Genome-Wide Identification and Expression Analysis of Hsp70 Gene Family of Procambarus clarkii Reveals Its Immune Role in Response to Bacterial Challenge After Non-Lethal Heat Shock
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Genome-Wide Identification and Protein Characteristic Analysis of the Hsp70 Gene Family
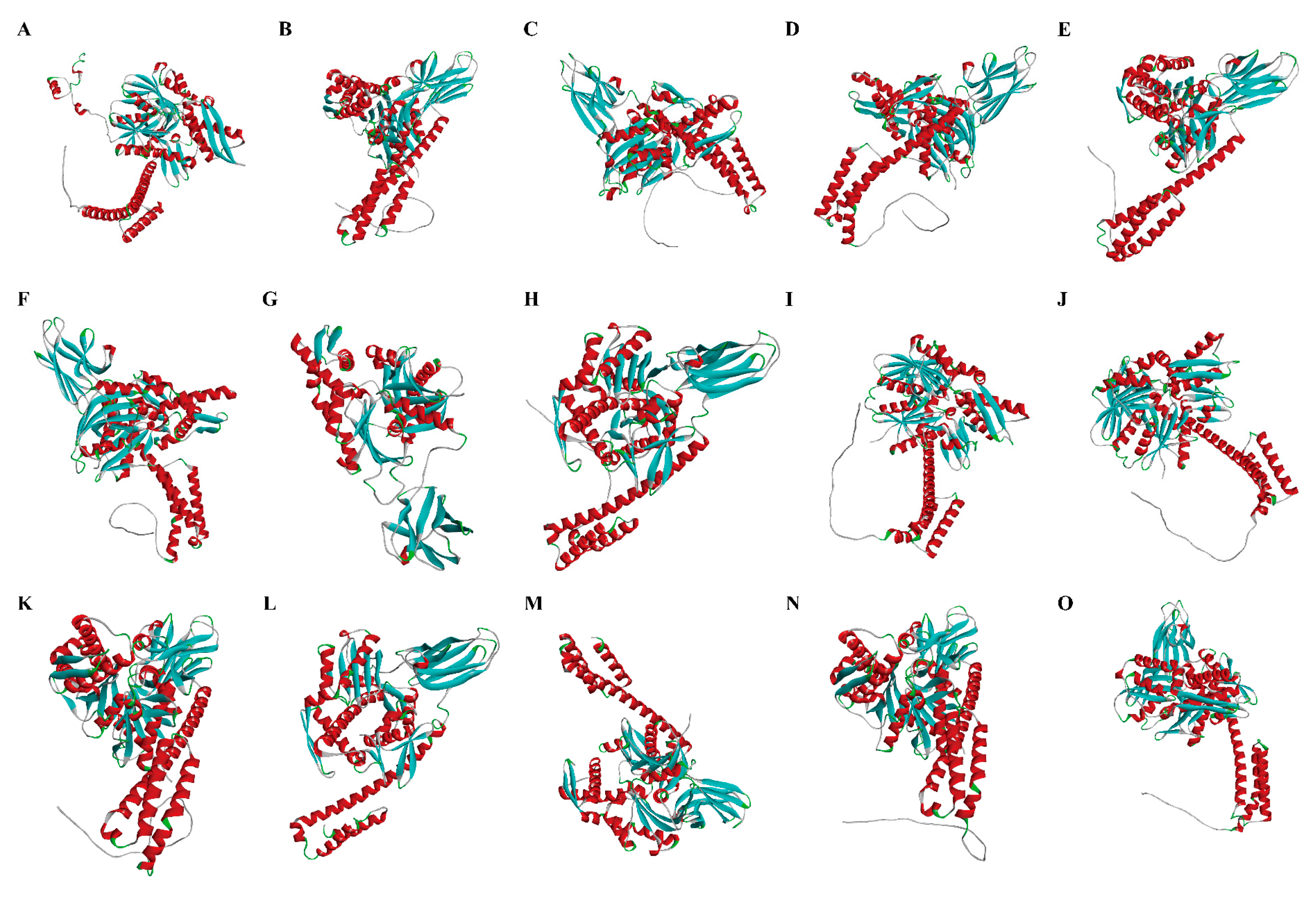
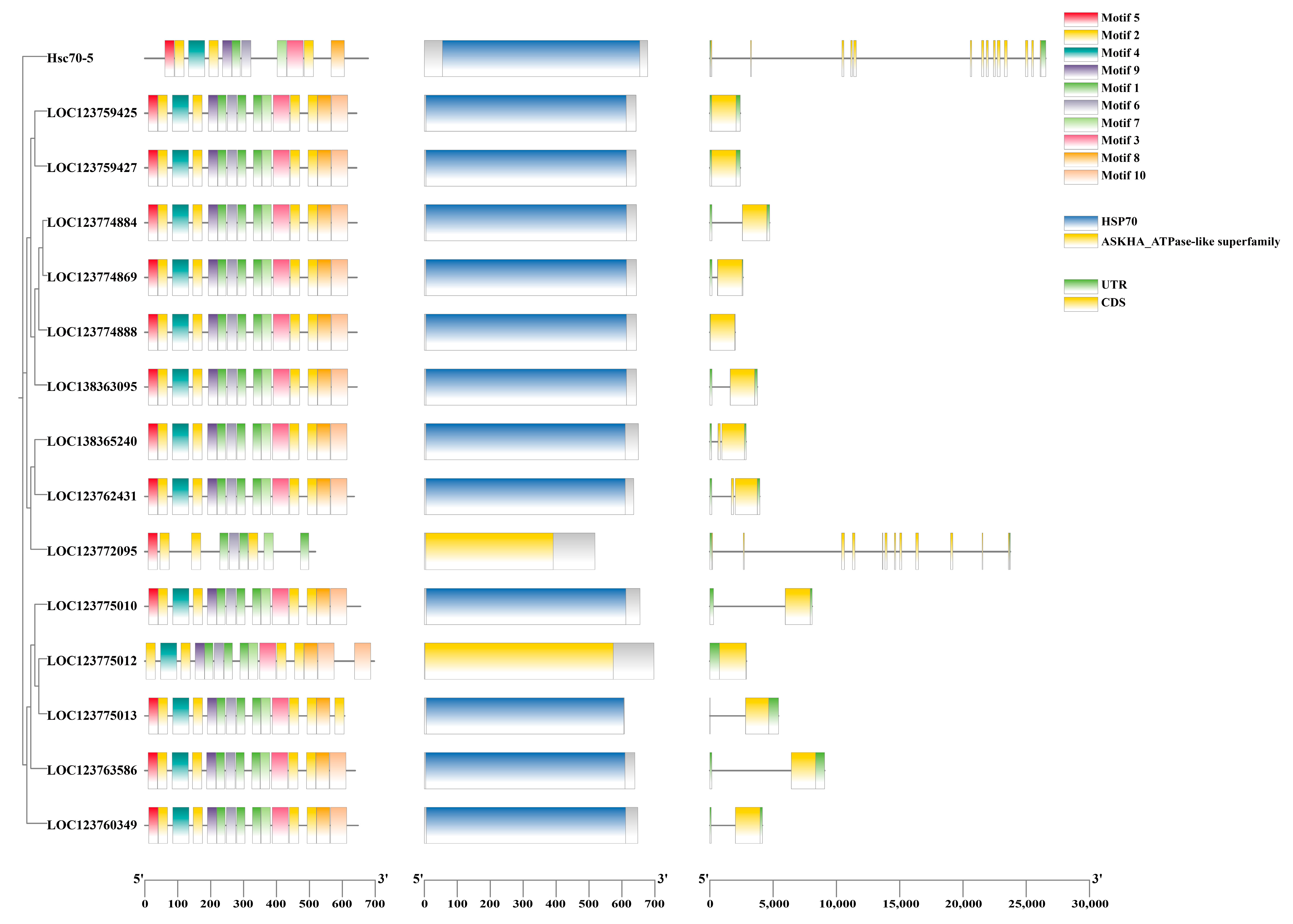
2.3. Gene Structure, Protein Domain, and 3-Dimensional Structural Analysis of P. clarkii Hsp70
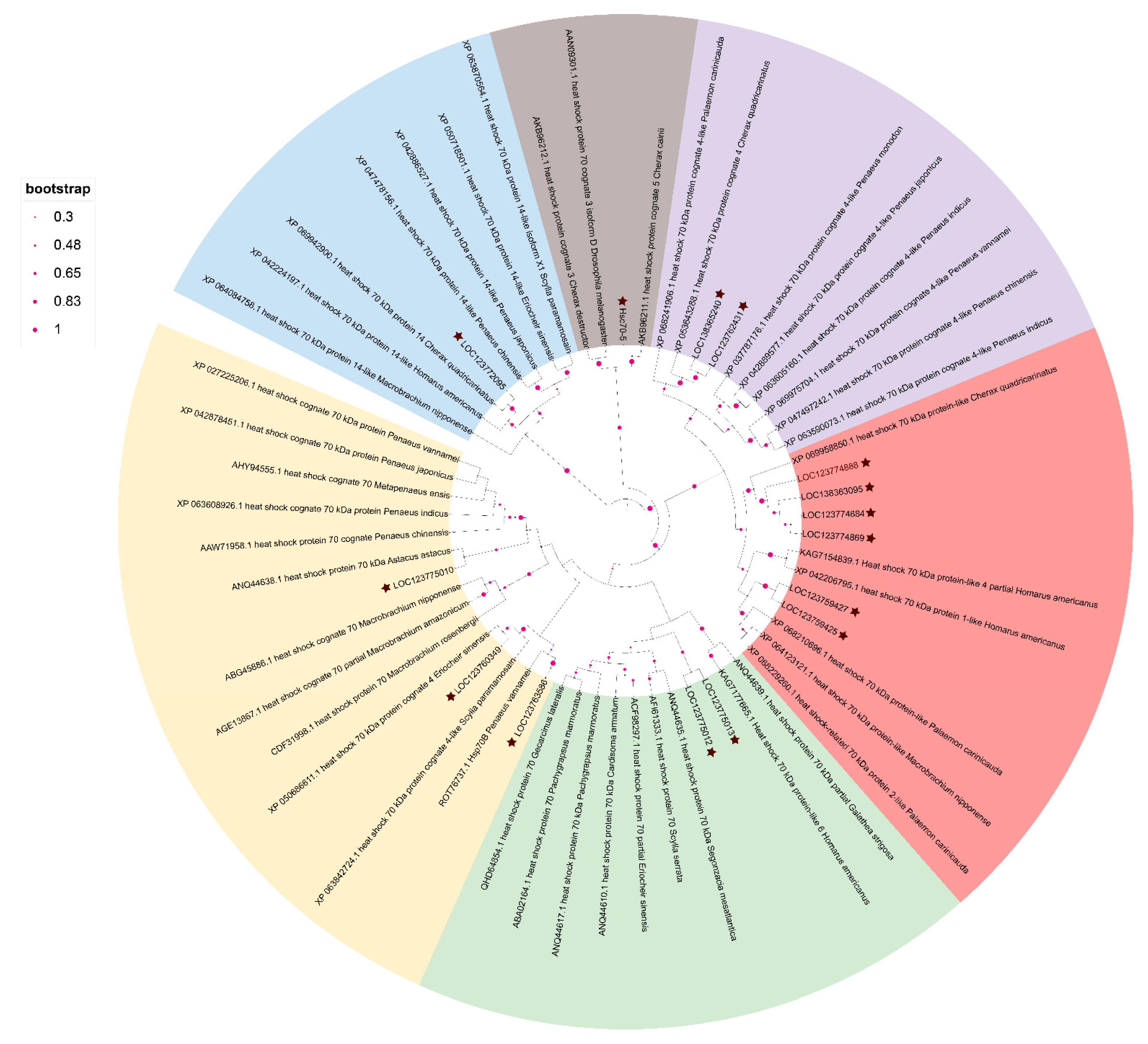
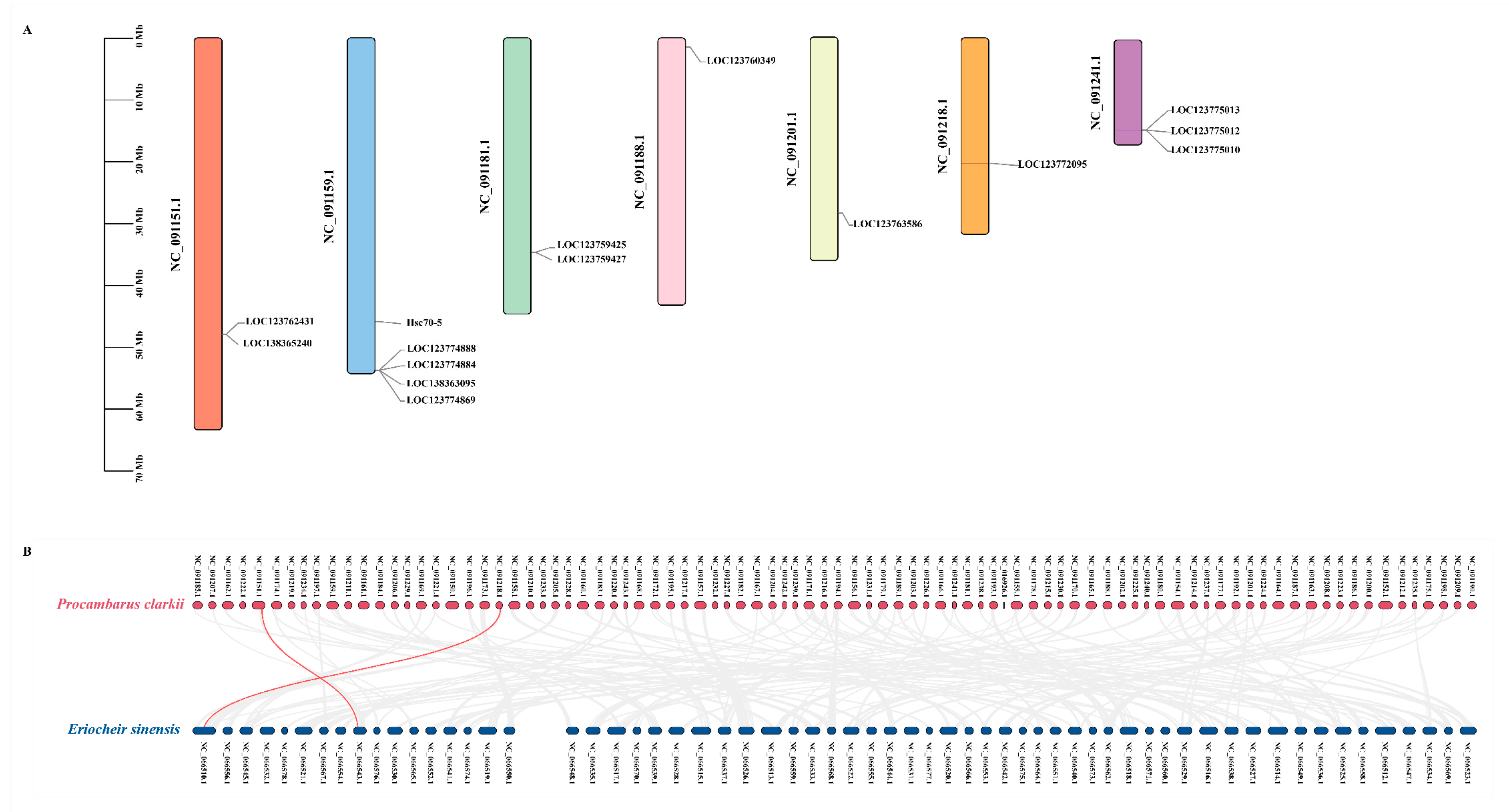
2.4. Chromosome Localization, Gene Duplication, and Phylogenetic Analysis of PcHsp70 Family
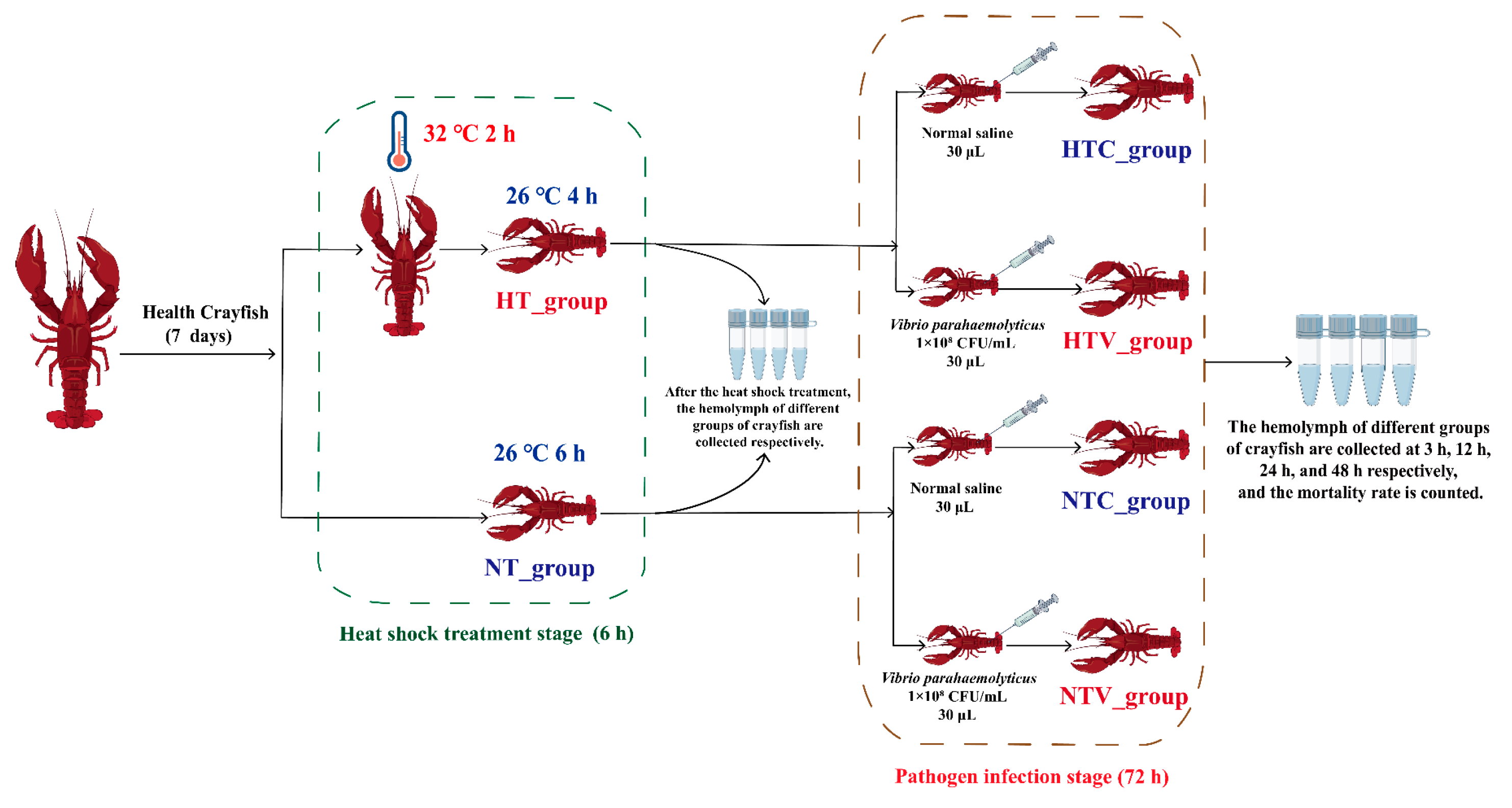
2.5. Animals and Sample Collection
- (1)
- Control group (NT_group): Crayfish were maintained in 26 °C water for 6 h without heat shock treatment.
- (2)
- Heat shock treatment group (HT_group): Crayfish were subjected to heat treatment in a 32 °C water bath for 2 h, followed by recovery in 26 °C water for 4 h.
- (1)
- HTC_group: The HT_group was injected with 30 μL of normal saline (NS).
- (2)
- HTV_group: The HT_group was injected with 30 μL of V. parahaemolyticus (1.0 × 108 CFU/mL).
- (3)
- NTC_group: The NT_group was injected with 30 μL of NS.
- (4)
- NTV_group: The NT_group was injected with 30 μL of V. parahaemolyticus (1.0 × 108 CFU/mL).
2.6. Survival Rate Statistics
2.7. The Expression Analysis of Hsp70 Genes in Response to Bacterial Challenge After NLHS
2.8. Suppression of Hsp70 Gene Expression by Double-Stranded RNA (dsRNA)
3. Results
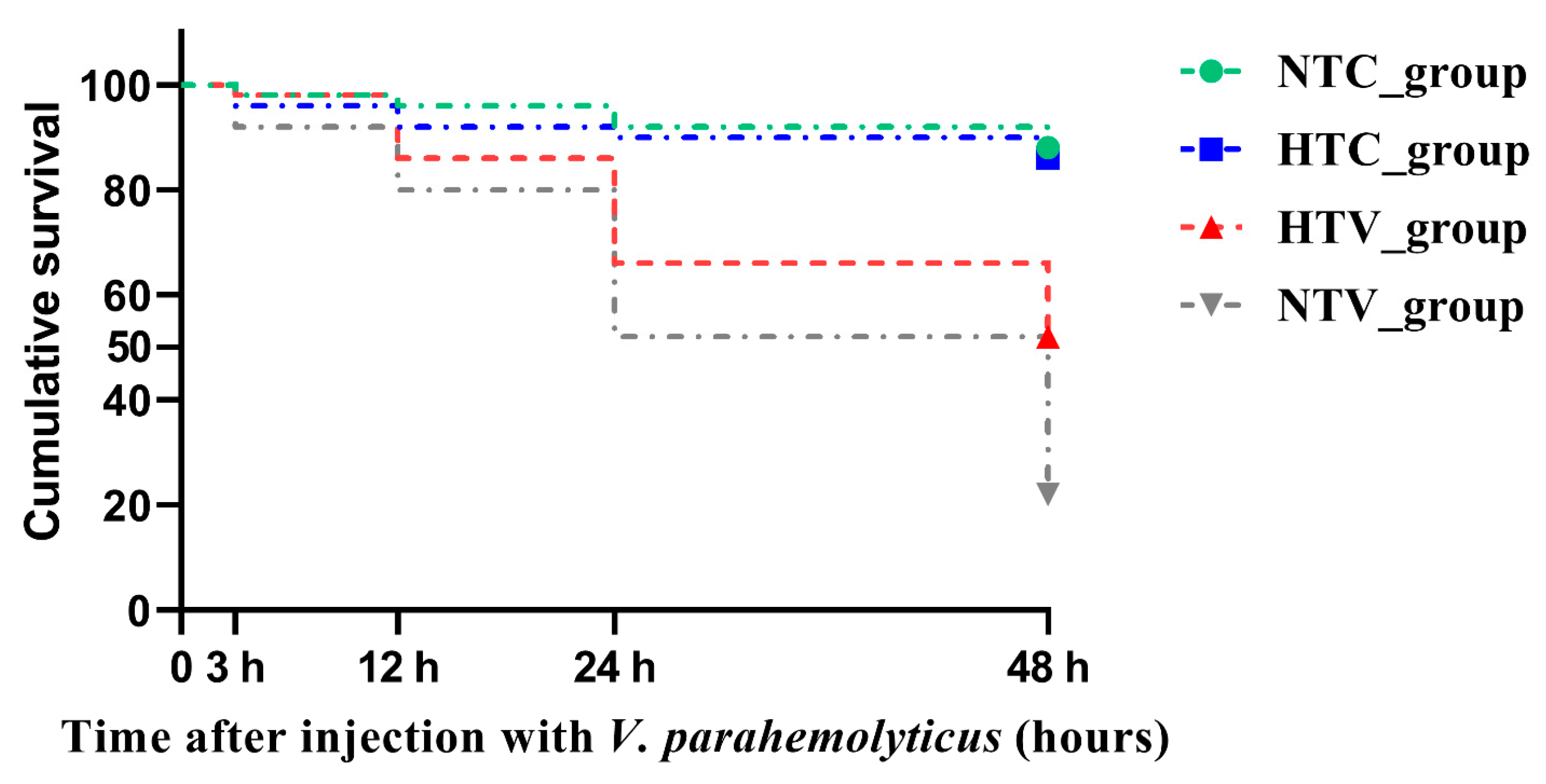
3.1. Survival Rate of P. clarkia Post Injection with V. parahaemolyticus Following NLHS
3.2. Identification and Characterization of Hsp70 Genes in P. clarkii
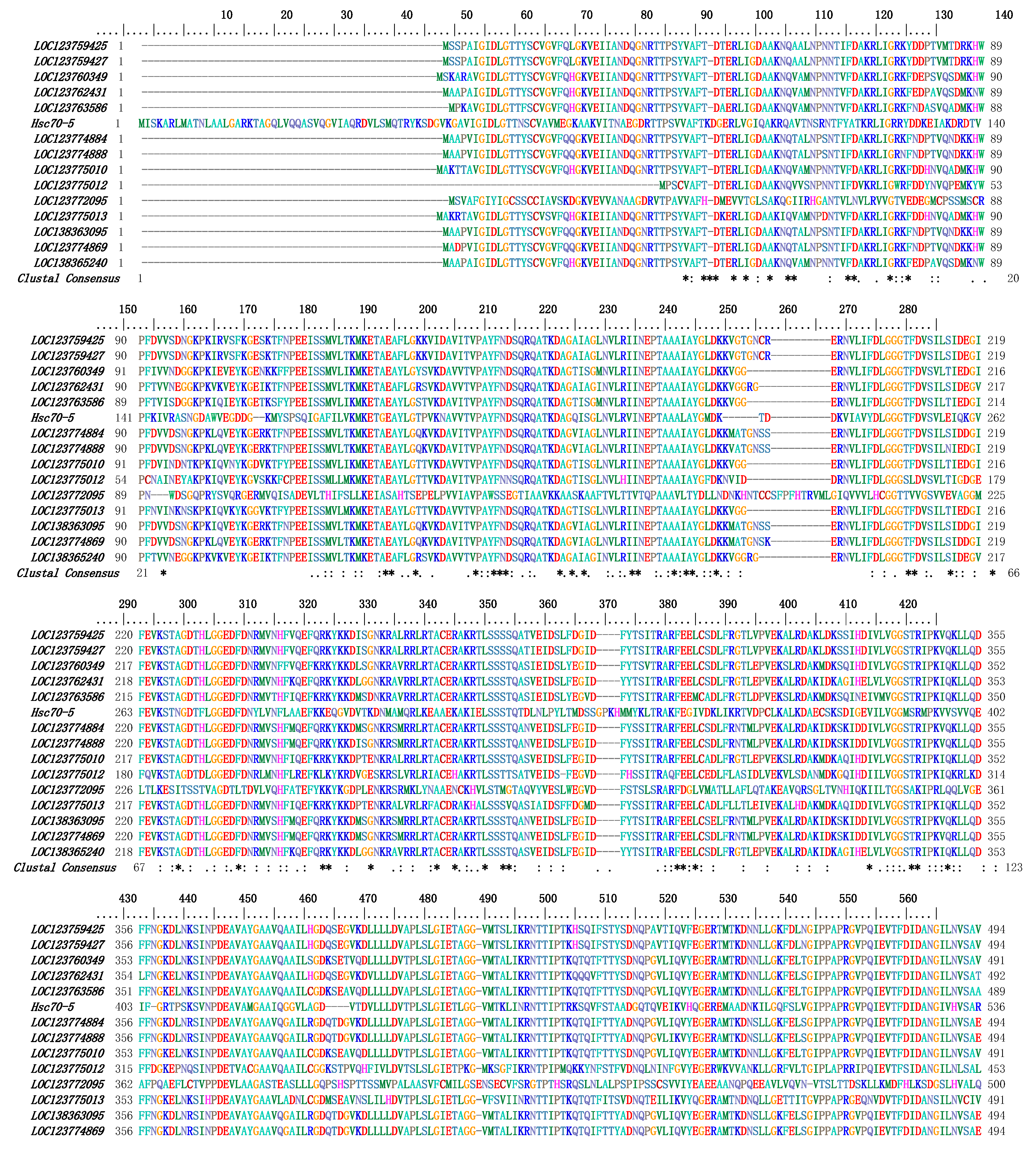
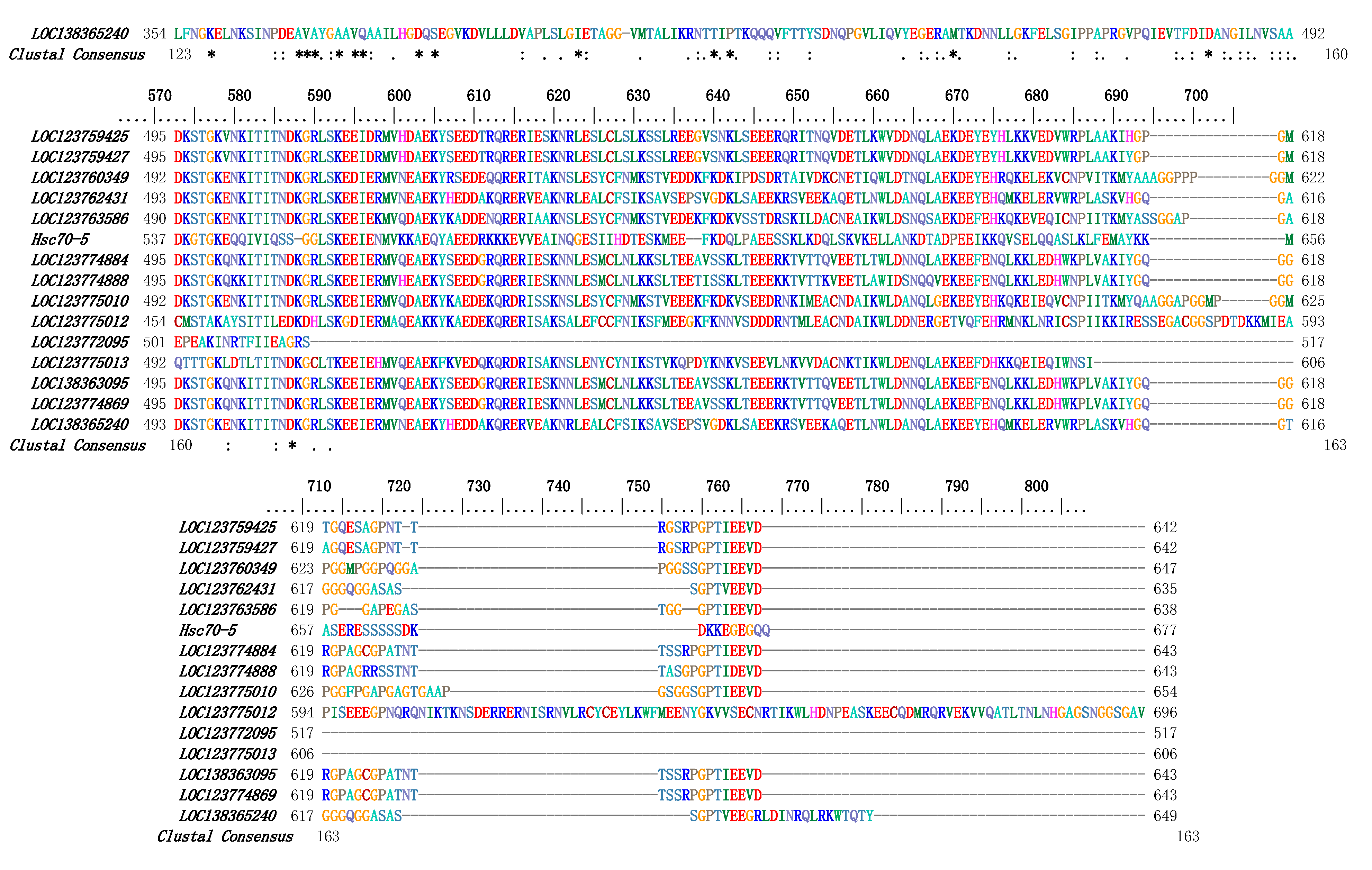
3.3. Multiple Sequence Alignment and Phylogenetic Analysis of PcHSP70s
3.4. Conserved Motifs, CDD Domain, and Gene Structure of Hsp70 Genes
3.5. Chromosomal Location and Gene Duplication Analysis of Hsp70 Genes
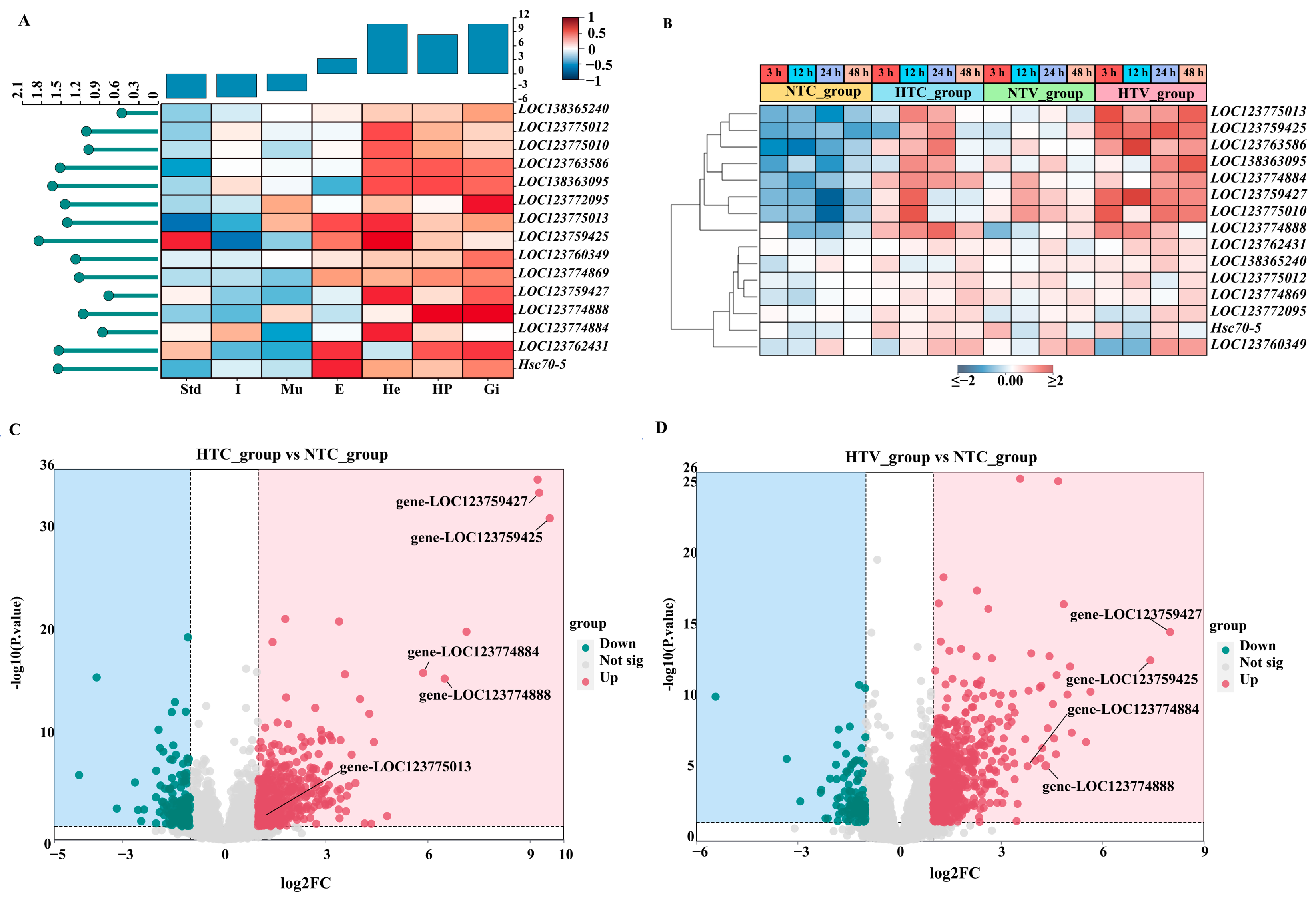
3.6. Expression Patterns of PcHsp70 Genes in Different Tissues
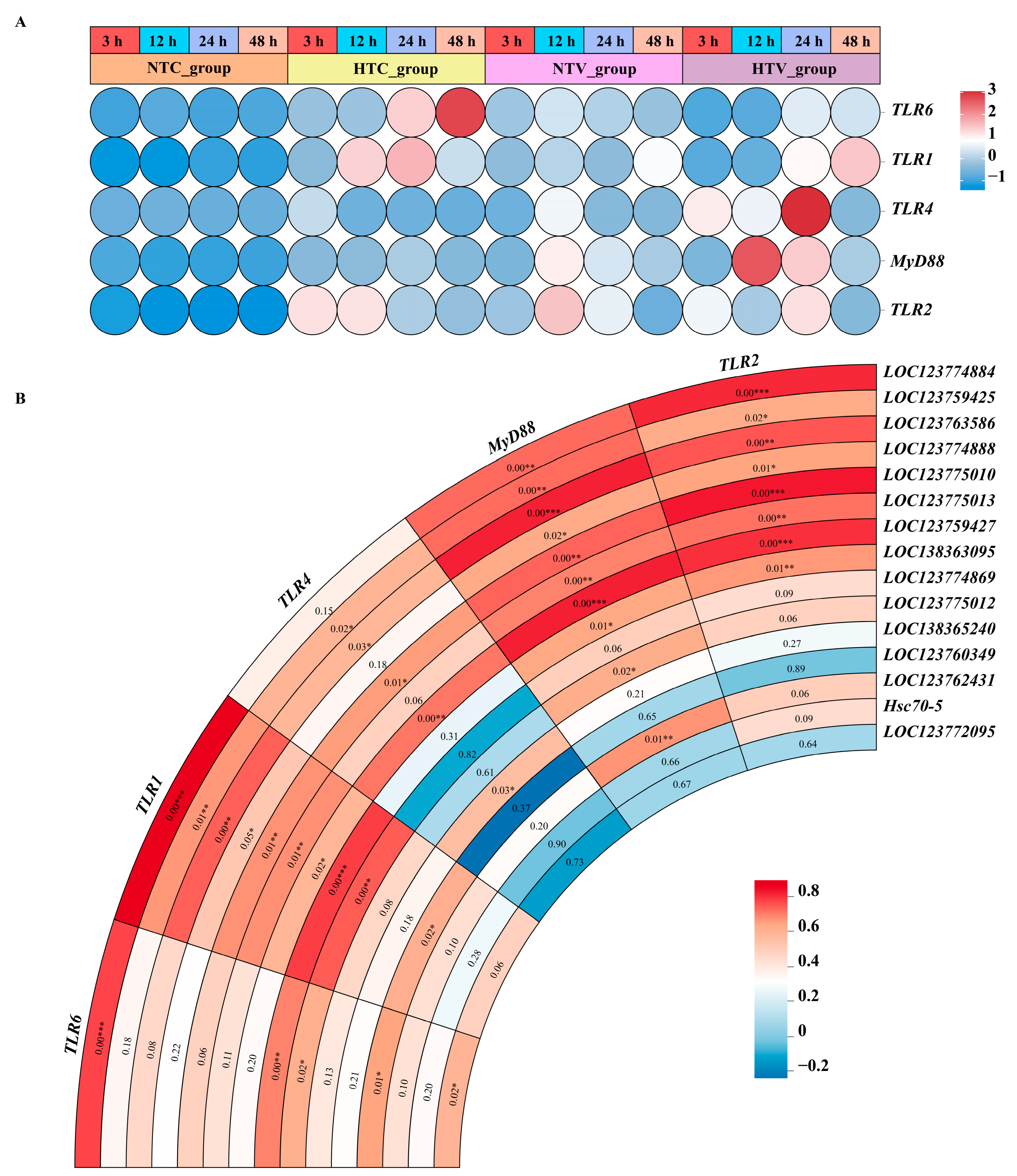
3.7. Expression Patterns of Hsp70 Genes in Response to Bacterial Challenge After NLHS
