1. Introduction
In the context of globalization, the laying hen industry, as an important part of the livestock industry, has always borne the heavy responsibility of meeting human demand for high-quality protein. Fatty liver syndrome (FLS) in laying hens is a nutritional and metabolic disease that frequently occurs in high-laying hens. The apparent characteristics of FLS in laying hens are a significant decrease in egg production rate, a significant increase in flock mortality, and the patho-anatomical features of hens with abundant abdominal fat reserves, enlarged livers, and a yellowish-brown or yellow color [
1]. The prevalence of FLS in laying hens is as high as 16% worldwide, and the disease affects feed intake, egg production, egg weight, and mortality of laying hens, causing great economic losses to the egg industry [
2]. Therefore, how to prevent and control the occurrence of FLS in laying hens has become a hot spot of current research.
Plant flavonoids are a class of secondary metabolites present in plants, belonging to polyphenolic compounds, which are widely found in the roots, stems, leaves and fruits of plants such as legumes, herbs, and tea. Studies have shown that flavonoids, as a kind of natural plant functional component, possess various biological characteristics such as antibacterial, anti-inflammatory, antioxidant, enhancing immunity, regulating lipid metabolism, and improving the health of the organism [
3,
4]. However, the application research of plant flavonoids in poultry production is relatively scarce. Currently, some studies have been conducted on citrus flavonoids [
5], mulberry-leaf flavonoids [
6], and total flavonoids from Epimedium [
7] in poultry. Tartary buckwheat flavonoids (TBF) are natural products extracted from Tartary buckwheat, which mainly contains natural active substances such as rutin and quercetin [
8]. The application research of TBF in poultry is relatively scarce. Studies on mice have confirmed that TBF has antioxidant, hypoglycemic, and anti-inflammatory effects [
9]. Studies have confirmed that TBF can effectively alleviate the vascular dysfunction and liver damage in mice induced by a high-fructose diet [
10]. In addition, TBF can alleviate insulin resistance and liver oxidative stress in mice caused by high levels of oxidized trimethylamine N-oxide [
11]. Based on this, we hypothesized that TBF could alleviate FLS in laying hens.
Vitamin D
3 (VD
3) is the traditional source of Vitamin D (VD) in laying hens’ diets, and 25-hydroxyvitamin D
3 (25-OHD) is an intermediate product of VD
3 metabolism; since 2006, 25-OHD has been widely used in the poultry industry as an alternative source of VD [
12]. 25-OHD circulates more easily in the blood and is the main form of circulation in the blood. Compared with VD
3, 25-OHD adds a hydroxyl group, and its water solubility is enhanced, so its absorption is neither affected by other fat-soluble vitamins, nor dependent on bile secretion and the microcluster structure formed by fat absorption. In addition, 25-OHD bypasses hepatic conversion, shortens the metabolic process of VD
3 in the organism, and has higher biological activity [
13]. The research has found that 25-OHD has a higher biological efficacy in calcium and phosphorus absorption, bone development, and immunity [
14,
15]. Furthermore, 25-OHD may directly regulate lipid metabolism via vitamin D receptor (VDR) in liver, suppressing lipogenic genes (e.g., SREBP-1c) and promoting fatty acid oxidation [
16]. Therefore, we hypothesize that the application of 25-OHD in FLS laying hens is more effective than VD
3, but no relevant research reports have been found so far.
The objective of this study is to establish a fatty liver syndrome (FLS) model in laying hens using a high-energy–low-protein (HELP) diet, and then to determine whether and how TBF and 25-OHD alone or in combination alleviated FLS in laying hens by measuring production performance, serum parameters, and liver gene expression related to lipid metabolism and gut microbial communities. This research can provide an important theoretical basis and data support for the prevention and mitigation of FLHS in laying hens.
2. Materials and Methods
2.1. Experimental Design and Animal Management
All experiments were performed in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the Institutional Animal Care and Use Committee of Xichang University (Protocol xcc2022012), China.
