Effects of Habitat Differences and Invasive Species Competition on Age and Growth of Triplophysa strauchii
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
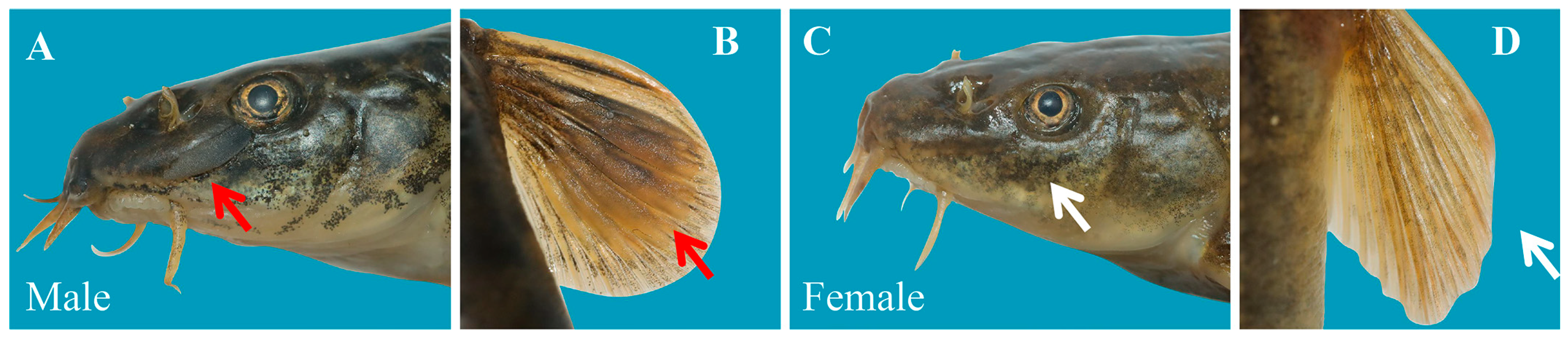
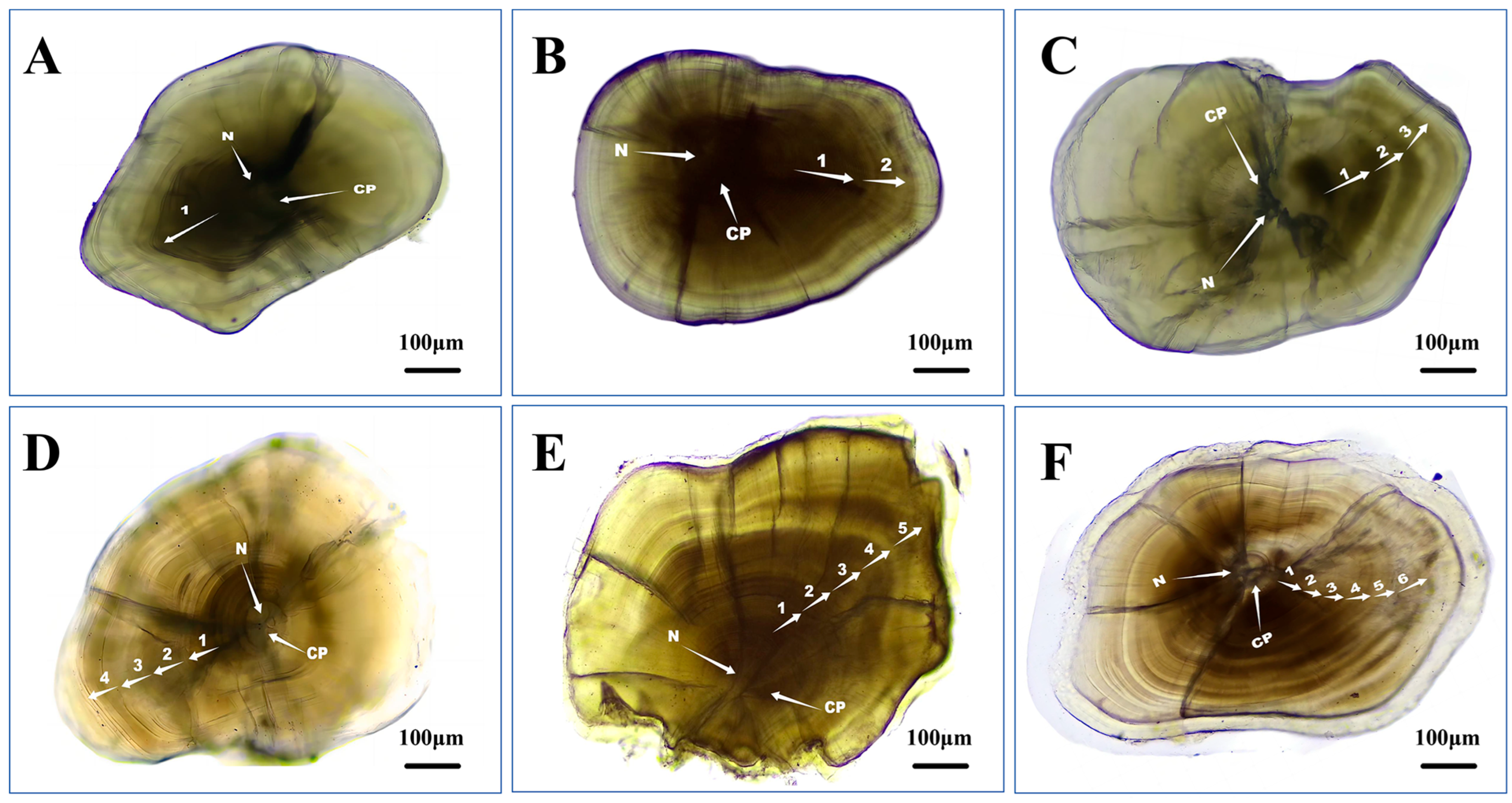
2.2. Lapillus Processing and Age Determination
2.3. Data Analysis
2.3.1. Length–Weight Relationship
2.3.2. Fulton’s Condition Factor
2.3.3. Growth Models
2.3.4. Statistical Analysis
3. Results
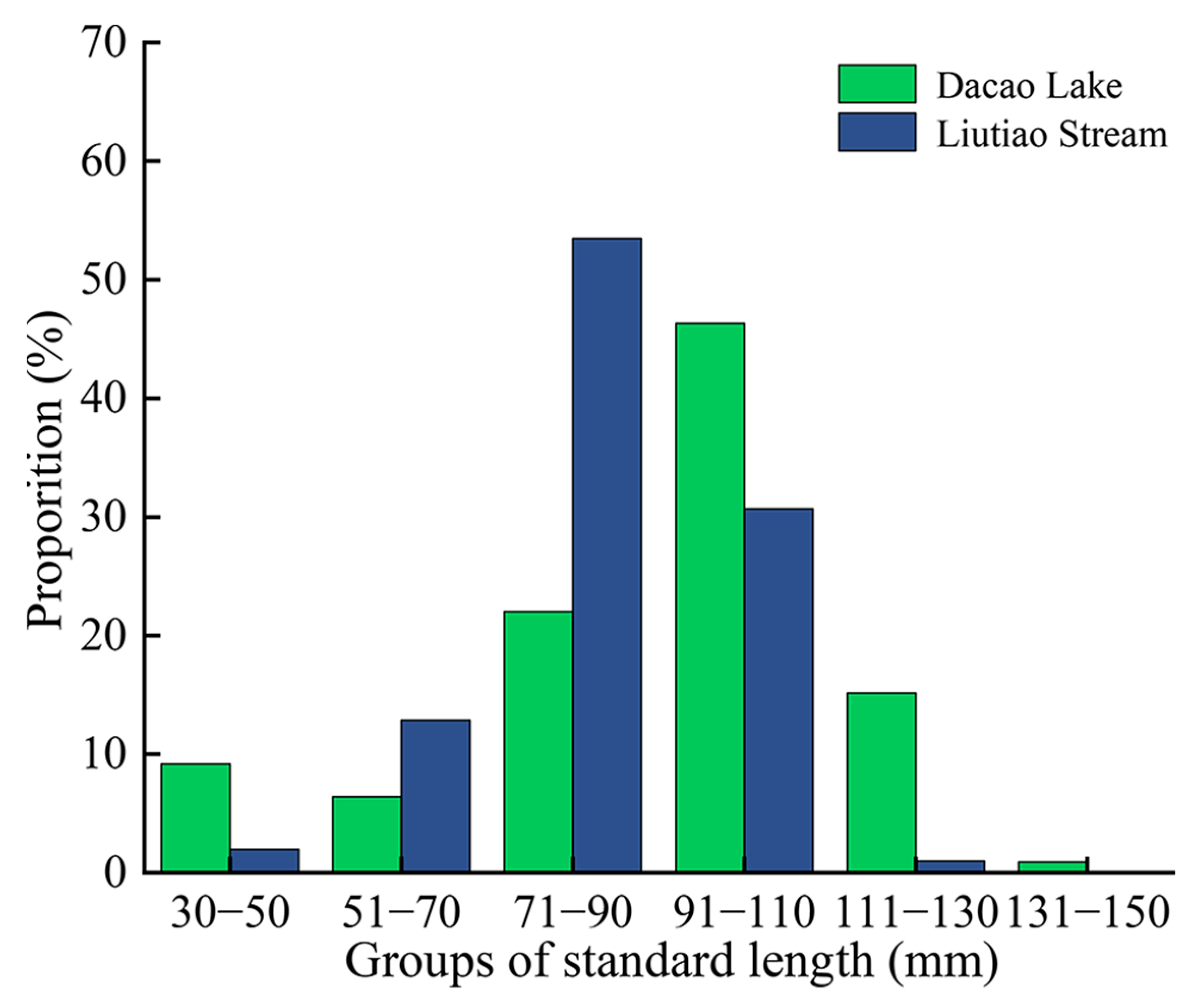
3.1. Body Length Distribution
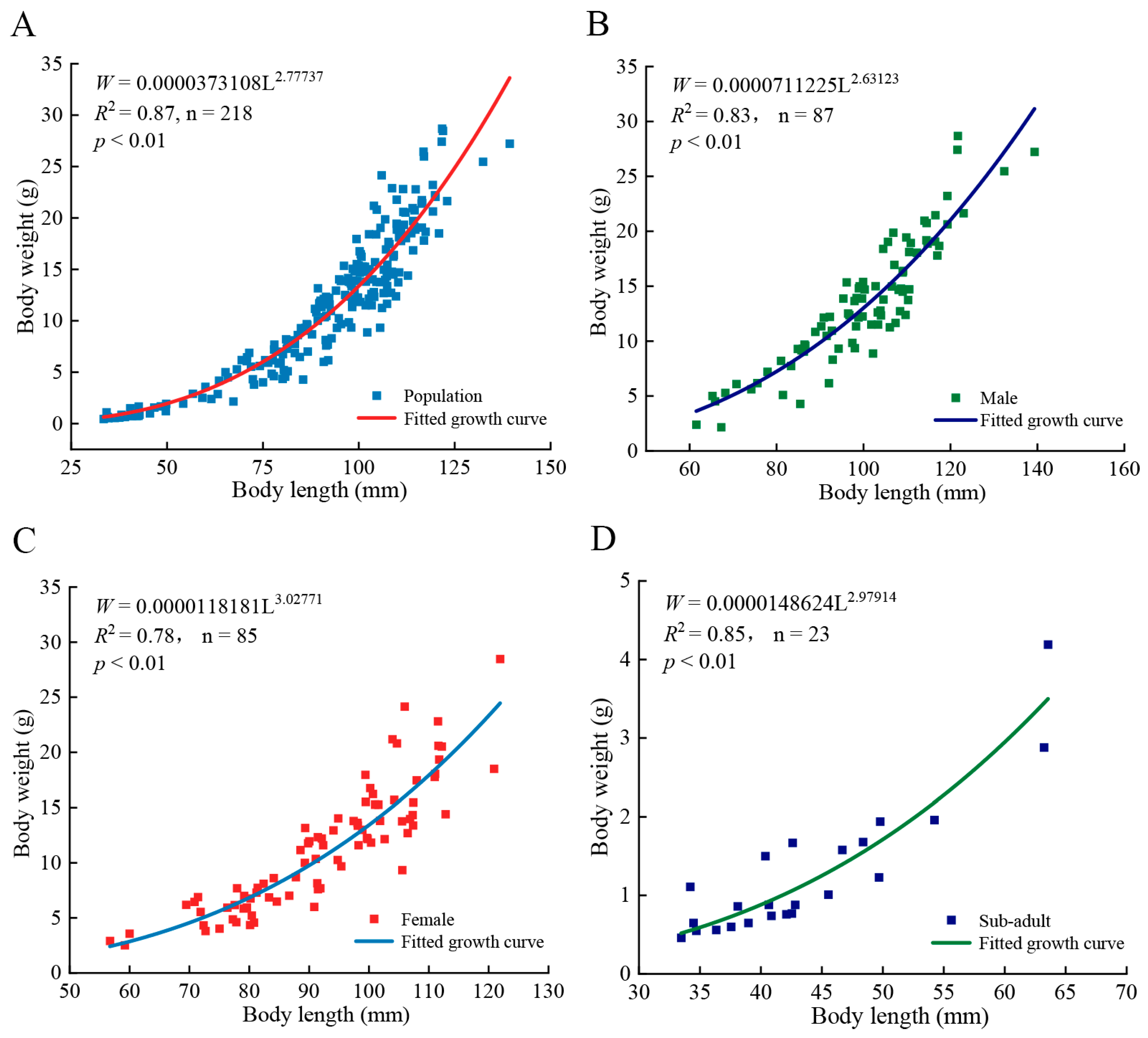
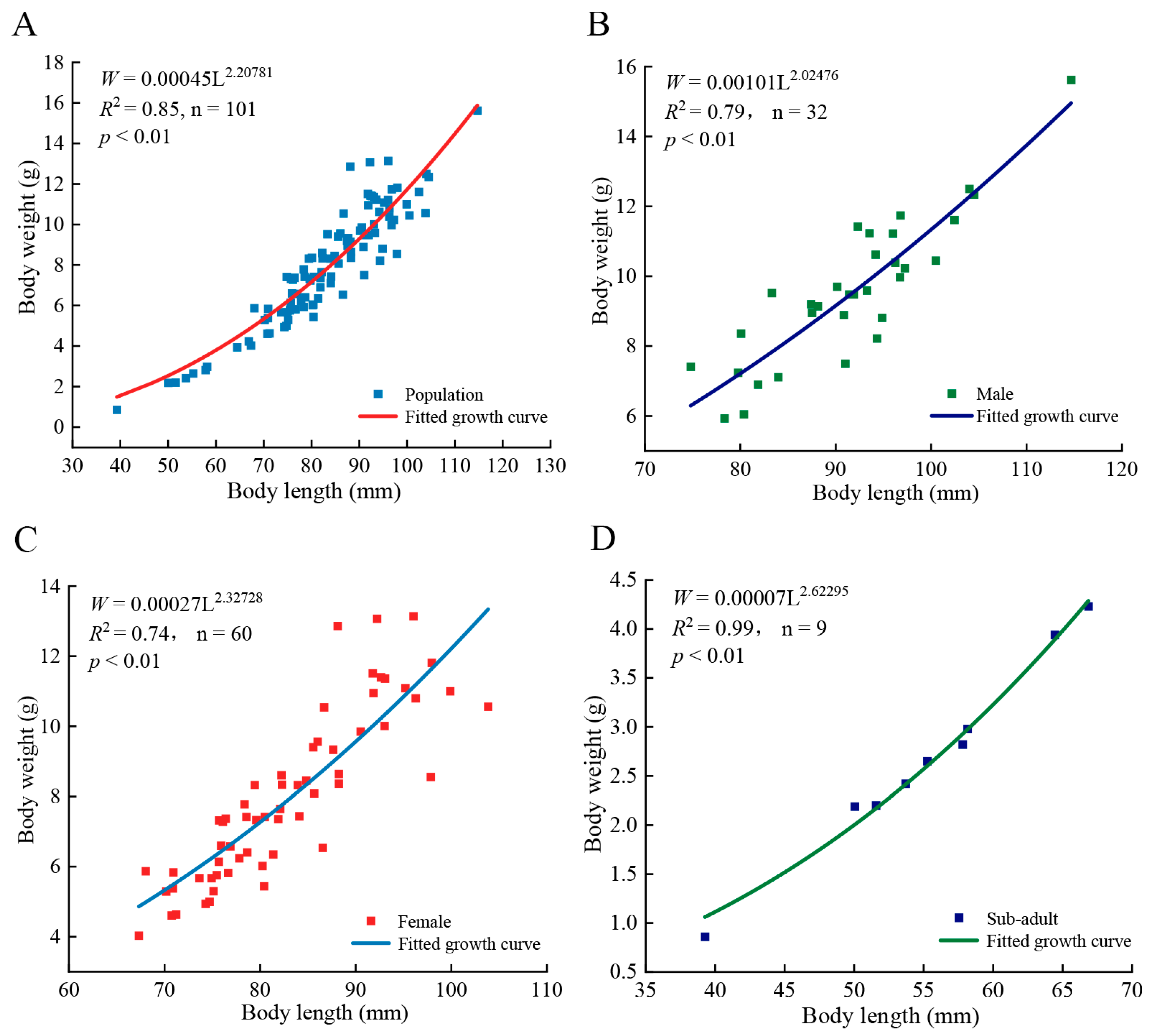
3.2. Length–Weight Relationship
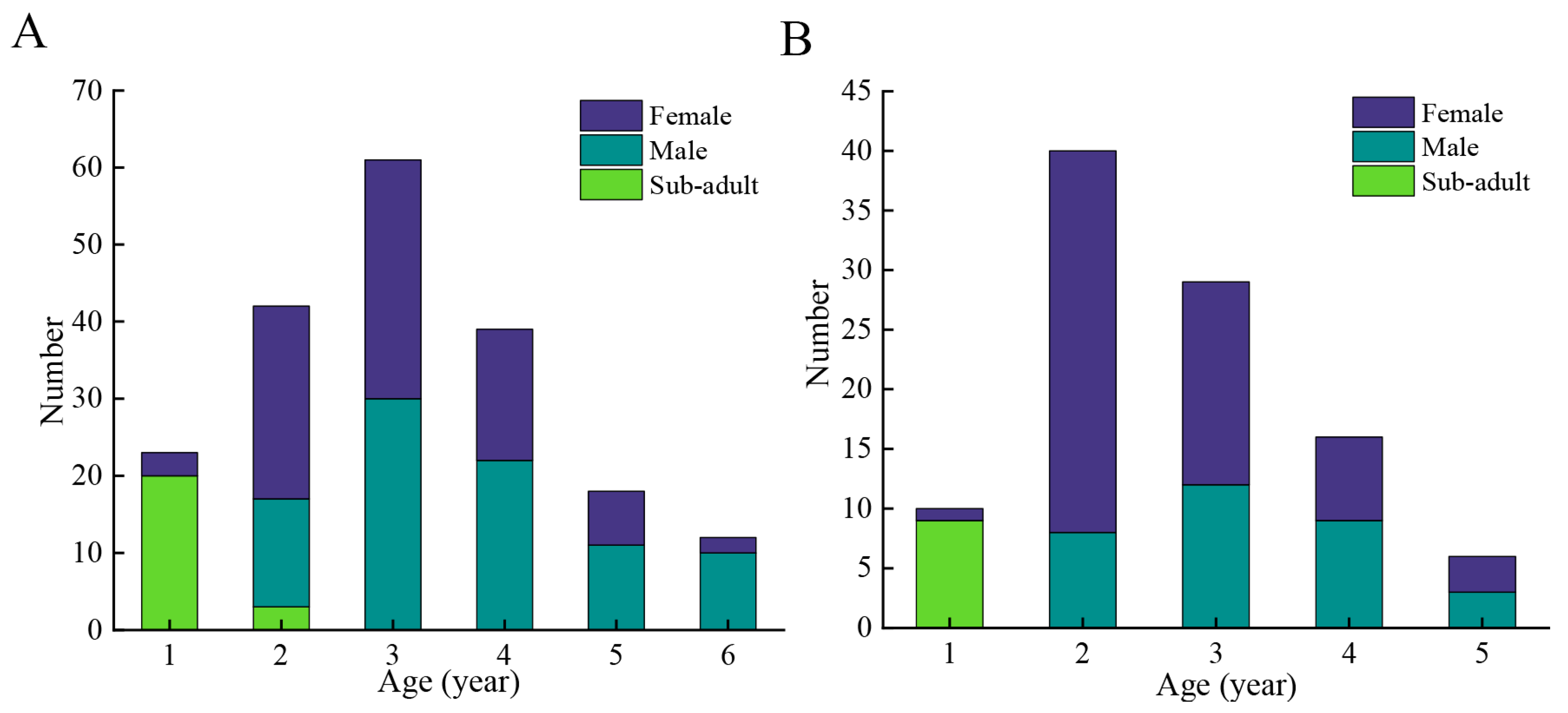
3.3. Age Structure
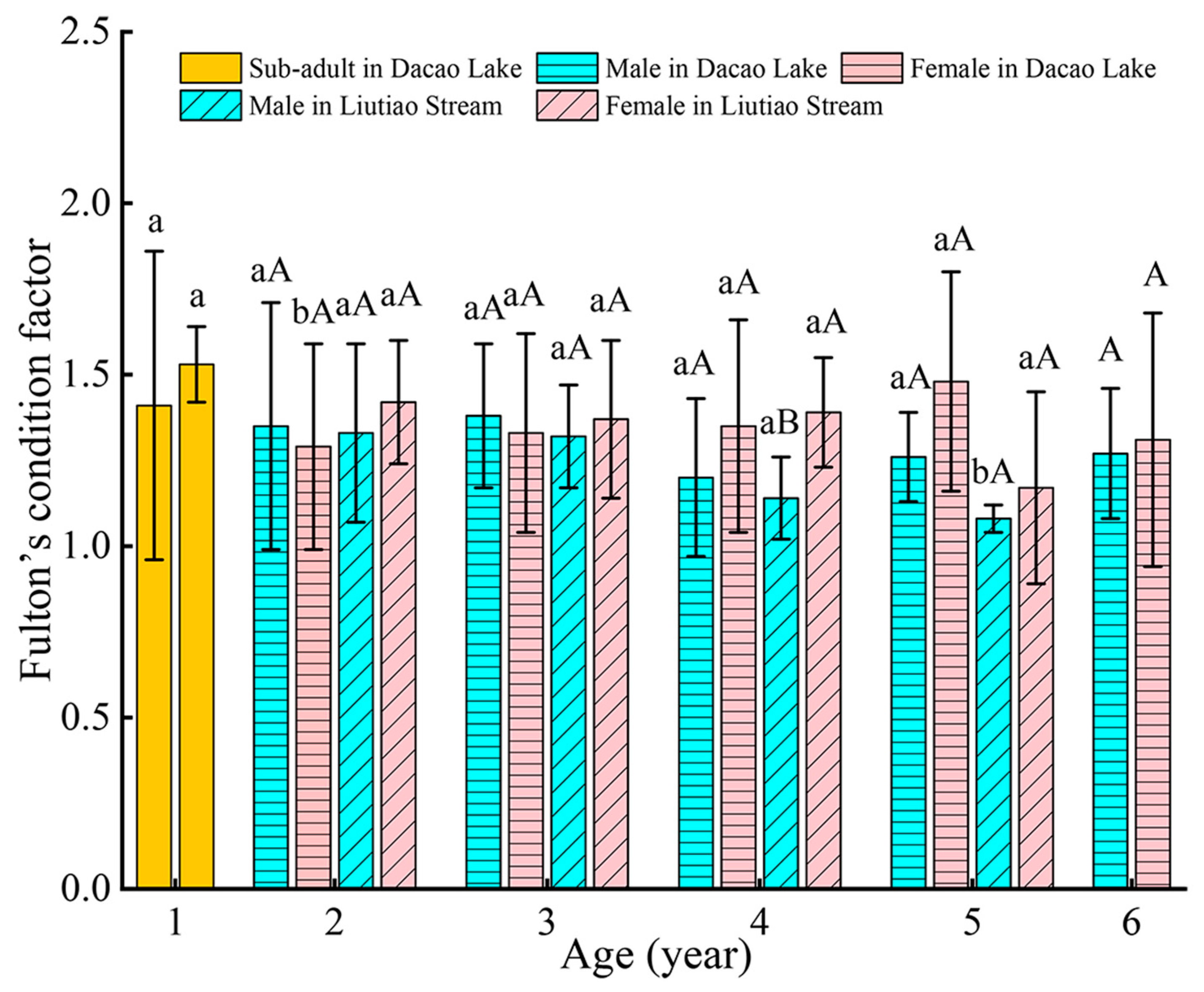
3.4. Fulton’s Condition Factor
3.5. Growth Models
4. Discussion
4.1. Growth Characteristics
4.2. Age Structure
4.3. Fulton’s Condition Factor
4.4. Growth Models
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Sampling Site (Time) | Species | Number | Proportion of Catch | Type | Feeding Habits | Activity Rhythm | Habitat Water Layer | References |
