Intraspecific Variations in Ecomorphological Functional Traits of Montane Stream-Dwelling Frogs Were Driven by Their Microhabitat Conditions
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
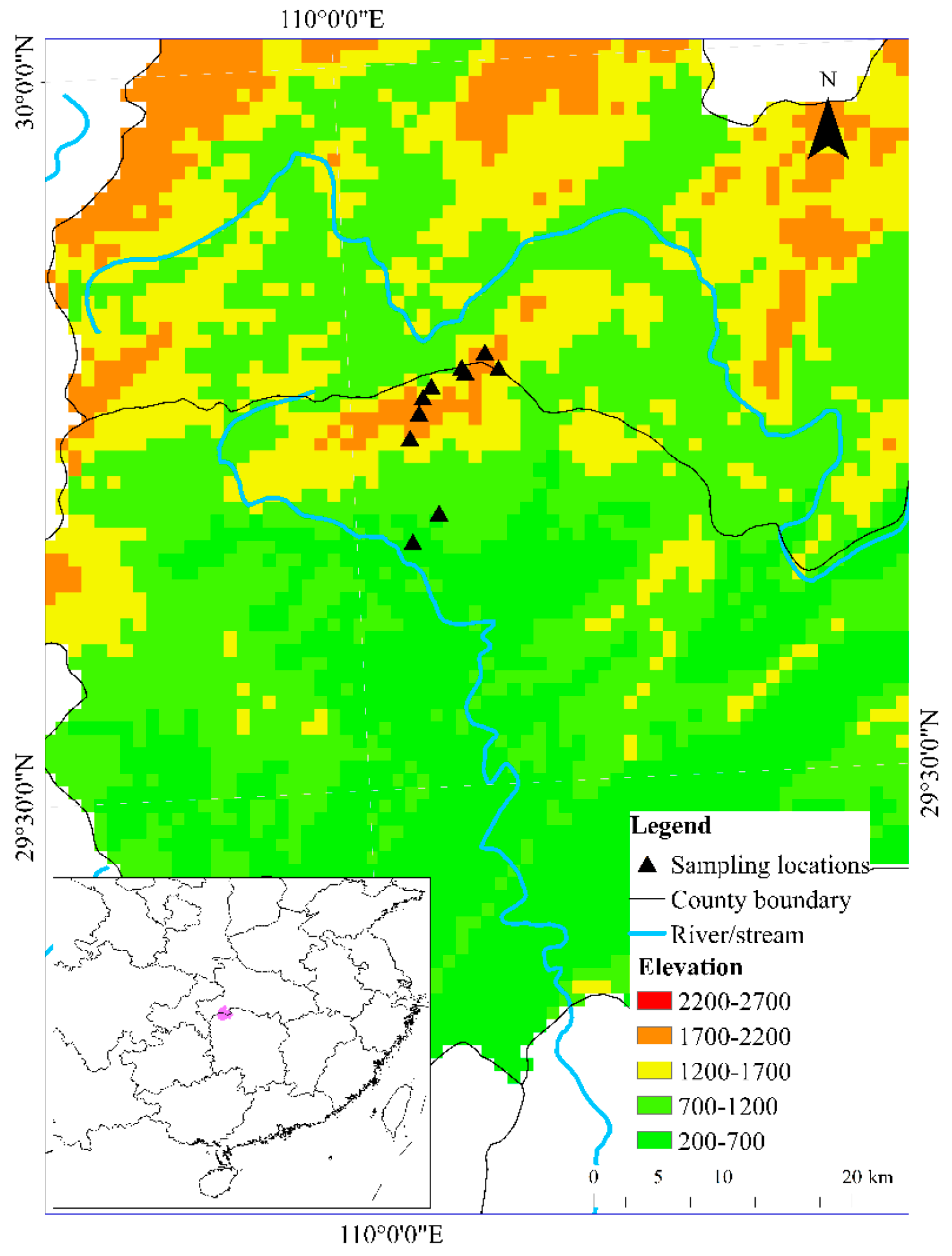
2.1. Study Area
2.2. Data Collection
2.3. Statistical Analyses
3. Results
3.1. Intra-Species Functional Morphology Difference vs. Elevation
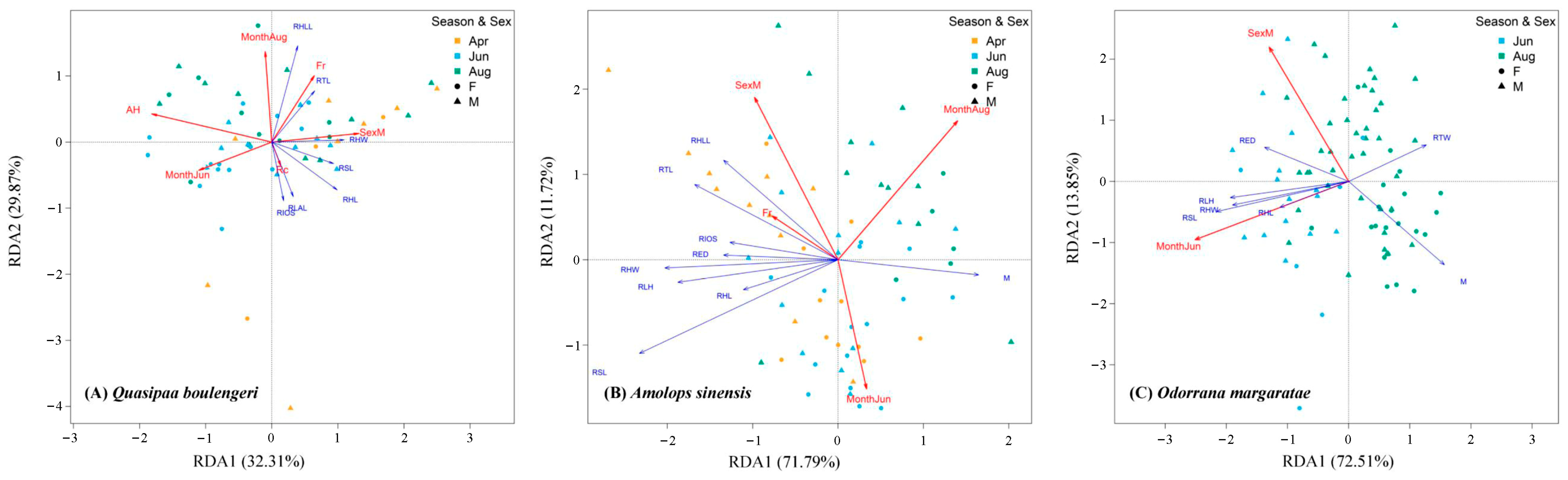
3.2. The Relationships Between Amphibians’ Functional Traits and Microhabitat Variables
3.3. Different Microhabitat Variables Affect Different Functional Traits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stuart-Smith, R.D.; Bates, A.E.; Lefcheck, J.S.; Duffy, J.E.; Baker, S.C.; Thomson, R.J.; Stuart-Smith, J.F.; Hill, N.A.; Kininmonth, S.J.; Airoldi, L.; et al. Integrating Abundance and Functional Traits Reveals New Global Hotspots of Fish Diversity. Nature 2013, 501, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Žagar, A.; Carretero, M.A.; Vrezec, A.; Drašler, K.; Kaliontzopoulou, A. Towards a Functional Understanding of Species Coexistence: Ecomorphological Variation in Relation to Whole-organism Performance in Two Sympatric Lizards. Funct. Ecol. 2017, 31, 1780–1791. [Google Scholar] [CrossRef]
- Zhao, T.; Grenouillet, G.; Pool, T.; Tudesque, L.; Cucherousset, J. Environmental Determinants of Fish Community Structure in Gravel Pit Lakes. Ecol. Freshw. Fish 2016, 25, 412–421. [Google Scholar] [CrossRef]
- Zhao, T.; Villéger, S.; Lek, S.; Cucherousset, J. High Intraspecific Variability in the Functional Niche of a Predator Is Associated with Ontogenetic Shift and Individual Specialization. Ecol. Evol. 2014, 4, 4649–4657. [Google Scholar] [CrossRef]
- Pease, A.A.; González-Díaz, A.A.; Rodiles-Hernández, R.; Winemiller, K.O. Functional Diversity and Trait–Environment Relationships of Stream Fish Assemblages in a Large Tropical Catchment. Freshw. Biol. 2012, 57, 1060–1075. [Google Scholar] [CrossRef]
