Thermographic Evaluation of the Stifle Region in Dogs with a Rupture of the Cranial Cruciate Ligament
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Assessment and Clinical Examination
2.2. Infrared Imaging and Data Collection
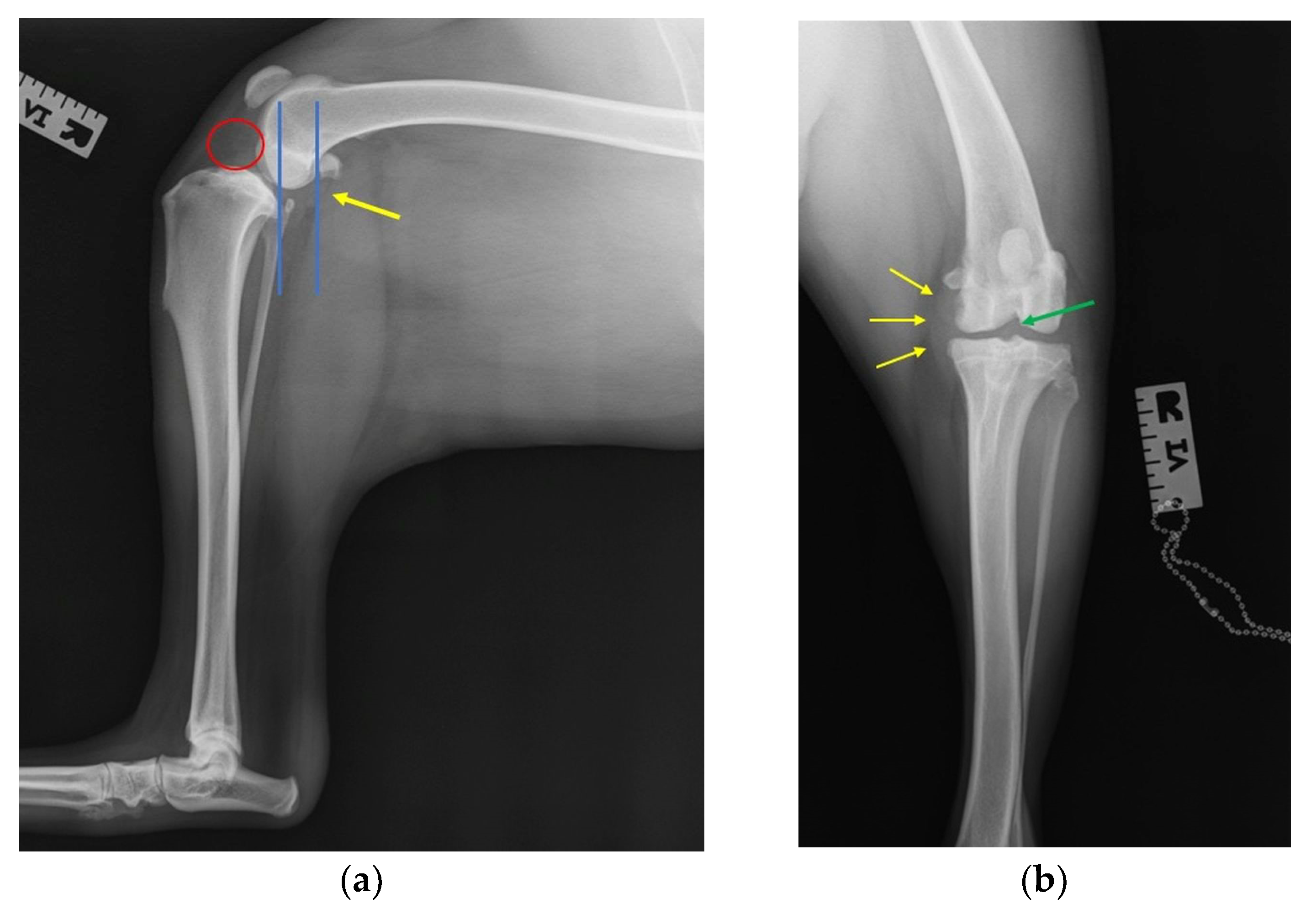
2.3. Radiographic Investigation
2.4. Statistical Analyses
3. Results
3.1. Thermography Result
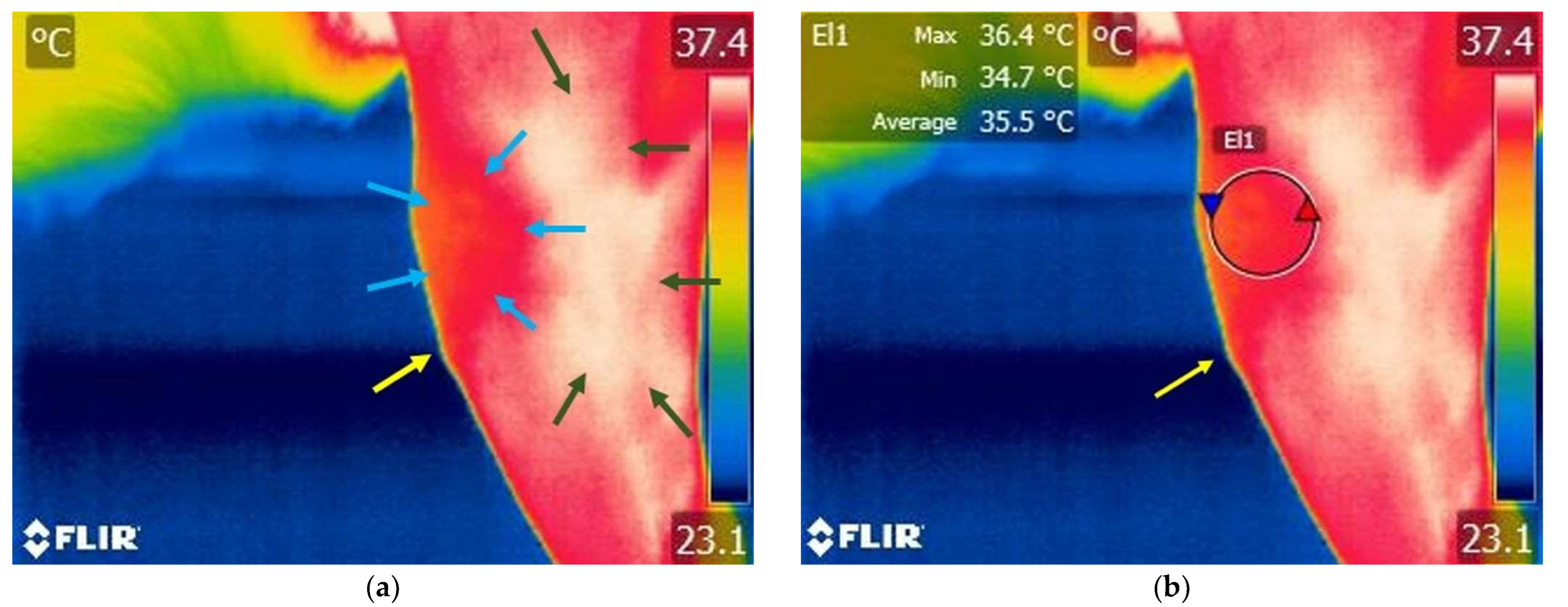
3.1.1. Thermography Scan of the Control Group
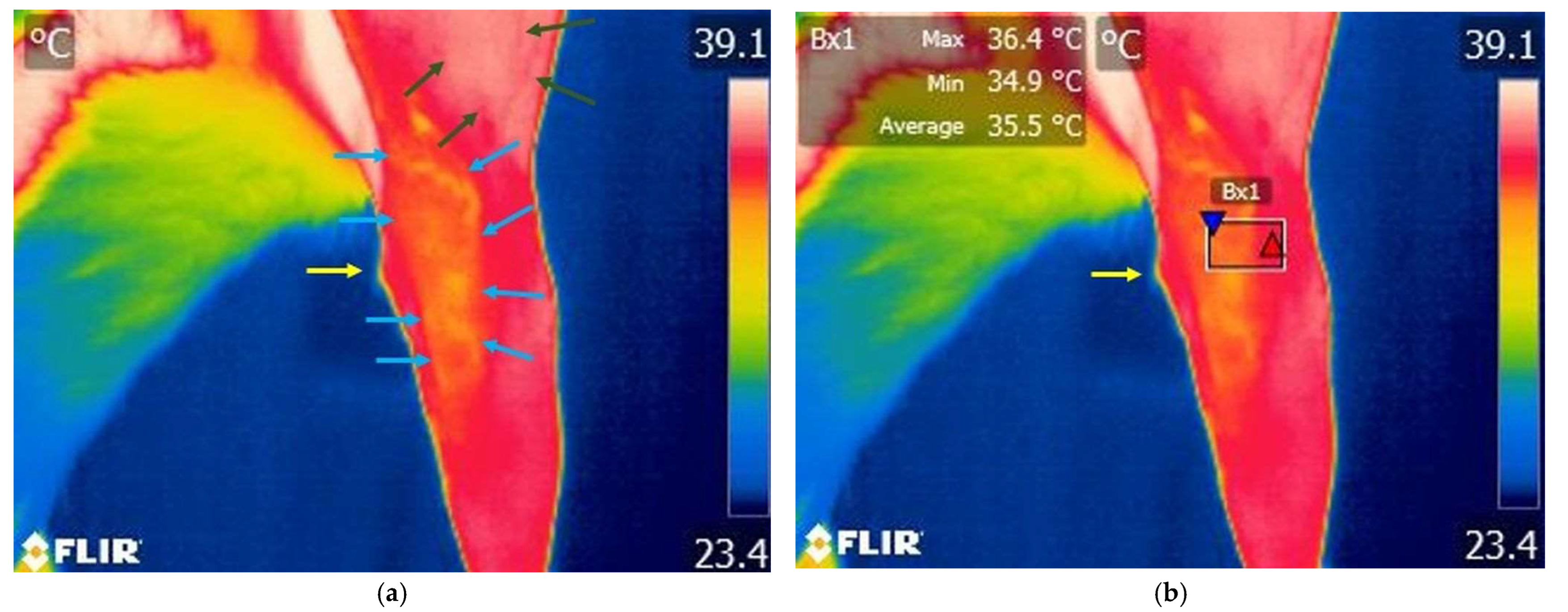
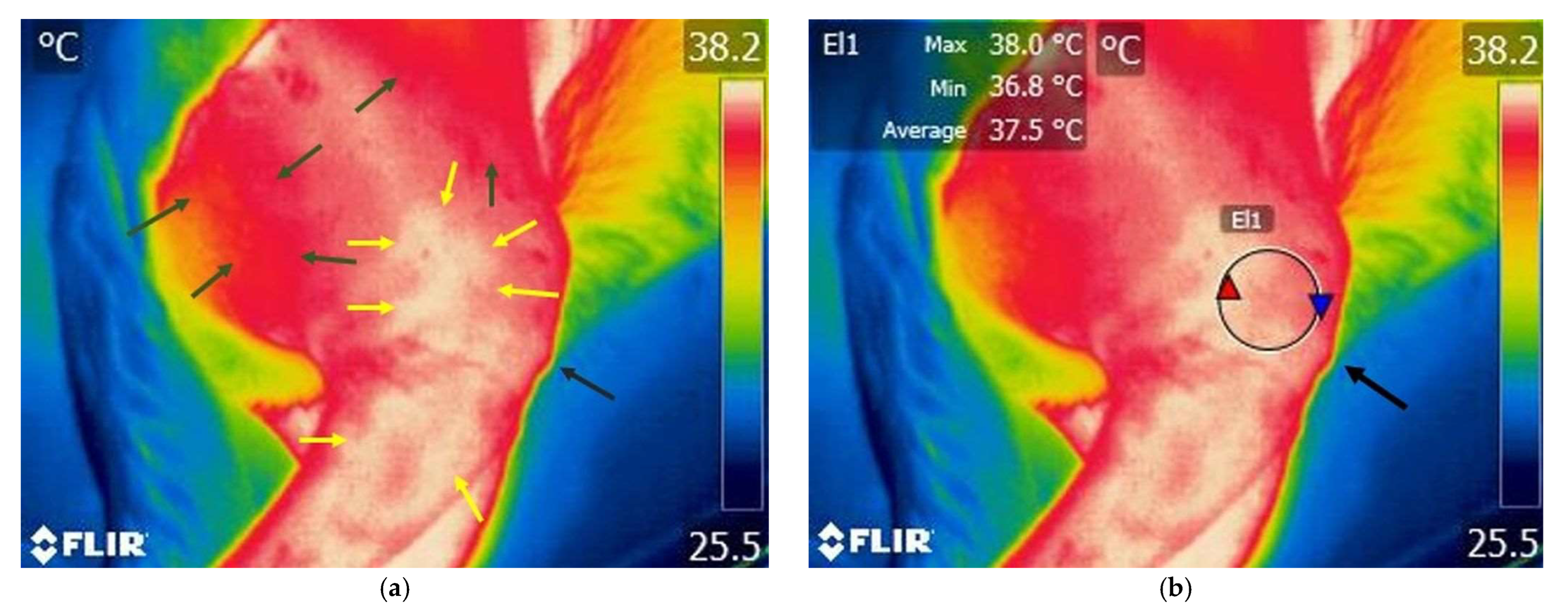
3.1.2. Thermography Scan of the Study Group
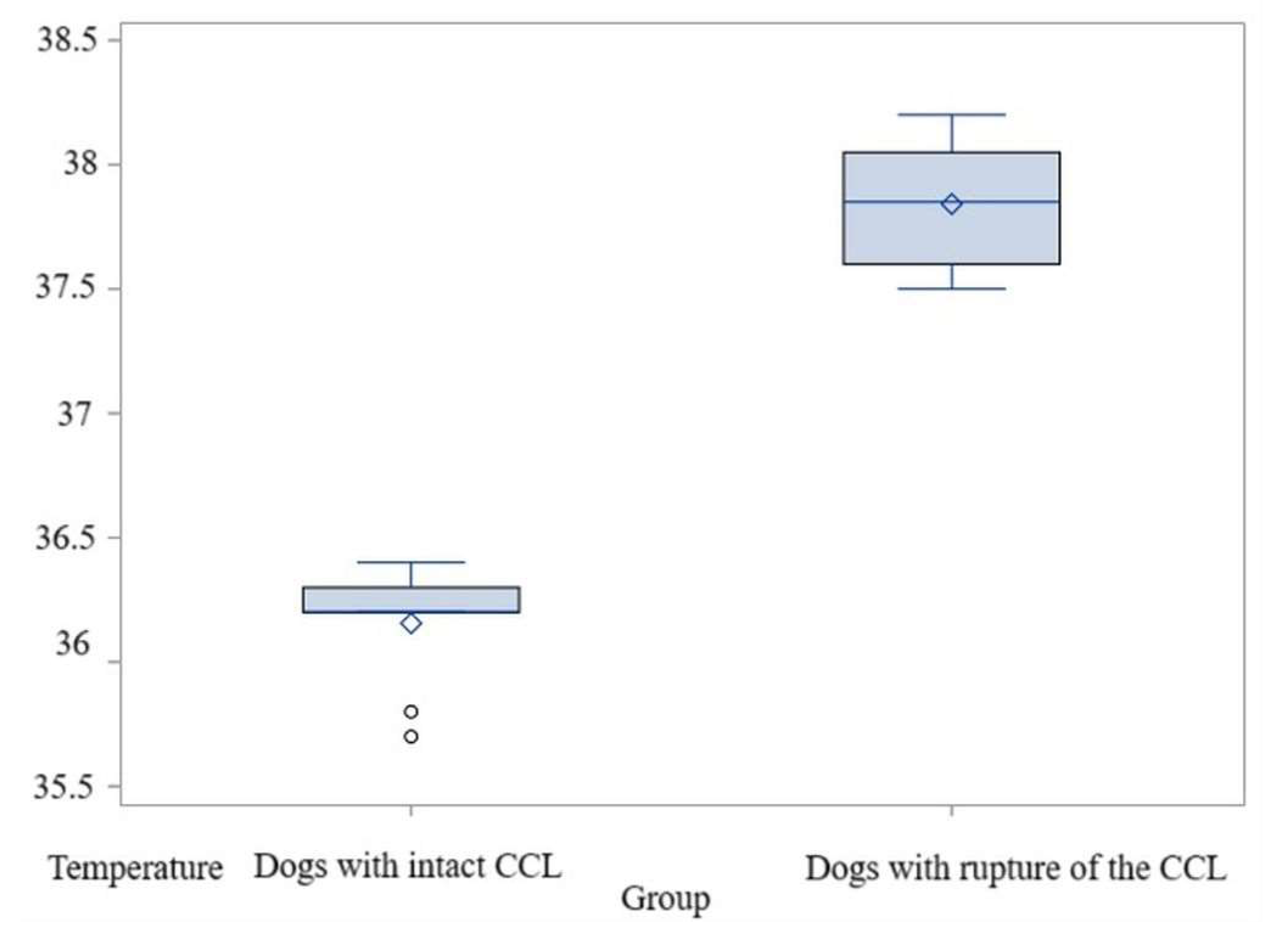
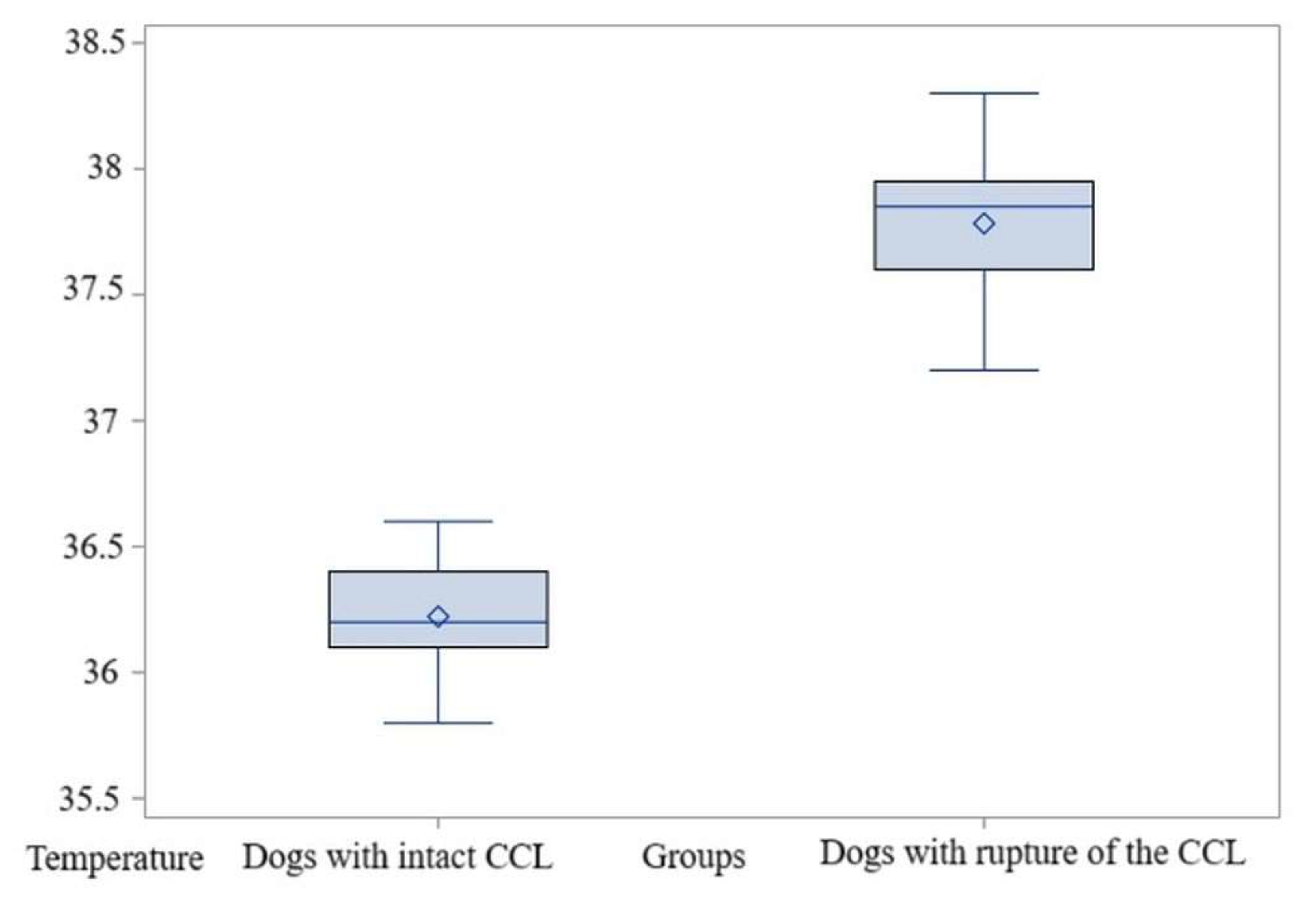
3.2. Comparison Within El1 Area
3.2.1. Comparisons for Maximum Temperature
3.2.2. Comparisons for Average Temperature
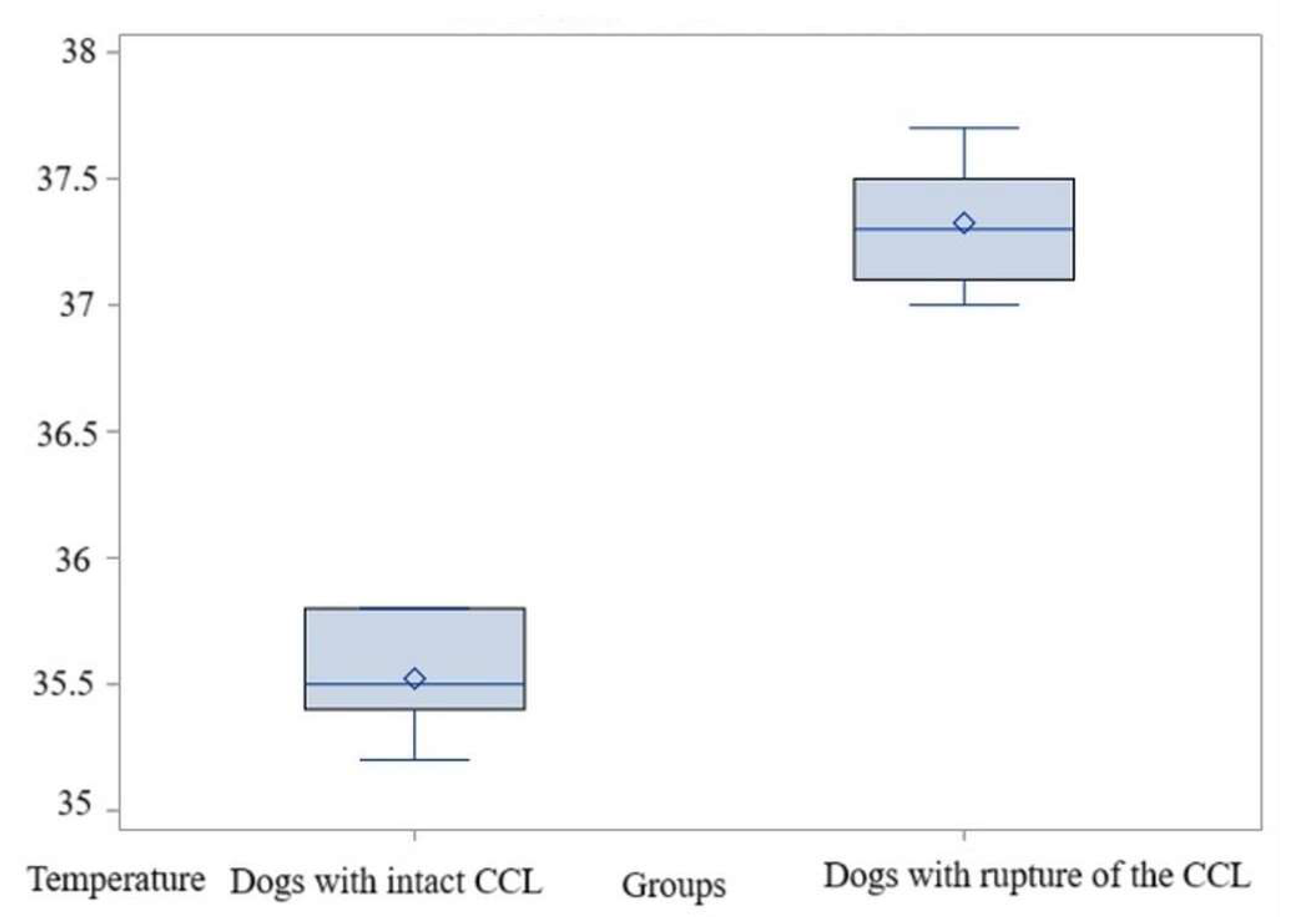
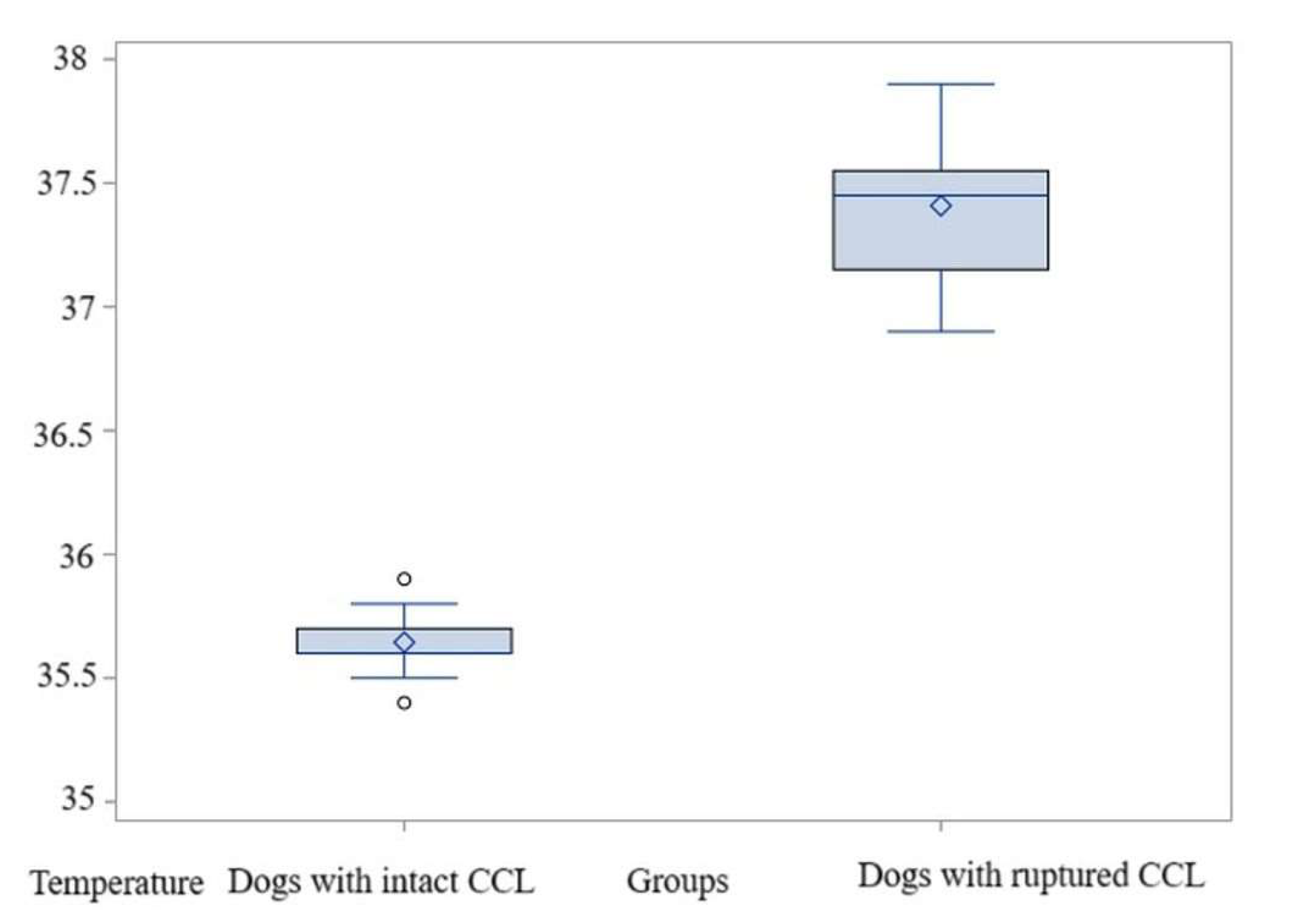
3.3. Comparison Within Bx1 Area
3.3.1. Comparisons for Maximum Temperature
3.3.2. Comparisons for Average Temperature
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rafla, M.; Yang, P. Canine Cranial Cruciate Ligament Disease (CCLD): A Concise Review of the Recent Literature. Animals 2025, 15, 1030. [Google Scholar] [CrossRef]
- Conzemius, M.G.; Evans, R.B.; Besancon, M.F.; Gordon, W.J.; Horstman, C.L.; Hoefle, W.D.; Nieves, M.A.; Wagner, S.D. Effect of surgical technique on limb function after surgery for rupture of the cranial cruciate ligament in dogs. J. Am. Vet. Med. Assoc. 2005, 226, 232–236. [Google Scholar] [CrossRef] [PubMed]
- de Rooster, H.G.; Van Ryssen, B.; van Bree, H. Diagnosis of cranial cruciate ligament injuries in dogs by tibial compression radiography. Vet. Rec. 1998, 142, 366–368. [Google Scholar] [CrossRef] [PubMed]
- Barrett, J.G.; Hao, Z.; Graf, B.K.; Kaplan, L.D.; Heiner, J.P.; Muir, P. Inflammatory changes in ruptured canine cranial and human anterior cruciate ligaments. Am. J. Vet. Res. 2005, 66, 2073–2080. [Google Scholar] [CrossRef]