|---|---|---|---|---|---|---|---|---|
| Dacao Lake (27 April 2024) | Triplophysa strauchii | 218 | 57.80% | Native | Omnivory | Nocturnal | Demersal | Unpublished data; [48] |
| Phoxinus grumi | 92 | 42.20% | Native | Omnivory (zooplankton) | Diurnal | Pelagic | Unpublished data | |
| Liutiao Stream (19 May 2024) | Triplophysa strauchii | 101 | 56.11% | Native | Omnivory | Nocturnal | Demersal | Unpublished data; [48] |
| Misgurnus anguillicaudatus | 55 | 30.56% | Alien | Omnivory | Nocturnal | Demersal | [43] | |
| Abbottina rivularis | 24 | 13.33% | Alien | Omnivory | Diurnal | Demersal | [42] |
| Age | Sex | Dacao Lake | Liutiao Stream | p-Value | ||
|---|---|---|---|---|---|---|
| Mean ± SD | Range | Mean ± SD | Range | |||
| 1 | Sub-adult | 43.29–7.75 b (n = 23) | 33.46–59.99 | 55.24 ± 8.16 a (n = 9) | 39.27–66.86 | 0.001 |
| 2 | Male | 74.48 ± 8.06 bA (n = 14) | 61.54–85.45 | 81.69 ± 5.75 aA (n = 8) | 74.80–94.32 | 0.038 |