In this experiment, we selected 450 35 wk-old Lohmann laying hens of similar weight, and then randomly divided them into five groups. Each treatment group consisted of 6 replicates, with 15 hens per replicate. Each replicate was housed in 5 cages (3 hens per cage), and each cage measured 60 cm in length, 35 cm in width, and 45 cm in height. Treatment hens was uniformly distributed in the layer house to minimize environment effects. Each experimental group was fed with a different treatment diet. The five treatment diets used in the experiment (detailed in
Table 1) were as follows: the control diet (basal diet), the high-energy–low-protein (HELP) diet, the HELP diet supplemented with 60 mg/kg Tartary buckwheat flavonoids (TBF), the HELP diet supplemented with 69 μg/kg 25-hydroxyvitamin D
3(25-OHD), and the HELP diet co-supplemented with 60 mg/kg TBF and 69 μg/kg 25-OHD. The hens were raised in fully enclosed chicken coops, with the indoor temperature maintained at around 24 °C. The lighting duration is 16 h, and continuous ventilation is maintained inside the chicken coop. All hens had free access to water via nipple drinkers and feed via feed troughs. The hens were fed diets in mash form during the experiment (36 to 45 wk of age). The formulas and nutritional levels of the diets are shown in
Table 2. The nutrition level of the basic diet in this experiment was prepared according to the feeding management manual of Lohmann laying hens with l phase, The energy level of HELP diet is 3050 kcal/kg, and the protein level is 12%. The TBF used in this experiment was obtained from Fufeng Snoot Biotechnology Co., Ltd. in Baoji City, China, with a purity level 50%. 25-OHD was provided by Shandong Haineng Bioengineering Co., Ltd. in Rizhao City, China, with a purity level 0.05%.