3.8. The Protein-Protein Interaction of HSP70s
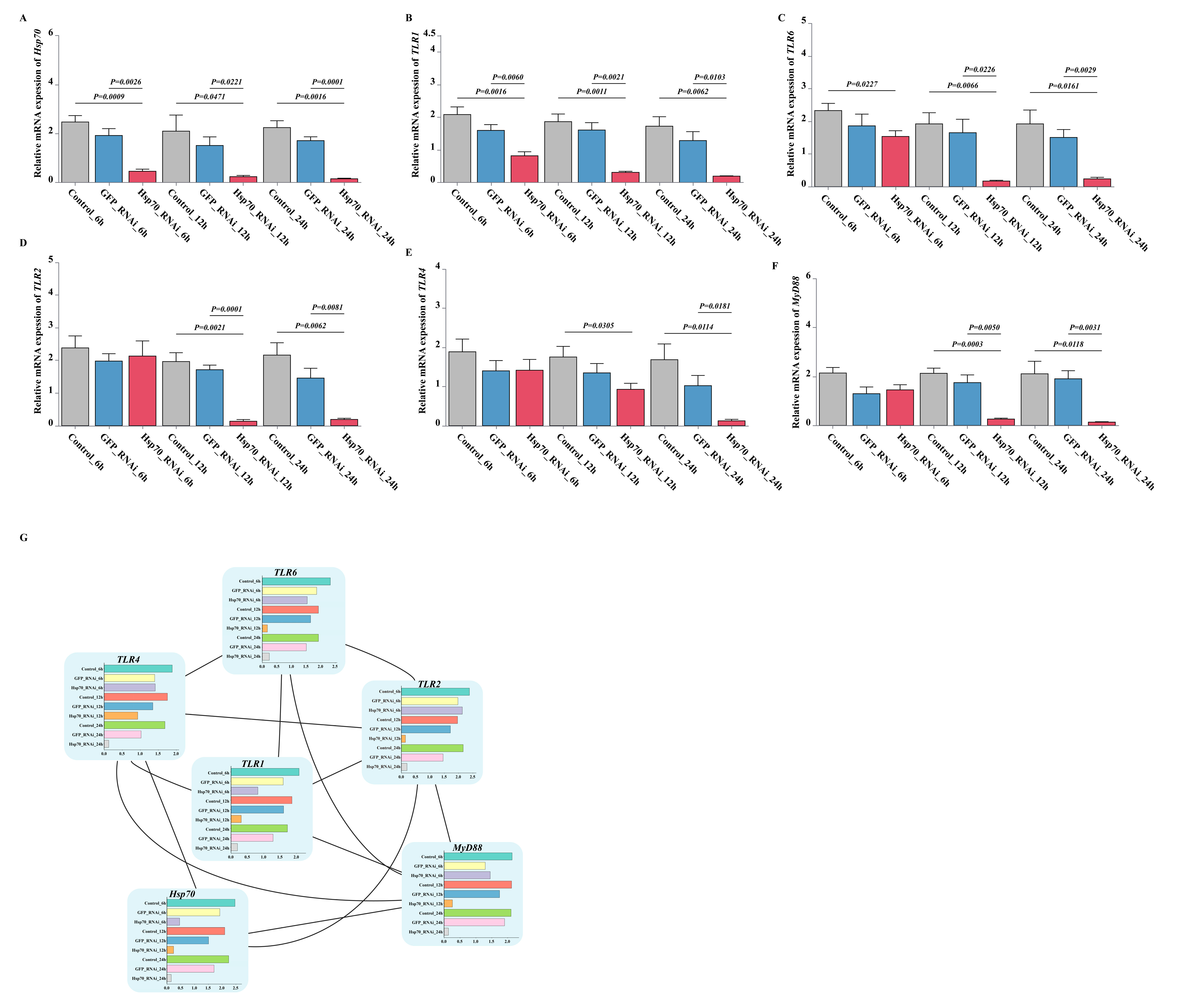
3.9. Effect of dsRNA Exposure Assay for Hsp70 Gene Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kazmi, S.S.U.H.; Wang, Y.Y.L.; Cai, Y.-E.; Wang, Z. Temperature effects in single or combined with chemicals to the aquatic organisms: An overview of thermo-chemical stress. Ecol. Indic. 2022, 143, 109354. [Google Scholar] [CrossRef]
- Li, R.; Bai, S.; Yang, D.; Dong, C. A crayfish Ras gene is involved in the defense against bacterial infection under high temperature. Fish Shellfish Immunol. 2019, 86, 608–617. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, A.; Arshad, A.; Sung, Y.Y.; Jasmani, S. Effect of thermal stress on Hsp70 gene expression and female reproductive performance of giant freshwater prawn, Macrobrachium rosenbergii. Aquac. Res. 2018, 49, 135–150. [Google Scholar] [CrossRef]
- Abbas, A.S.A. The Challenge of Securing Future Food Production for Aquaculture Species under Environmental Change: Enhancing Physiological Performance Under Environmental Stress. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2024. [Google Scholar]
- Roberts, R.J.; Agius, C.; Saliba, C.; Bossier, P.; Sung, Y.Y. Heat shock proteins (chaperones) in fish and shellfish and their potential role in relation to fish health: A review. J. Fish Dis. 2010, 33, 789–801. [Google Scholar] [CrossRef] [PubMed]
- Junprung, W.; Nanakorn, Z.; Norouzitallab, P.; Supungul, P.; Vanrompay, D.; Bossier, P.; Tassanakajon, A. Thermal adaptation affects expression and regulation of metabolism-, stress-, and immune-related genes in Artemia franciscana populations. Aquac. Rep. 2024, 39, 102511. [Google Scholar] [CrossRef]
- Loc, N.H.; MacRae, T.H.; Musa, N.; Bin Abdullah, M.D.D.; Abdul Wahid, M.E.; Sung, Y.Y. Non-Lethal Heat Shock Increased Hsp70 and Immune Protein Transcripts but Not Vibrio Tolerance in the White-Leg Shrimp. PLoS ONE 2013, 8, e73199. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Aballay, A. Heat-shock transcription factor (HSF)-1 pathway required for Caenorhabditis elegans immunity. Proc. Natl. Acad. Sci. USA 2006, 103, 13092–13097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pan, L.; Tong, R.; Li, Y.; Si, L.; Chen, Y.; Li, D. The exploration of neuroendocrine regulation of crustacean hyperglycemic hormone (CHH) on innate immunity of Litopenaeus vannamei under ammonia-N stress. Mol. Immunol. 