- Hatmough, C.H.W.; Oores, A.N.P.M.; Agalhaes, R.I.S.O.M.; Amb, C.H.R.L. Factors affecting width of the canine femorotibial joint. Vet. Radio Ultrasound 2008, 49, 129–134. [Google Scholar] [CrossRef]
- Restucci, B.; Sgadari, M.; Fatone, G.; Valle, G.D.; Aragosa, F.; Caterino, C.; Ferrara, G.; Niebauer, G.W. Immunoexpression of Relaxin and Its Receptors in Stifle Joints of Dogs with Cranial Cruciate Ligament Disease. Animals 2022, 12, 819. [Google Scholar] [CrossRef]
- Johnson, J.M.; Johnson, A.L. Cranial cruciate ligament rupture. Pathogenesis, diagnosis, and postoperative rehabilitation. Vet. Clin. N. Am. Small Anim. Pract. 1993, 23, 717–733. [Google Scholar] [CrossRef]
- McCready, D.J.; Ness, M.G. Diagnosis and management of meniscal injury in dogs with cranial cruciate ligament rupture: A systematic literature review. J. Small Anim. Pract. 2016, 57, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Markovszky, A.K.; Weber, C.; Biksi, O.; Danes, M.; Dumitrescu, E.; Muselin, F.; Tufarelli, V.; Puvača, N.; Cristina, R.T. Is ECLIA Serum Cortisol Concentration Measurement, an Accurate Indicator of Pain Severity in Dogs with Locomotor Pain? Animals 2020, 10, 2036. [Google Scholar] [CrossRef]
- Spinella, G.; Arcamone, G.; Valentini, S. Cranial Cruciate Ligament Rupture in Dogs: Review on Biomechanics, Etiopathogenetic Factors and Rehabilitation. Vet. Sci. 2021, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Harasen, G. Diagnosing rupture of the cranial cruciate ligament. Can. Vet. J. 2002, 43, 475–476. [Google Scholar] [PubMed]
- Loughin, C.A.; Marino, D.J. Evaluation of thermographic imaging of the limbs of healthy dogs. Am. J. Vet. Res. 2007, 68, 1064–1069. [Google Scholar] [CrossRef] [PubMed]