2.2. Data Record and Sample Collection
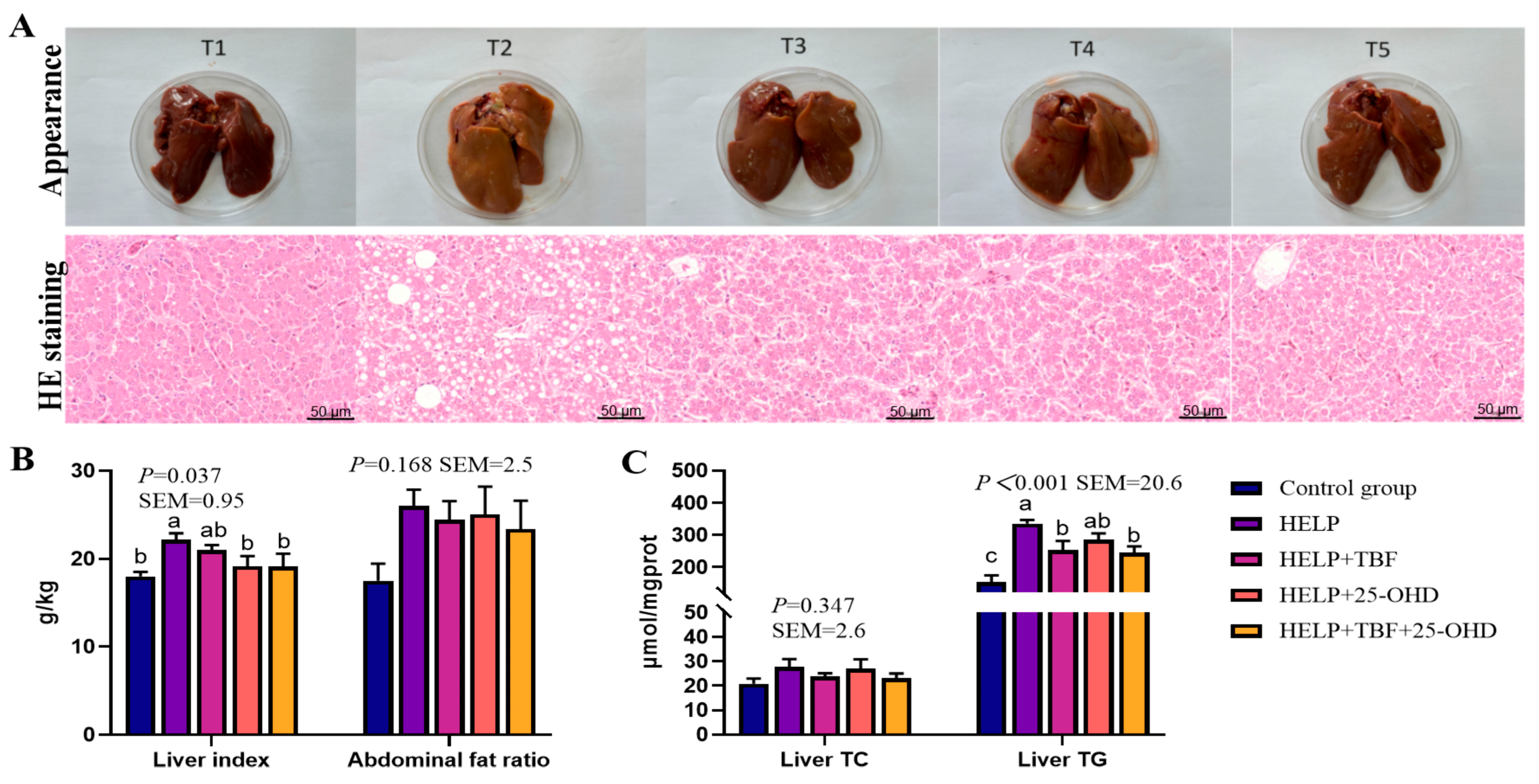
During the experiment, daily laying performance was documented for each replicate, and the death of the hens were recorded daily. Then hen-day egg production (HDEP), average egg weight (AEW), egg mass, feed conversion ratio (FCR), average daily feed intake (ADFI) at the laying stage were calculated. At the 8th wk of the experiment, one laying hen from each replicate was randomly selected, and 30 laying hens in total were fasted for 12 h. Blood was collected from the wing vein and centrifuged at 3000 rpm for 10 min, The upper serum samples were stored at a temperature of −20 °C for future testing purposes. The laying hens were weighed and then slaughtered. Fresh liver and abdominal fat were weighed to calculate the liver index and abdominal fat percentage. The complete liver tissue from all experimental hens underwent systematic photographic documentation. Liver samples were stored at −80 °C prior to RNA extraction, while adjacent tissue sections were fixed in 4% paraformaldehyde for subsequent histopathological assessment.
2.3. Egg Quality Analysis
In the 8th week of the experiment, for each repetition, 3 eggs were selected and their quality was measured. An egg multitester (EMT-7300, Robot-mation Co., Ltd., Tokyo, Japan) was used to evaluate Haugh unit, albumen height and yolk color. Eggshell strength was measured using an Eggshell Force Gauge Model II (Robot-mation Co., Ltd.). Eggshell thickness was measured at the large end, equatorial region, and small end of the egg with an Eggshell Thickness Gauge (Robot-mation Co., Ltd.).