2021, 139, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Repsilber, D.; Martinetz, T.; Björklund, M. Adaptive Dynamics of Regulatory Networks: Size Matters. EURASIP J. Bioinform. Syst. Biol. 2009, 2009, 618502. [Google Scholar] [CrossRef]
- Rehman, A.; Atif, R.M.; Qayyum, A.; Du, X.; Hinze, L.; Azhar, M.T. Genome-wide identification and characterization of HSP70 gene family in four species of cotton. Genomics 2020, 112, 4442–4453. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.E.; Hofmann, G.E. Heat-shock proteins, molecular chaperones, and the stress response: Evolutionary and ecological physiology. Annu. Rev. Physiol. 1999, 61, 243–282. [Google Scholar] [CrossRef] [PubMed]
- Richter-Landberg, C.; Goldbaum, O. Stress proteins in neural cells: Functional roles in health and disease. Cell. Mol. Life Sci. CMLS 2003, 60, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sen, S.S.; Sukumaran, V. Role of HSP70 in cytoplasm protection against thermal stress in rohu, Labeo rohita. Fish Shellfish Immunol. 2014, 41, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Welch, W.J.; Gething, M.-j.; Clarke, A.R.; Viitanen, P.; Lund, P.; Haas, I.G.; Georgopoulos, C.; Ellis, R.J.; Laskey, R.A.; Lorimer, G.H. Heat shock proteins functioning as molecular chaperones: Their roles in normal and stressed cells. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 1993, 339, 327–333. [Google Scholar] [CrossRef]
- Moseley, P.L. Heat shock proteins and heat adaptation of the whole organism. J. Appl. Physiol. 1997, 83, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Deng, Z.; Xu, S.; Zhang, H.; Han, Z. Genome-wide identification and low-salinity stress analysis of the Hsp70 gene family in swimming crab (Portunus trituberculatus). Int. J. Biol. Macromol. 2022, 208, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Daugaard, M.; Rohde, M.; Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 2007, 581, 3702–3710. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.V.; Roy, S.; Behera, B.K.; Das, B.K. Heat Shock Proteins (Hsps) in Cellular Homeostasis: A Promising Tool for Health Management in Crustacean Aquaculture. Life 2022, 12, 1777. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Han, X.; Sun, S.; Jin, S.; Liu, M.; Han, Z. Genome-Wide Identification and Transcriptome Analysis of the Hsp70 Gene Family in Monodonta labio Reveals Its Role in Response to Nanoplastics Stress. Genes 2024, 15, 291. [Google Scholar] [CrossRef] [PubMed]
- Grewal, H.S.; Yoshinaga, T.; Ehsan, H.; Yu, E.; Kaneko, G. A genome-wide screening of the 70 kDa heat shock protein (HSP70) genes in the rotifer Brachionus plicatilis sensu stricto with a characterization of two heat-inducible HSP70 genes. Cell Stress Chaperones 2023, 28, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Fan, K.-p.; Hua, X.-t.; Liu, Y.-f.; Zhang, Z.-q.; Li, X.-h.; Liu, Y.; Liu, P.-f. HSP70 gene expression responses to the temperature stress in pufferfish (Takifugu Rubripes). Biosci. Biotechnol. Biochem. 2021, 85, 1088–1096. [Google Scholar] [CrossRef] [PubMed]
- Tukaj, S. Heat Shock Protein 70 as a Double Agent Acting Inside and Outside the Cell: Insights into Autoimmunity. Int. J. Mol. Sci. 2020, 21, 5298. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Jin, S.; Shou, C.; Han, Z. Hsp70 Gene Family in Sebastiscus marmoratus: The Genome-Wide Identification and Transcriptome Analysis under Thermal Stress. Genes 2023, 14, 1779. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.; Wang, J.; Liu, C.; Gao, X.; Zhang, Y.; Ding, J.; Hou, C.-C.; Zhu, J.; Lou, B.; Shen, W.; et al. Hif-1α/Hsf1/Hsp70 signaling pathway regulates redox homeostasis and apoptosis in large yellow croaker (Larimichthys crocea) under environmental hypoxia. Zool. Res. 2021, 42, 746–760. [Google Scholar] [CrossRef] [PubMed]
- Ribeil, J.A.; Zermati, Y.; Vandekerckhove, J.; Cathelin, S.; Kersual, J.; Dussiot, M.; Coulon, S.; Moura, I.C.; Zeuner, A.; Kirkegaard-Sørensen, T.; et al. Hsp70 regulates erythropoiesis by preventing caspase-3-mediated cleavage of GATA-1. Nature 2007, 445, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Hishiya, A.; Kitazawa, T.; Takayama, S. BAG3 and Hsc70 interact with actin capping protein CapZ to maintain myofibrillar integrity under mechanical stress. Circ. Res. 2010, 107, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Oficialdegui, F.J.; Sánchez, M.I.; Clavero, M. One century away from home: How the red swamp crayfish took over the world. Rev. Fish Biol. Fish. 2020, 30, 121–135. [Google Scholar] [CrossRef]