| Female | 77.88 ± 4.35 aA (n = 25) | 69.46–84.59 | 76.62 ± 4.28 aB (n = 32) | 68.03–87.64 | 0.279 | |
| 3 | Male | 94.34 ± 4.97 aA (n = 30) | 85.72–102.76 | 90.55 ± 3.54 bA (n = 12) | 83.31–96.74 | 0.021 |
| Female | 94.35 ± 4.50 aA (n = 31) | 86.67–101.84 | 87.28 ± 4.18 bB (n = 17) | 82.10–97.85 | 0.000 | |
| 4 | Male | 105.90 ± 2.76 aA (n = 22) | 100.25–109.83 | 96.58 ± 3.36 bA (n = 9) | 91.01–102.45 | 0.000 |
| Female | 104.81 ± 3.23 aA (n = 17) | 99.39–110.97 | 94.16 ± 2.34 bA (n = 7) | 91.77–97.95 | 0.000 | |
| 5 | Male | 112.55 ± 2.50 aA (n = 11) | 109.09–116.96 | 107.73 ± 6.02 aA (n = 3) | 104.01–114.68 | 0.298 |
| Female | 110.96 ± 2.28 aA (n = 7) | 105.95–112.79 | 99.92 ± 3.91 bA (n = 3) | 96.03–103.85 | 0.000 | |
| 6 | Male | 122.68 ± 7.46 A (n = 10) | 116.43–139.32 | |||
| Female | 121.40 ± 0.72 A (n = 2) | 120.89–121.91 | ||||
| Model | Dacao Lake | R2 | RSS | Liutiao Stream | R2 | RSS |
|---|---|---|---|---|---|---|
| Von Bertalanffy | 0.9393 | 6.346 | 0.9982 | 2.9738 | ||
| Logistic | 0.9309 | 7.234 | 0.8352 | 2.7506 | ||