2.4. Serum Parameters
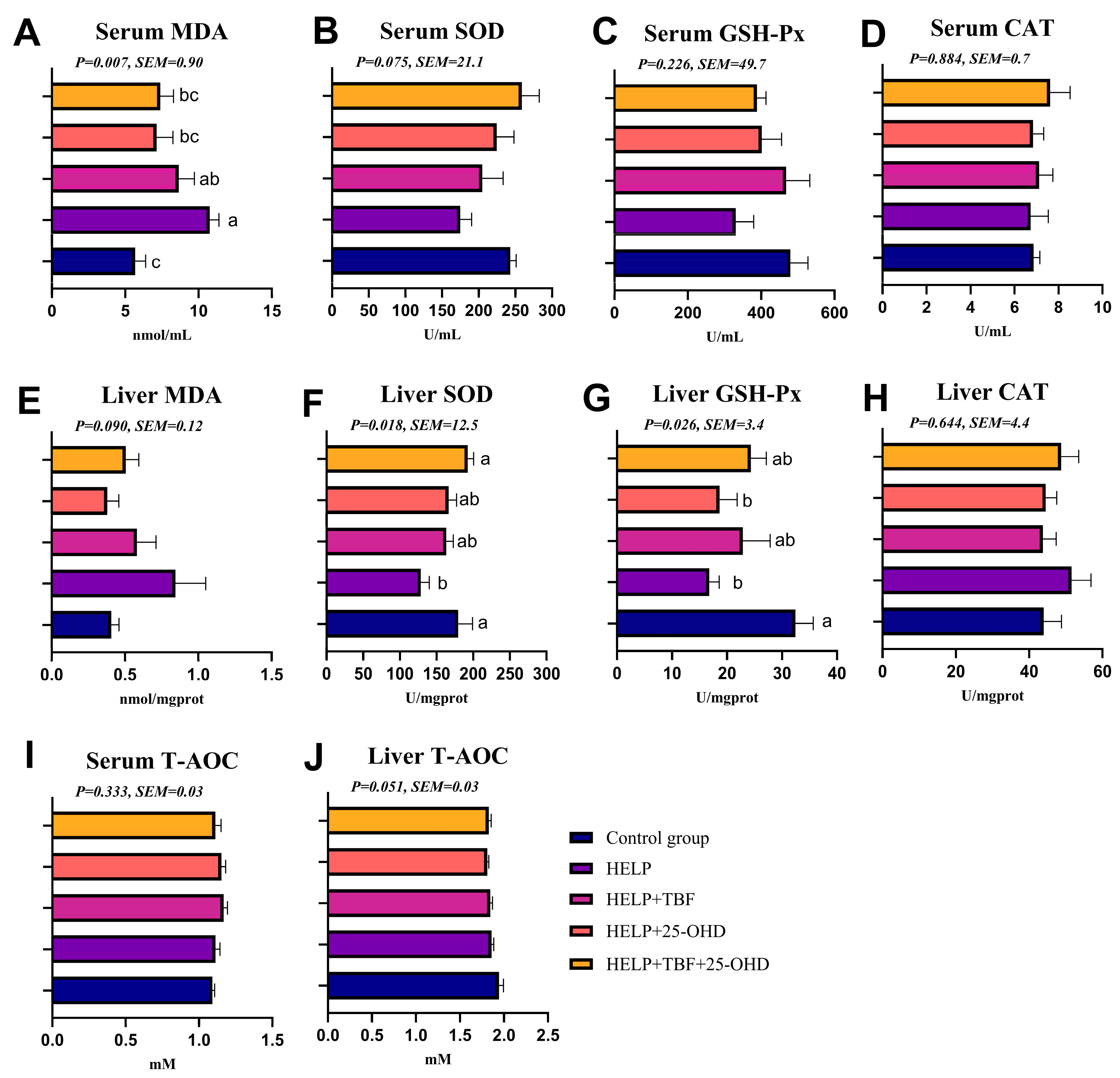
Serum parameters were determined including serum biochemical parameters and serum antioxidant capacity indexes. Serum total cholesterol (TC), triglycerides (TG), aspartate aminotransferase (AST), alkaline phosphatase (ALP), glucose (GLU), low-density lipoprotein cholesterol (LDL-C), high-density lipoproteincholesterol (HDL-C) were measured by an automatic biochemistry analyzer (Shenzhen Mindray Biomedical Electronics Co., Ltd., BS-460, Shenzhen, China). Serum non-esterified fatty acid (NEFA), malondialdehyde (MDA), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase, (CAT), and total antioxidant capacity (T-AOC) were detected by a specific assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Liver Antioxidant Capacity Parameters, TG and TC Contents
The levels of MDA, SOD, GSH-Px, CAT, T-AOC, TG, and TC in the liver were determined using the colorimetric method. Specific assay kits from Nanjing Jiancheng Bioengineering Institute (Nanjing, China) were employed for these measurements.
2.6. Hematoxylin-Eosin Staining
Hematoxylin-Eosin (H&E) staining was performed following the method described in previous studies [
17]. Liver tissues were fixed in 4% paraformaldehyde. After fixation, the tissues were dehydrated, embedded, and sectioned into slices approximately 5 μm thick. Subsequently, the sections were stained with hematoxylin and eosin. A microscope slide scanning imaging system (SQS-600P, Shenzhen Shengqiang Technology Co., Ltd., Shenzhen, China) was used to evaluate pathological changes in the liver.