- Oficialdegui, F.J.; Clavero, M.; Sánchez, M.I.; Green, A.J.; Boyero, L.; Michot, T.C.; Klose, K.; Kawai, T.; Lejeusne, C. Unravelling the global invasion routes of a worldwide invader, the red swamp crayfish (Procambarus clarkii). Freshw. Biol. 2019, 64, 1382–1400. [Google Scholar] [CrossRef]
- Ministry of Agriculture and Rural Affairs. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2024. [Google Scholar]
- Yuan, G.; Zhu, L.; Jiang, X.; Zhang, J.; Pei, C.; Zhao, X.; Li, L.; Kong, X. Diagnosis of co-infection with white spot syndrome virus and Aeromonas veronii in red swamp crayfish Procambarus clarkii. Aquaculture 2021, 532, 736010. [Google Scholar] [CrossRef]
- Junprung, W.; Supungul, P.; Tassanakajon, A. HSP70 and HSP90 are involved in shrimp Penaeus vannamei tolerance to AHPND-causing strain of Vibrio parahaemolyticus after non-lethal heat shock. Fish Shellfish Immunol. 2017, 60, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Rakhshaninejad, M.; Zheng, L.; Nauwynck, H. Shrimp (Penaeus vannamei) survive white spot syndrome virus infection by behavioral fever. Sci. Rep. 2023, 13, 18034. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Luo, L. Genome-wide identification of Hsp70/110 genes in rainbow trout and their regulated expression in response to heat stress. PeerJ 2020, 8, e10022. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Xu, H.; Han, Z. Genome-wide identification of Hsp70 genes in the large yellow croaker (Larimichthys crocea) and their regulated expression under cold and heat stress. Genes 2018, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Yuan, Z.; Yu, S.; Yang, Y.; Li, Y.; He, C. Genome-wide identification of HSP70/110 genes in sea cucumber Apostichopus japonicus and comparative analysis of their involvement in aestivation. Comp. Biochem. Physiol. Part D Genom. Proteom. 2018, 28, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, H.; DeBarry, J.D.; Tan, X.; Li, J.; Wang, X.; Lee, T.-h.; Jin, H.; Marler, B.; Guo, H. MCScanX: A toolkit for detection and evolutionary analysis of gene synteny and collinearity. Nucleic Acids Res. 2012, 40, e49. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Huan, P.; Liu, B. RNAi assay in primary cells: A new method for gene function analysis in marine bivalve. Mol. Biol. Rep. 2012, 39, 8209–8216. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol. J. 2017, 15, 405–414. [Google Scholar] [CrossRef] [PubMed]
