spixii Wagler, 1831) Oocytes. Animals 2025, 15, 2403. https://doi.org/10.3390/ani15162403
Aquino LVC, Olindo SL, Silva YLF, Silva VD, Oliveira LRM, Oliveira MF, Pereira AF. Effect of Epidermal Growth Factor and 6-Dimethylaminopurine on In Vitro Maturation and Artificial Activation of Spix’s Yellow-Toothed Cavy (Galea spixii Wagler, 1831) Oocytes. Animals. 2025; 15(16):2403. https://doi.org/10.3390/ani15162403
Chicago/Turabian StyleAquino, Leonardo V. C., Samara L. Olindo, Yara L. F. Silva, Vinícius D. Silva, Lhara R. M. Oliveira, Moacir F. Oliveira, and Alexsandra F. Pereira. 2025. "Effect of Epidermal Growth Factor and 6-Dimethylaminopurine on In Vitro Maturation and Artificial Activation of Spix’s Yellow-Toothed Cavy (Galea spixii Wagler, 1831) Oocytes" Animals 15, no. 16: 2403. https://doi.org/10.3390/ani15162403
APA StyleAquino, L. V. C., Olindo, S. L., Silva, Y. L. F., Silva, V. D., Oliveira, L. R. M., Oliveira, M. F., & Pereira, A. F. (2025). Effect of Epidermal Growth Factor and 6-Dimethylaminopurine on In Vitro Maturation and Artificial Activation of Spix’s Yellow-Toothed Cavy (Galea spixii Wagler, 1831) Oocytes. Animals, 15(16), 2403. https://doi.org/10.3390/ani15162403