- Karpouzi, V.S.; Stergiou, K.I. The Relationships between Mouth Size and Shape and Body Length for 18 Species of Marine Fishes and Their Trophic Implications. J. Fish Biol. 2003, 62, 1353–1365. [Google Scholar] [CrossRef]
- Kang, D.; Zhao, C.; Sun, Z.; Chen, G.; Feng, J.; Zhu, W.; Huang, Y.; Zhao, T. Effects of Microhabitat Features on the Intraspecific Variability of the Distribution and Functional Traits in a Highest Elevational Distributed Lizard. Ecol. Evol. 2024, 14, e10902. [Google Scholar] [CrossRef]
- Grant, P.R. Ecology and Evolution of Darwin’s Finches; Princeton University Press: Princeton, NJ, USA, 1999. [Google Scholar]
- González-Suárez, M.; Revilla, E. Variability in Life-history and Ecological Traits Is a Buffer against Extinction in Mammals. Ecol. Lett. 2013, 16, 242–251. [Google Scholar] [CrossRef]
- Zhao, T.; Khatiwada, J.R.; Zhao, C.; Feng, J.; Sun, Z. Elevational Patterns of Amphibian Functional and Phylogenetic Structures in Eastern Nepal Himalaya. Divers. Distrib. 2022, 28, 2475–2488. [Google Scholar] [CrossRef]
- Toussaint, A.; Brosse, S.; Bueno, C.G.; Pärtel, M.; Tamme, R.; Carmona, C.P. Extinction of Threatened Vertebrates Will Lead to Idiosyncratic Changes in Functional Diversity across the World. Nat. Commun. 2021, 12, 5162. [Google Scholar] [CrossRef]
- Daniels, A. IUCN Morella Spathulata: The IUCN Red List of Threatened Species 2020. Available online: https://www.iucnredlist.org/ (accessed on 21 January 2025).
- McCain, C.M.; Colwell, R.K. Assessing the Threat to Montane Biodiversity from Discordant Shifts in Temperature and Precipitation in a Changing Climate: Climate Change Risk for Montane Vertebrates. Ecol. Lett. 2011, 14, 1236–1245. [Google Scholar] [CrossRef]
- Peng, X.-W.; Lan, J.; Sun, Z.-J.; Zhu, W.-B.; Zhao, T. Distinct Amphibian Elevational and Seasonal Phylogenetic Structures Are Determined by Microhabitat Variables in Temperate Montane Streams. Animals 2022, 12, 1673. [Google Scholar] [CrossRef]