- Marino, D.J.; Loughin, C.A. Diagnostic Imaging of the Canine Stifle: A Review. Vet. Surg. 2010, 39, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Banfield, C.M. Magnetic resonance arthrography of the canine stifle joint: Technique and applications in eleven military dogs. Vet. Radiol. Ultrasound 2000, 41, 200–213. [Google Scholar] [CrossRef]
- Plesman, R.; Gilbert, P.; Campbell, J. Detection of meniscal tears by arthroscopy and arthrotomy in dogs with cranial cruciate ligament rupture: A retrospective, Vet. Comp. Orthop. Traumatol. 2013, 26, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Arnault, F.; Cauvin, E.; Viguier, E.; Kraft, E.; Sonet, J.; Carozzo, C. Diagnostic value of ultra- sonography to assess stifle lesions in dogs after cranial cruciate ligament rupture: 13 cases. Vet. Comp. Orthop. Traumatol. 2009, 22, 479–485. [Google Scholar] [CrossRef]
- Neto, G.A.C.; Machado, N.A.F.; Barbosa-Filho, J.A.D.; Marques, J.I.; Leite, P.G.; de Andrade, H.A.F.; de Sousa, A.M.; Santos, J.C.S.D.; de Sousa, A.C.; da Silva Sousa, W.; et al. Infrared thermography as a non-invasive method to quantify the heat stress response in weaned piglets after road transport in a semi-arid region. Int. J. Biometeorol. 2025, 69, 633–642. [Google Scholar] [CrossRef]
- Roberto, J.V.B.; de Souza, B.B. Use of infrared thermography in veterinary medicine and animal production. J. Anim. Behav. Biometeorol. 2014, 2, 73–84. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Titto, C.G.; Orihuela, A.; Martínez-Burnes, J.; Gómez-Prado, J.; Torres-Bernal, F.; Flores-Padilla, K.; Carvajal-de la Fuente, V.; Wang, D. Physiological and Behavioral Mechanisms of Thermoregulation in Mammals. Animals 2021, 11, 1733. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Ogi, A.; Villanueva-García, D.; Martínez-Burnes, J.; Hernández-Avalos, I.; Olmos-Hernández, A.; Mora-Medina, P.; Domínguez-Oliva, A.; Mota-Rojas, D. Application of Infrared Thermography in the Rehabilitation of Patients in Veterinary Medicine. Animals 2024, 14, 696. [Google Scholar] [CrossRef] [PubMed]