2.7. Real-Time Quantitative PCR
Total RNA was extracted from liver tissues using the Tissue Total RNA Isolation Kit (YEASEN, Shanghai, China), followed by cDNA synthesis using the PrimeScrip™ RT Reagent Kit (Takara Bio, Dalian, China). The primers were designed by using NCBI in
Table 3. Real-time PCR was performed using a real-time fluorescence quantitative PCR system (Chengdu LILAI, Chengdu, China) and carried out on the QuantStudio™ 3 instrument (Thermo Fisher Scientific, Waltham, MA, USA). Relative mRNA expression levels were calculated using the 2−ΔΔCt method, with β-actin serving as the housekeeping gene to normalize gene transcription.
2.8. 16S rRNA Sequencing
The microbial genomic DNA was extracted from the contents of the hen’s colon for sequencing analysis of the 16S rRNA gene. We used specific primers to amplify the V3-V4 region of the bacterial 16S gene. Subsequently, a DNA library was constructed using standard Illumina reagents and sequenced in dual-end mode on the NovaSeq 6000 platform. The raw sequences were truncated during processing, during which the PCR primers were removed. Sequences with expected errors were removed. Through filtering, denoising, and by joining the read segments, the sequences were grouped into operational taxonomic units (OTUs), and species annotation and abundance analysis were performed to determine the taxonomic composition of the samples. After completing these steps, other analyses were also conducted, such as α-diversity assessment and β-diversity assessment, to reveal the differences between individual samples and among different sample groups.
2.9. Statistical Analysis
Statistical analyses of the data from this trial were conducted using SPSS software (version 25.0; IBM Inc., New York, NY, USA). Meanwhile, GraphPad Prism version 8.0.2 (GraphPad Software, La Jolla, CA, USA) was employed for generating figures and formatted to a resolution of over 600 dpi. The data were reported as means along with pooled standard errors of the means (SEM). One way analysis of variance (ANOVA) was performed for significance testing, and a p value less than 0.05 was defined as statistically significant.
4. Discussion
Widely known as a noninfectious condition, FLS frequently plagues laying hens, adversely affecting their productive performance. Feeding hens with HELP diets has been widely recognized as being able to experimentally induce FLS in hens, and this has been reported in many studies [
2,
18,
19,
20,
21,
22]. Consistent with the findings of this study, previous research has shown that feeding laying hens with the HELP diet leads to a significant decrease in their egg production rate, egg weight, and average egg weight, and also results in a substantial increase in FCR [
23]. Furthermore, analysis of our research data indicates that the addition of TBF and 25-OHD to the HELP diet does not result in any enhancement of production performance. A previous study also found that the addition of citrus flavonoids did not enhance egg quality and production performance [
5]. In another similar study, adding 0.5 or 2 g of total flavonoids from Rhizoma Drynariae to the diet per kilogram had no effect on the egg production rate, feed intake, and feed conversion ratio of laying hens [
24]. The application effect of 25-OHD in laying hens is controversial. Numerous studies have shown that in the diet of laying hens containing VD3, adding 25-OHD additionally does not improve the laying performance and egg quality [
12,
25,
26]. However, the research also found that dietary supplementation with 50 μg/kg of 25-OHD and 2000 IU/kg of VD3 improved eggshell strength, thick albumen height, and the Haugh unit [
27]. In this study, supplementing the HELP diet with 25-OHD improved eggshell strength, albumen height, and the Haugh unit. This evidence demonstrates that, specifically for laying hens with FLS, adding 25-OHD to their feed contributes to a notable enhancement in egg quality.