- Wen-Bo, Z.; Chun-Lin, Z.; Chun-Lin, L.; Bei, Z.; Dan, X.; Wei, Z.; Tian, Z.; Jian-Ping, J. Spatial and Temporal Patterns of Amphibian Species Richness on Tianping Mountain, Hunan Province, China. Zool. Res. 2020, 41, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, C.; Wang, Y. Ecological Correlates of Extinction Risk in Chinese Amphibians. Divers. Distrib. 2019, 25, 1586–1598. [Google Scholar] [CrossRef]
- Hero, J.; Williams, S.E.; Magnusson, W.E. Ecological Traits of Declining Amphibians in Upland Areas of Eastern Australia. J. Zool. 2005, 267, 221–232. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Zhong, M.-J.; Zhang, J.; Si, X.-F.; Yang, S.-N.; Jiang, J.-P.; Hu, J.-H. Multidimensional Amphibian Diversity and Community Structure along a 2600 m Elevational Gradient on the Eastern Margin of the Qinghai-Tibetan Plateau. Zool. Res. 2022, 43, 40–51. [Google Scholar] [CrossRef]
- Grundel, R.; Beamer, D.A.; Glowacki, G.A.; Frohnapple, K.J.; Pavlovic, N.B. Opposing Responses to Ecological Gradients Structure Amphibian and Reptile Communities across a Temperate Grassland–Savanna–Forest Landscape. Biodivers. Conserv. 2015, 24, 1089–1108. [Google Scholar] [CrossRef]
- Keller, A.; Rödel, M.-O.; Linsenmair, K.E.; Grafe, T.U. The Importance of Environmental Heterogeneity for Species Diversity and Assemblage Structure in Bornean Stream Frogs. J. Anim. Ecol. 2009, 78, 305–314. [Google Scholar] [CrossRef]
- Khatiwada, J.R.; Zhao, T.; Chen, Y.; Wang, B.; Xie, F.; Cannatella, D.C.; Jiang, J. Amphibian Community Structure along Elevation Gradients in Eastern Nepal Himalaya. BMC Ecol. 2019, 19, 19. [Google Scholar] [CrossRef]
- Anderson, M.J. Distance-Based Tests for Homogeneity of Multivariate Dispersions. Biometrics 2006, 62, 245–253. [Google Scholar] [CrossRef]
- Peters, M.K.; Hemp, A.; Appelhans, T.; Behler, C.; Classen, A.; Detsch, F.; Ensslin, A.; Ferger, S.W.; Frederiksen, S.B.; Gebert, F.; et al. Predictors of Elevational Biodiversity Gradients Change from Single Taxa to the Multi-Taxa Community Level. Nat. Commun. 2016, 7, 13736. [Google Scholar] [CrossRef] [PubMed]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 23 January 2025).