- Soroko, M.; Górniak, W.; Howell, K.; Zielińska, P.; Dudek, K.; Eberhardt, M.; Kalak, P.; Korczyński, M. Changes in Body Surface Temperature Associated with High-Speed Treadmill Exercise in Beagle Dogs Measured by Infrared Thermography. Animals 2021, 11, 2982. [Google Scholar] [CrossRef]
- Zaha, C.; Cărpinișan, L.; Schuszler, L.; Paula, N.; Căsălean, T.; Florea, T.; Cristina, V.; Sicoe, B.; Rujescu, C.; Dascălu, R. Thermographic Scan of the Thoracolumbar Area in Dogs with Acute Intervertebral Disc Extrusion (IVDE): A Retrospective Study. Life 2025, 15, 68. [Google Scholar] [CrossRef]
- Infernuso, T.; Loughin, C.A.; Marino, D.J.; Umbaugh, S.E.; Solt, P.E. Thermal Imaging of Normal and Cranial Cruciate Ligament-Deficient Stifles in Dogs. Vet. Surg. 2010, 39, 410–417. [Google Scholar] [CrossRef]
- Igna, C.; Mavromatis, S.; Bumb, D.; Sicoe, B.; Zaha, C.; Schuszler, L. Thermal Imaging of the Dogs with Cranial Cruciate Ligaments Ruptures. Lucr. Stiint. Med. Vet. 2017, 50, 232–241. [Google Scholar]
- Aulakh, K.S.; Dongaonkar, K.R.; Barnes, K.; Gines, A.J.; Bordelon, J.T.; Hulse, D.; Aulakh, H.K.; Liu, C.C. Influence of orthopedic examination on lameness scores and interobserver and intraobserver agreement in dogs with naturally occurring elbow osteoarthritis. Vet. Surg. 2020, 49, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Grossbard, B.P.; Loughin, C.A.; Marino, D.J.; Marino, L.J.; Sackman, J.; Umbaugh, S.E.; Solt, P.S.; Afruz, J.; Leando, P.; Lesser, M.L.; et al. Medical infrared imaging (Thermography) of Type I thoracolumbar disk disease in chondrodystrophic dogs. Vet. Surg. 2014, 43, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Garcia del Carvalho, J.R.; del Puppo, D.; Thayssa de Oliveira, L.; Agassi de Sales, N.A.; Yamamoto Silva, A.C.; Ribeiro, G.; Nielsen de Almeida, F.; Gomes Alves, B.; Honorato Gatto, I.R.; Vieira Ramos, G.; et al. Functional infrared thermography imaging can be used to assess the effectiveness of Maxicam Gel ® in pre-emptively treating transient synovitis and lameness in horses. Front. Vet. Sci. 2024, 11, 1399815. [Google Scholar] [CrossRef]