- Jeyachandran, S.; Chellapandian, H.; Park, K.; Kwak, I.-S. A Review on the Involvement of Heat Shock Proteins (Extrinsic Chaperones) in Response to Stress Conditions in Aquatic Organisms. Antioxidants 2023, 12, 1444. [Google Scholar] [CrossRef] [PubMed]
- Rehman, S.U.; Nadeem, A.; Javed, M.; Hassan, F.-U.; Luo, X.; Khalid, R.B.; Liu, Q. Genomic Identification, Evolution and Sequence Analysis of the Heat-Shock Protein Gene Family in Buffalo. Genes 2020, 11, 1388. [Google Scholar] [CrossRef] [PubMed]
- Fang, Z.; Sun, Y.; Zhang, X.; Wang, G.; Li, Y.; Wang, Y.; Zhang, Z. Responses of HSP70 Gene to Vibrio parahaemolyticus Infection and Thermal Stress and Its Transcriptional Regulation Analysis in Haliotis diversicolor. Molecules 2019, 24, 162. [Google Scholar] [CrossRef] [PubMed]
- Innan, H.; Kondrashov, F.A. The evolution of gene duplications: Classifying and distinguishing between models. Nat. Rev. Genet. 2010, 11, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Knighton, L.E.; Nitika; Omkar, S.; Truman, A.W. The C-terminal domain of Hsp70 is responsible for paralog-specific regulation of ribonucleotide reductase. PLoS Genet. 2022, 18, e1010079. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Su, S.; Song, C.Y.; Yu, F.; Zhou, J.; Li, J.-l.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Bao, J.; Xing, Y.; Feng, C.; Li, X.; Chen, Q. Proteomic Analysis of the Hemolymph After Metschnikowia bicuspidata Infection in the Chinese Mitten Crab Eriocheir sinensis. Front. Immunol. 2021, 12, 659723. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Das, B.K.; Dhar, S.; Bisai, K.; Pande, G.S.J.; Zheng, X.; Parida, S.N.; Adhikari, A.; Jana, A.K. Ecytonucleospora hepatopenaei (EHP) disease prevalence and mortality in Litopenaeus vannamei: A comparative study from Eastern India shrimp farms. BMC Microbiol. 2024, 24, 523. [Google Scholar] [CrossRef] [PubMed]