Serum biochemical indices serve as reliable indicators for assessing metabolic status, nutritional state, and physiological or pathological alterations in animals. In this study, it was also found that the contents of LDL-C and TG in the serum of laying hens in the HELP group significantly increased, and the addition of TBF and 25-OHD to the HELP diet could reduce the contents of LDL-C and TG in the serum. Research has demonstrated that plant extracts contain natural molecular compounds that can effectively regulate lipid metabolism in poultry [
28]. A recently published study has demonstrated that adding mulberry leaf extract rich in flavonoids to the HELP diet can significantly reduce the levels of TG, low-density lipoprotein and very low-density lipoprotein in laying hens [
23]. Polyphenols, which include flavonoids, similarly regulate lipid profiles, as Chang et al. (2025) showed that Myrica rubra pomace polyphenols modulated metabolic markers in diabetic mice, supporting their role in reducing TG and LDL [
29]. When 0.2% and 0.4% of green tea catechins were added to the feed of laying hens, it was found that the levels of TC, TG, and LDL in the plasma could be reduced [
30]. When adding 100 and 200 g/kg of the flavonoid compound baicalin to broiler diets, it significantly reduced the levels of TC, TG, and LDL-C in their blood serum [
31]. At present, there is no direct evidence indicating that 25-OHD has a direct impact on the serum indicators related to lipid metabolism in laying hens. However, considering its roles in calcium and phosphorus metabolism, liver function regulation, and lipoprotein biosynthesis, it may regulate blood lipid levels through indirect pathways. There remains a significant research gap in understanding the regulatory pathways and causal relationship between 25-OHD and serum lipid profiles. Notably, lipid metabolism is governed by complex signaling networks, Chu et al. (2022) revealed that MC-LR exacerbates liver lipid disorders in obese mice via the PI3K/AKT/mTOR/SREBP1 pathway, highlighting potential pathways that 25-OHD might interact with but whose involvement remains uncharacterized [
32]. Furthermore, Zhang et al. (2025) identified an association between serum vitamin D levels and cardiovascular disease in type 2 diabetic patients, a condition linked to lipid profile abnormalities, yet the underlying mechanisms connecting vitamin D to lipid regulation remain unclear [
33]. Therefore, future investigations should be designed to address this knowledge gap.
In addition, in this study, we demonstrated that supplementing a HELP diet with TBF and 25-OHD not only enhanced the antioxidant capacities of the liver and serum but also reduced the expression of inflammatory factor genes in the liver, with the combined supplementation of the two substances yielding a more pronounced effect. Studies have shown that adding 2 g/kg of Rhizoma Drynariae total flavonoids to the diet can significantly increase the serum T-AOC and GSH-px activities of aged cage-layer hens [
24]. The inclusion of dandelion flavonoid extract in the diet significantly elevated the activities of T-AOC, T-SOD, and GSH-Px in the plasma of spent laying hens [
34]. The anti-inflammatory effects of flavonoids have been extensively studied. Research has shown that quercetin (a well-known flavonoid) can inhibit the increase in the expression levels of interleukin-1β and TLR-4 in the small intestinal mucosa induced by lipopolysaccharide [
35]. Consistent with our findings, earlier studies observed that vitamin D supplementation can attenuate inflammatory markers, particularly under severe inflammatory conditions [
36]. The latest research results on sows also indicate that 25-OHD can improve the oxidative stress state in their plasma, enhance the antioxidant capacity of the placenta, and alleviate the oxidative stress condition of the placenta [
37].
Nutritional imbalance, especially the imbalance between protein and energy in the feed (similar to what we observed in the HELP feed), is the main cause of FLS [
38]. This is supported by findings that low protein (13%) combined with high fat-derived energy (3000 kcal/kg) disrupts lipoprotein assembly (due to insufficient protein for apolipoprotein synthesis), leading to hepatic lipid accumulation and hemorrhage—key features of FLS [
39]. As expected, the current research shows that the liver in the HELP group appears pale yellow and is heavily laden with lipid droplets. Birds mainly rely on liver tissue for fat synthesis and metabolism. An increase in fatty acid synthesis and a disruption in fatty acid oxidation and decomposition can lead to fat accumulation in the liver [
40]. High-energy diets have been confirmed to induce excessive liver fat deposition (up to 56.66% in dry matter) in laying hens, while natural additives like propolis can reduce liver fat ratio to 28.50–32.00%, mirroring the effects of TBF and 25-OHD observed in this study [
41]. The process of fatty acid synthesis from scratch involves three distinct steps: fatty acid synthesis, fatty acid elongation, and subsequently the assembly into triglycerides [
42]. Interestingly, in the present study, the dietary co-administration of TBF and 25-OHD was found to effectively alleviate hepatic lipid accumulation and liver injury induced by a HELP diet in laying hens with FLS, this is primarily mediated by downregulating hepatic lipogenic genes including ACC, FAS, GPAT1, ChREBP1, LXRα, SREBP-1c, SREBP-2, and FABP. Previous studies have also shown that adding mulberry leaf flavonoids to the diet can significantly reduce the expression level of SREBP-1c mRNA in the liver of aged laying hens [
6]. Similar studies have found that adding 0.8 and 1.2% mulberry leaf extract to the diet of laying hens can significantly reduce the relative expression levels of FASN, PPAR-γ, and SREBP-1c mRNA in the liver [
43]. Beyond the lipogenic gene pathways discussed, flavonoids like TBF may also regulate lipid metabolism through bile acid metabolism and intestinal FXR signaling. Cai et al. demonstrated in 2025 that caffeic acid phenethyl ester alleviates obesity via such routes, supporting the potential of related compounds in these pathways [
44]. The number of investigations regarding the impacts of 25-OHD on lipid metabolism within the liver of laying hens is relatively limited. Hence, further research in this area is highly warranted.