- Alves, J.C.; Santos, A.; Jorge, P.; Lavrador, C.; Carreira, L.M. Evaluation of digital thermography imaging to assess and monitor treatment of police working dogs with naturally occurring hip osteoarthritis. BMC Vet. Res. 2021, 17, 180. [Google Scholar] [CrossRef]
- Arthurs, G. Orthopaedic examination of the dog: 2. Pelvic limb. Practice 2011, 33, 172–179. [Google Scholar] [CrossRef]
- Martinez, S.A.; Might, K.R.; Gay, J.M. Evaluation of the Drawer Test and the Tibial Compression Test for Differentiating Between Cranial and Caudal Stifle Subluxation Associated with Cruciate Ligament Instability. Vet. Surg. 2013, 42, 392–397. [Google Scholar] [CrossRef]
- Casas-Alvarado, A.; Martínez-Burnes, J.; Mora-Medina, P.; Hernández-Avalos, I.; Domínguez-Oliva, A.; Lezama-García, K.; Gómez-Prado, J.; Mota-Rojas, D. Thermal and Circulatory Changes in Diverse Body Regions in Dogs and Cats Evaluated by Infrared Thermography. Animals 2022, 12, 789. [Google Scholar] [CrossRef]
- Sanchis-sánchez, E.; Vergara-hernández, C.; Cibrián, R.M.; Salvador, R.; Codoñer-Franch, P. Infrared Thermal Imaging in the Diagnosis of Musculoskeletal Injuries: A Systematic Review and meta-analysis. Am. J. Roentgenol. 2014, 203, 875–882. [Google Scholar] [CrossRef]
- Banik, R.T.; Sia, T.; Ibrahim, M.M.; Sivanesan, E.; Uhelski, M.; Pena, A.; Streicher, J.M.; Simone, D.A. Increases in local skin temperature correlate with spontaneous foot lifting and heat hyperalgesia in both incisional inflammatory models of pain. Pain. Rep. 2023, 8, e1097. [Google Scholar] [CrossRef] [PubMed]
- Nomura, R.H.C.; de Freitas, I.B.; Guedes, R.L.; Araujo, F.F.; Neves Mafra, A.C.D.; Ibanez, J.F.; Dornbusch, P.T. Thermographic images from healthy knees between dogs with long and short hair. Cienc. Rural. 2018, 48, 12. [Google Scholar] [CrossRef]