- Oksanen, J. Multivariate Analysis of Ecological Communities in R: Vegan Tutorial. Available online: https://cran.r-project.org/web/packages/vegan/index.html (accessed on 23 January 2025).
- Revelle, W. Psych: Procedures for Psychological, Psychometric, and Personality Research 2007, 2.4.12. Available online: https://cran.r-project.org/web/packages/psych/ (accessed on 23 January 2025).
- Cui, K.; Yang, S.; Hu, J. Trophic Niche Adaptation of Mountain Frogs around the Sichuan Basin: Individual Specialization and Response to Climate Variations. Front. Zool. 2024, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Secor, S.M.; Faulkner, A.C. Effects of Meal Size, Meal Type, Body Temperature, and Body Size on the Specific Dynamic Action of the Marine Toad, Bufo Marinus. Physiol. Biochem. Zool. 2002, 75, 557–571. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C. Über Die Verhältnisse der Wärmeökonomie der Thiere Zu Ihrer Grösse. Göttinger Stud. 1847, 3, 595–708. [Google Scholar]
- Blackburn, T.M.; Gaston, K.J.; Loder, N. Geographic Gradients in Body Size: A Clarification of Bergmann’s Rule. Divers. Distrib. 1999, 5, 165–174. [Google Scholar] [CrossRef]
- Gardner, J.L.; Peters, A.; Kearney, M.R.; Joseph, L.; Heinsohn, R. Declining Body Size: A Third Universal Response to Warming? Trends Ecol. Evol. 2011, 26, 285–291. [Google Scholar] [CrossRef]
- Schäuble, C.S. Variation in Body Size and Sexual Dimorphism across Geographical and Environmental Space in the Frogs Limnodynastes tasmaniensis and L. peronii: Geographical Variation in Two Limnodynastes. Biol. J. Linn. Soc. 2004, 82, 39–56. [Google Scholar] [CrossRef]
- Cante-Bazán, E.; Luría-Manzano, R. Seasonal Variation of the Relationship between Body Condition and Fluctuating Asymmetry in Two Sympatric Ranid Frogs. Herpetol. J. 2024, 34, 92–99. [Google Scholar] [CrossRef]
- Fei, L.; Ye, C.; Jiang, J. Colorful Illustrations of Amphibians in China, 1st ed.; Sichuan Publishing Group, Sichuan Science and Technology Press: Chengdu, China, 2010. [Google Scholar]
- Zhao, C.; Feng, J.; Sun, Z.; Zhu, W.; Chang, J.; Fan, W.; Jiang, J.; Yue, B.; Zhao, T. Intraspecific Variation in Microhabitat Selection in Reintroduced Chinese Giant Salamanders. Curr. Zool. 2023, 69, 121–127. [Google Scholar] [CrossRef]
- Richards, C.T. Kinematics and Hydrodynamics Analysis of Swimming Anurans Reveals Striking Inter-Specific Differences in the Mechanism for Producing Thrust. J. Exp. Biol. 2010, 213, 621–634. [Google Scholar] [CrossRef]


| Functional Traits | Code | Measure | Ecological Meaning |
|---|---|---|---|
| Mass (F/L) | M | Log (M + 1) | Volume, muscle mass |
| Relative head length (F) | RHL | HL/SVL | Predatory and feeding capabilities |
| Relative head width (F) | RHW | HW/SVL | Predatory and feeding capabilities |
| Relative snout length (F) | RSL | SL/SVL | Predatory and feeding capabilities |