- Lyu, L.; Wen, H.; Li, Y.; Li, J.-F.; Zhao, J.; Zhang, S.; Song, M.; Wang, X. Deep Transcriptomic Analysis of Black Rockfish (Sebastes schlegelii) Provides New Insights on Responses to Acute Temperature Stress. Sci. Rep. 2018, 8, 9113. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen Recognition and Innate Immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Sarrand, J.; Baglione, L.; Parisis, D.; Soyfoo, M. The Involvement of Alarmins in the Pathogenesis of Sjögren’s Syndrome. Int. J. Mol. Sci. 2022, 23, 5671. [Google Scholar] [CrossRef] [PubMed]
- Vabulas, R.M.; Ahmad-Nejad, P.; Ghose, S.; Kirschning, C.J.; Issels, R.D.; Wagner, H. HSP70 as Endogenous Stimulus of the Toll/Interleukin-1 Receptor Signal Pathway. J. Biol. Chem. 2002, 277, 15107–15112. [Google Scholar] [CrossRef] [PubMed]
- Asea, A.A.A.; Rehli, M.; Kabingu, E.; Boch, J.A.; Baré, O.; Auron, P.E.; Stevenson, M.A.; Calderwood, S.K. Novel Signal Transduction Pathway Utilized by Extracellular HSP70. J. Biol. Chem. 2002, 277, 15028–15034. [Google Scholar] [CrossRef] [PubMed]
| Gene_Name | Gene_ID | Chromosome | Annotation | Number of Amino Acid | Molecular Weight | Theoretical pI | Instability Index | Aliphatic Index | Grand Average of Hydropathicity |
|---|---|---|---|---|---|---|---|---|---|
| LOC123759425 | XM_045744490.2 | NC_091181.1 | heat shock 70 kDa protein 1-like | 642 | 70,854.91 | 5.73 | 34.11 | 83.99 | −0.469 |
| LOC123759427 | XM_045744492.2 | NC_091181.1 | heat shock 70 kDa protein 1 | 642 | 70,864.95 | 5.67 | 35 | 84.3 | −0.462 |
| LOC123760349 | XM_045745963.2 | NC_091188.1 | heat shock 70 kDa protein cognate 4 | 647 | 71,168.32 | 5.28 | 35.85 | 77.6 | −0.483 |
| LOC123762431 | XM_045748943.2 | NC_091151.1 | heat shock 70 kDa protein cognate 4 | 635 | 69,433.29 | 5.49 | 33.81 | 82.33 | −0.457 |
| LOC123763586 | XM_045750772.2 | NC_091201.1 | heat shock cognate 71 kDa protein | 638 | 69,882.92 | 5.23 | 37.94 | 77.7 | −0.443 |
| Hsc70-5 | XM_045769046.2 | NC_091159.1 | heat shock 70 kDa protein cognate 5 | 677 | 73,443.6 | 5.73 | 32.52 | 84.11 | −0.376 |
| LOC123774884 | XM_045769563.2 | NC_091159.1 | heat shock 70 kDa protein | 643 | 71,026.16 | 5.41 | 38.04 | 80.39 | −0.52 |
| LOC123774888 | XM_045769576.2 | NC_091159.1 | heat shock 70 kDa protein-like | 643 | 71,043.14 | 5.6 | 37.71 | 81.29 | −0.528 |