| Gompertz | 0.9360 | 6.953 | 0.8454 | 5.9596 |
References
- Beamish, R.J.; McFarlane, G.A. The forgotten requirement for age validation in fisheries biology. Trans. Am. Fish. Soc. 1983, 112, 735–743. [Google Scholar] [CrossRef]
- Morat, F.; Wicquart, J.; Schiettekatte, N.M.D.; de Sinéty, G.; Bienvenu, J.; Casey, J.M.; Brandl, S.J.; Vii, J.; Carlot, J.; Degregori, S.; et al. Individual back-calculated size-at-age based on otoliths from Pacific coral reef fish species. Sci. Data 2020, 7, 370. [Google Scholar] [CrossRef] [PubMed]
- Legaki, A.; Leonhard, I.; Mytilineou, C.; Anastasopoulou, A. Dentex maroccanus Valenciennes, 1830 Otolith Morphology, Age, and Growth in the Aegean Sea (E. Mediterranean). Animals 2024, 14, 3151. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.J.; Xie, C.X.; Zhou, X.J.; Ma, B.S.; Huo, B. Age and growth characteristics of Schizomycosis younghusbandi Regan, 1905 in the Yarlung Tsangpo River in Tibet, China. J. Appl. Ichthyol. 2014, 30, 948–954. [Google Scholar] [CrossRef]