- Mota-Rojas, D.; Ogi, A.; Villanueva-García, D.; Hernández-Ávalos, I.; Casas-Alvarado, A.; Domínguez-Oliva, A.; Lendez, P.; Ghezzi, M. Thermal Imaging as a Method to Indirectly Assess Peripheral Vascular Integrity and Tissue Viability in Veterinary Medicine: Animal Models and Clinical Applications. Animals 2024, 14, 142. [Google Scholar] [CrossRef]
- Kwon, C.J.; Brundage, C.M. Quantifying body surface temperature differences in canine coat types using infrared thermography. J. Therm. Biol. 2019, 82, 18–22. [Google Scholar] [CrossRef]
- Woo, S.C.; Lee, J.; Millis, D.L.; Drum, M.G. Thermographic Evaluation of the Duration of Skin Cooling After Cryotherapy in Dogs Following Tibial Plateau Leveling Osteotomy Surgery. Front. Vet. Sci. 2022, 31, 9. [Google Scholar] [CrossRef]
- Markovszky, A.K.; Szabo, J.A.; Margineanu, A.V.; Dumitrescu, E.; Muselin, F.; Cristina, R.T. The value of a combined drug and laser therapy protocol in lameness monitored with thermographic means—A clinical study in 20 horses. Rev. Rom. Med. Vet. 2021, 31, 47–52. [Google Scholar]
- Jerram, R.M.; Walker, A.M. Cranial cruciate ligament injury in the dog: Pathophysiology, diagnosis and treatment. N. Z. Vet. J. 2003, 51, 149–158. [Google Scholar] [CrossRef]
- Griffon, D.J. A review of the pathogenesis of canine cranial cruciate ligament disease. Vet. Surg. 2010, 39, 399–409. [Google Scholar] [CrossRef]
- Rooster, H. Cranial Cruciate Ligament Disease in the Dog: Contributions to Etiology, Diagnosis and Treatment. Ph.D. Thesis, Ghent University, Ghent, Belgium, 2001. [Google Scholar]
- Schmutterer, J.M.; Augat, P.; Greinwald, M.; Meyer-Lindenberg, A. Meniscal load distribution in canine stifles. Vet. Comp. Orthop. Traumatol. 2024, 37, 230–235. [Google Scholar] [PubMed]