| LOC123775010 | XM_045769739.2 | NC_091241.1 | heat shock cognate 70 kDa protein | 654 | 71,709.98 | 5.27 | 37.97 | 78.01 | −0.467 |
| LOC123775012 | XM_045769740.2 | NC_091241.1 | heat shock cognate 71 kDa protein-like | 696 | 78,070.67 | 5.98 | 38.32 | 79.44 | −0.467 |
| LOC123772095 | XM_069311168.1 | NC_091218.1 | heat shock 70 kDa protein 14 isoform X1 | 517 | 55,113.17 | 5.89 | 44.58 | 94.08 | 0.145 |
| LOC123775013 | XM_069318964.1 | NC_091241.1 | heat shock cognate 71 kDa protein-like | 606 | 67,565.77 | 5.51 | 35.67 | 87.84 | −0.344 |
| LOC138363095 | XM_069322069.1 | NC_091159.1 | heat shock 70 kDa protein-like | 643 | 71,054.21 | 5.41 | 37.4 | 80.39 | −0.519 |
| LOC123774869 | XM_069322070.1 | NC_091159.1 | heat shock 70 kDa protein-like | 643 | 71,139.28 | 5.41 | 37.14 | 80.23 | −0.534 |
| LOC138365240 | XM_069325507.1 | NC_091151.1 | heat shock-related 70 kDa protein 2-like | 649 | 71,249.39 | 5.79 | 33.51 | 81.91 | −0.484 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Cai, X.; Yue, S.; Chen, Z.; Sun, Y.; Cheng, L.; Xi, Y.; Wang, S. Genome-Wide Identification and Expression Analysis of Hsp70 Gene Family of Procambarus clarkii Reveals Its Immune Role in Response to Bacterial Challenge After Non-Lethal Heat Shock. Animals 2025, 15, 2150. https://doi.org/10.3390/ani15142150
Zhang X, Cai X, Yue S, Chen Z, Sun Y, Cheng L, Xi Y, Wang S. Genome-Wide Identification and Expression Analysis of Hsp70 Gene Family of Procambarus clarkii Reveals Its Immune Role in Response to Bacterial Challenge After Non-Lethal Heat Shock. Animals. 2025; 15(14):2150. https://doi.org/10.3390/ani15142150
Chicago/Turabian StyleZhang, Xin, Xiuhong Cai, Shirui Yue, Zhangxuan Chen, Yulong Sun, Lei Cheng, Yewen Xi, and Shunchang Wang. 2025. "Genome-Wide Identification and Expression Analysis of Hsp70 Gene Family of Procambarus clarkii Reveals Its Immune Role in Response to Bacterial Challenge After Non-Lethal Heat Shock" Animals 15, no. 14: 2150. https://doi.org/10.3390/ani15142150
APA StyleZhang, X., Cai, X., Yue, S., Chen, Z., Sun, Y., Cheng, L., Xi, Y., & Wang, S. (2025). Genome-Wide Identification and Expression Analysis of Hsp70 Gene Family of Procambarus clarkii Reveals Its Immune Role in Response to Bacterial Challenge After Non-Lethal Heat Shock. Animals, 15(14), 2150. https://doi.org/10.3390/ani15142150