- Campana, S.E. Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J. Fish Biol. 2001, 59, 197–242. [Google Scholar] [CrossRef]
- Weatherley, A.H.; Gill, H.S. The Biology of Fish Growth; Academic Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Polat, N.; Bostanci, D.; Yilmaz, S. Comparable age determination in different bony structures of Pleuronectes flesus luscus Pallas, 1811 inhabiting the Black Sea. Turk. J. Zool. 2001, 25, 441–446. [Google Scholar]
- Willmes, M.; Sturrock, A.M.; Cordoleani, F.; Hugentobler, S.; Meek, M.H.; Whitman, G.; Evans, K.; Palkovacs, E.P.; Stauffer-Olsen, N.J.; Johnson, R.C. Integrating otolith and genetic tools to reveal intraspecific biodiversity in a highly impacted salmon population. J. Fish Biol. 2024, 105, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Tang, J.B.; Wang, Q.L.; Sun, Z.H.; Yu, S.S.; Si, F. Effect of Temperature on the Early Development of Paralichthys olivaceus Otoliths. Animals 2025, 15, 814. [Google Scholar] [CrossRef] [PubMed]
- Sapota, M.R.; Dąbrowska, V. Shapes of otoliths in some Baltic fish and their proportions. Oceanol. Hydrobiol. Stud. 2019, 48, 296–304. [Google Scholar] [CrossRef]
- Phelps, Q.E.; Edwards, K.R.; Willis, D.W. Precision of five structures for estimating age of common carp. N. Am. J. Fish. Manag. 2007, 27, 103–105. [Google Scholar] [CrossRef]
- Schulz-Mirbach, T.; Ladich, F.; Plath, M.; Heß, M. Enigmatic ear stones: What we know about the functional role and evolution of fish otoliths. Biol. Rev. 2019, 94, 457–482. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Chen, S.G.; Zi, F.Z.; Ge, J.M.; Chang, D.S.; Song, Y.; Xie, C.X. Age and Growth of Triplophysa (Hedinichthys) yarkandensis (Day, 1877) in the Tarim River in Xinjiang, China. Pak. J. Zool. 2024, 56, 1771–1780. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Wang, J.L.; Li, P.L.; Liu, J.C.; Liu, Y.B.; Wang, T.; Liu, K.; Zhao, P.; Zhang, J. Age identification and growth model selection of Triplophysa pseudoscleroptera in the middle and upper reaches of the Yellow River. J. N. Agric. 2023, 51, 105–111. [Google Scholar]