- Chuang, C.; Ramaker, M.A.; Kaur, S.; Csomos, R.A.; Kroner, K.T.; Bleedorn, J.A.; Schaefer, S.L.; Muir, P. Radiographic risk factors for contralateral rupture in dogs with unilateral cranial cruciate ligament rupture. PLoS ONE 2014, 9, e106389. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Crawford, A.H.; De Decker, S. Paper Clinical presentation and outcome of dogs treated medically or surgically for thoracolumbar intervertebral disc protrusion. Vet. Rec. 2017, 180, 569. [Google Scholar] [CrossRef] [PubMed]
- D’anjou, M.-A.; Moreau, M.; Troncy, É.; Martel-Pelletier, J.; Abram, F.; Raynauld, J.-P.; Pelletier, J.-P. Osteophytosis, Subchondral Bone Sclerosis, Joint Effusion and Soft Tissue Thickening in Canine Experimental Stifle Osteoarthritis: Comparison Between 1.5 T Magnetic Resonance Imaging and Computed Radiography. Vet. Surg. 2008, 37, 166–177. [Google Scholar] [CrossRef]
- Blond, L.; Thrall, D.E.; Roe, S.C.; Chailleux, N.; Robertson, I.D. Diagnostic accuracy of magnetic resonance imaging for meniscal tears in dogs affected with naturally occuring cranial cruciate ligament rupture. Vet. Radiol. Ultrasound 2008, 49, 425–431. [Google Scholar] [CrossRef]
- Subedi, S.; Umbaugh, S.E.; Fu, J.; Marino, D.J.; Loughin, C.A.; Sackman, J. Thermographic image analysis as a pre-screening tool for the detection of canine bone cancer. In Applications of Digital Image Processing XXXVII; SPIE: San Diego, CA, USA, 2014. [Google Scholar] [CrossRef]
- Markovszky, A.K.; Danes, M.; Dumitrescu, E.; Muselin, F.; Stancu, A.; Becskei, Z.; Puvača, N.; Cristina, R.T. The importance of professional based questionnaire in the dog’s acute and chronic pain evaluation. J. Hell. Vet. Med. Soc. 2021, 72, 3229–3238. [Google Scholar] [CrossRef]
- Zaha, C.; Schuszler, L.; Dascalu, R.; Nistor, P.; Florea, T.; Imre, K.; Rujescu, C.; Sicoe, B.; Igna, C. Evaluation of Thermal Changes of the Sole Surface in Horses with Palmar Foot Pain: A Pilot Study. Biology 2023, 12, 423. [Google Scholar] [CrossRef]