| Relative diameter of eye (F) | RED | ED/SVL | Prey detection and discovery of natural predators |
| Relative interorbital space (F) | RIOS | IOS/SVL | Prey detection and discovery of natural predators |
| Relative length of lower arm (L) | RLAL | LAL/SVL | Jumping, locomotion performance |
| Relative length of hand (L) | RLH | LH/SVL | Jumping, locomotion performance |
| Relative hindlimb length (L) | RHLL | HLL/SVL | Swimming style, digging nests, and jumping |
| Relative tibia length (L) | RTL | TL/SVL | Swimming style, digging nests, and jumping |
| Relative width of tibia (L) | RTW | WT/SVL | Swimming style, digging nests, and jumping |
| Functional Traits | Q. boulengeri | A. sinensis | O. margaratae | |||
|---|---|---|---|---|---|---|
| PC1 (28.58%) | PC2 (16.74%) | PC1 (41.52%) | PC2 (12.66%) | PC1 (32.55%) | PC2 (18.50%) | |
| M | −0.53 | −0.16 | 0.84 | −0.84 | 0.92 | −0.24 |
| RHL | 0.31 | 1.02 | −0.94 | −0.16 | −0.92 | −0.40 |
| RHW | 1.17 | 0.35 | −1.41 | 0.05 | −1.31 | 0.02 |
| RSL | 0.88 | 0.37 | −1.14 | 0.35 | −1.22 | −0.46 |
| RED | 0.93 | −0.10 | −0.91 | −0.40 | −0.73 | −0.57 |
| RIOS | 0.24 | 0.95 | −0.81 | 0.10 | −0.73 | −0.36 |
| RLAL | −0.27 | 0.92 | −0.40 | 0.40 | −0.87 | 0.46 |
| RLH | 1.10 | 0.22 | −1.27 | 0.25 | −1.22 | −0.11 |
| RHLL | 1.01 | −0.89 | −1.20 | −0.56 | −0.56 | 1.41 |
| RTL | 1.04 | −0.38 | −1.23 | −0.09 | −0.88 | 1.09 |
| RTW | −0.38 | −0.09 | −0.31 | −1.36 | 0.60 | 1.10 |
| Microhabitat Variables | PC1 (49.85%) | PC2 (17.95%) |
|---|---|---|
| AT | −1.67 | −0.76 |
| AH | 0.59 | 0.60 |
| Wt | −1.89 | 0.03 |
| pH_w | 0.68 | 0.75 |
| Wd | −1.81 | −0.57 |
| Ww | −1.51 | 1.36 |
| Cd | 1.78 | 0.61 |
| Rc | 0.36 | −1.78 |
| Fr | −1.49 | 0.90 |
| Ele | 2.03 | −0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, X.; Kang, D.; Chen, G.; Hu, S.; Sun, Z.; Zhao, T. Intraspecific Variations in Ecomorphological Functional Traits of Montane Stream-Dwelling Frogs Were Driven by Their Microhabitat Conditions. Animals 2025, 15, 2243. https://doi.org/10.3390/ani15152243
Peng X, Kang D, Chen G, Hu S, Sun Z, Zhao T. Intraspecific Variations in Ecomorphological Functional Traits of Montane Stream-Dwelling Frogs Were Driven by Their Microhabitat Conditions. Animals. 2025; 15(15):2243. https://doi.org/10.3390/ani15152243
Chicago/Turabian StylePeng, Xiwen, Da Kang, Guangfeng Chen, Suwen Hu, Zijian Sun, and Tian Zhao. 2025. "Intraspecific Variations in Ecomorphological Functional Traits of Montane Stream-Dwelling Frogs Were Driven by Their Microhabitat Conditions" Animals 15, no. 15: 2243. https://doi.org/10.3390/ani15152243
APA StylePeng, X., Kang, D., Chen, G., Hu, S., Sun, Z., & Zhao, T. (2025). Intraspecific Variations in Ecomorphological Functional Traits of Montane Stream-Dwelling Frogs Were Driven by Their Microhabitat Conditions. Animals, 15(15), 2243. https://doi.org/10.3390/ani15152243