The gut microbiota plays a pivotal role in maintaining energy homeostasis, regulating lipid metabolism, and safeguarding intestinal health, thereby ensuring the harmonious operation of the gut-liver axis. Imbalances in the gut microbiota are closely associated with liver damage, the development of FLS, and obesity [
45]. Recent studies have shown that gut microbial metabolites like
Faecalibacterium prausnitzii-derived butyric acid can regulate key metabolic enzymes via post-translational modifications—by competitively inhibiting GAPDH lysine 263 lactylation and promoting its butyrylation—thereby reshaping glycolytic metabolism [
46]. In the intestinal microbiota of FLS laying hens, it was observed that the abundance of the Proteobacteria phylum increased, while the relative structures of the Firmicutes phylum and the Bacteroidetes phylum changed [
47]. Previous studies have also found that in laying hens with severe liver fibrosis and fibrosis syndrome, the number of Firmicutes in their cecum has decreased, the characteristic manifestation is a significant reduction in the abundance of Lactobacillus, while the abundance of
Roseobacter and
Pseudomonas has increased [
48]. Similarly, in the present study, we observed that, relative to the control group, the abundance of
Firmicutes_D in the HELP group decreased. Conversely, when TBF and 25-OHD were added to the HELP diet, a tendency towards an increase in the abundance of
Firmicutes_D was noted. A previous study found that Lactobacillus supplements can improve the development of non-alcoholic fatty liver disease by reorganizing the intestinal microbiota and metabolites [
49]. Plant-derived compounds like Litsea cubeba essential oil also regulate gut microbiota, as Chen et al. (2023) reported it increased the abundance of
Oscillospiraceae_UCG-005, Faecalibacterium,
Blautia, and
Coprococcus in pig fecal microflora [
50]. Over a period of 35 days, when 45 g/d of mulberry leaf flavonoids were administered to water buffaloes, the abundance of the Proteobacteria phylum in the rumen significantly increased, while the relative abundances of the Actinobacteria phylum, Bacteroidetes phylum and Chlorobacteria phylum decreased [
51]. Adding mulberry leaf extract to the HELP diet significantly reduced the relative abundance of Desulfurization bacteria at the phylum level, and significantly increased the relative abundance of Desulfurization bacteria at the genus level [
23]. Studies on rats have shown that intraperitoneal injection of 5 μg/kg dose of 1,25(OH)2D3 can reverse the intestinal microbiota imbalance caused by a high-fat diet. This is achieved by increasing the relative abundance of Lactobacillus and reducing the relative abundances of Acetobacter, Rosebacter, and Flavobacter [
52]. In this study, we also found that feeding the HELP diet to laying hens significantly reduced the abundance of Lactobacillus; however, adding TBF and 25-OHD concurrently to the HELP diet reversed the decrease in the abundance of Lactobacillus. Such microbiota-regulating effects align with findings by He et al. (2024), who showed that bioactive compounds (graveoline) alleviated liver injury partly by modulating gut microbial balance, including changes in beneficial genera like Lactobacillus [
53]. This indicates that the combined use of TBF and 25-OHD can alleviate and prevent FLS in laying hens by regulating the intestinal microbiota of the hens.