- Burke-Smith, A.; Collier, J.; Jones, I.A. Comparison of non-invasive imaging modalities: Infrared thermography, spectrophotometric intracutaneous analysis and laser Doppler imaging for the assessment of adult burns. Burns 2015, 41, 1695–1707. [Google Scholar] [CrossRef]
- Redaelli, V.; Bergero, D.; Zucca, E.; Ferrucci, F.; Costa, L.N.; Crosta, L.; Luzi, F. Journal of Equine Veterinary Science Use of Thermography Techniques in Equines: Principles and Applications. J. Equine Vet. Sci. 2014, 34, 345–350. [Google Scholar] [CrossRef]


| Score of lameness | 2 | 3 | 4 |
| Dogs with CCL | 7 | 3 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Căsălean, T.; Zaha, C.; Schuszler, L.; Dascălu, R.; Sicoe, B.; Cojocaru, R.; Călugărița, A.; Rujescu, C.; Degi, J.; Cristina, R.T. Thermographic Evaluation of the Stifle Region in Dogs with a Rupture of the Cranial Cruciate Ligament. Animals 2025, 15, 2317. https://doi.org/10.3390/ani15152317
Căsălean T, Zaha C, Schuszler L, Dascălu R, Sicoe B, Cojocaru R, Călugărița A, Rujescu C, Degi J, Cristina RT. Thermographic Evaluation of the Stifle Region in Dogs with a Rupture of the Cranial Cruciate Ligament. Animals. 2025; 15(15):2317. https://doi.org/10.3390/ani15152317
Chicago/Turabian StyleCăsălean, Tudor, Cristian Zaha, Larisa Schuszler, Roxana Dascălu, Bogdan Sicoe, Răzvan Cojocaru, Andrei Călugărița, Ciprian Rujescu, Janos Degi, and Romeo Teodor Cristina. 2025. "Thermographic Evaluation of the Stifle Region in Dogs with a Rupture of the Cranial Cruciate Ligament" Animals 15, no. 15: 2317. https://doi.org/10.3390/ani15152317
APA StyleCăsălean, T., Zaha, C., Schuszler, L., Dascălu, R., Sicoe, B., Cojocaru, R., Călugărița, A., Rujescu, C., Degi, J., & Cristina, R. T. (2025). Thermographic Evaluation of the Stifle Region in Dogs with a Rupture of the Cranial Cruciate Ligament. Animals, 15(15), 2317. https://doi.org/10.3390/ani15152317