- Başusta, N.; Dürrani, Ö. Sexual dimorphism in the otolith shape of shi drum, Umbrina cirrosa (L.), in the eastern Mediterranean Sea: Fish size-otolith size relationships. J. Fish Biol. 2021, 99, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Bao, Z.Y.; Wang, Q.Z. Advances on research and application of age determination by hard tissues in fish: A review. J. Dalian Ocean. Univ. 2021, 36, 1071–1080. [Google Scholar]
- Shi, F.; Sun, J.; Lin, X.; Liu, H. Otolith ontogeny and increment formation in larval Tanichthys albonubes. Chin. J. Zool. 2006, 41, 10–16. [Google Scholar]
- Midway, S.R.; Wagner, T.; Arnott, S.A.; Biondo, P.; Martinez-Andrade, F.; Wadsworth, T.F. Spatial and temporal variability in growth of Southern Flounder (Paralichthys lethostigma). Fish. Res. 2015, 167, 323–332. [Google Scholar] [CrossRef]
- Noring, A.M.; Sass, G.G.; Midway, S.R.; VanDeHey, J.A.; Raabe, J.K.; Isermann, D.A.; Kampa, J.M.; Parks, T.P.; Lyons, J.; Jennings, M.J. Pelagic forage versus abiotic factors as drivers of Walleye growth in northern Wisconsin lakes. Adv. Limnol. 2021, 66, 207–223. [Google Scholar] [CrossRef]
- Huntsman, B.M.; Martin, R.W.; Patten, K.A. Effects of temperature and spatial scale on Rio Grande cutthroat trout growth and abundance. Trans. Am. Fish. Soc. 2018, 147, 480–496. [Google Scholar] [CrossRef] [PubMed]
- Caves, S.; Baumann, J.R.; Stich, D.S. Density-Dependent Changes in Grass Carp Growth and Mortality in Long-Term Aquatic Plant Management. N. Am. J. Fish. Manag. 2021, 41, 355–365. [Google Scholar] [CrossRef]
- Yin, M.C. Fish Ecology; China Agriculture Press: Beijing, China, 1995. [Google Scholar]
- Flinn, S.A.; Midway, S.R. Trends in growth modeling in fisheries science. Fishes 2021, 6, 1. [Google Scholar] [CrossRef]
- Nelson, J.S.; Grande, T.C.; Wilson, M.V.H. Fishes of the World; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Xiao, H.; Dai, Y.G. A review of study on diversity of Triplophysa in China. Fish. Sci. 2011, 30, 53–57. [Google Scholar]
- Wang, Y.J.; Shen, Y.J.; Feng, C.G.; Zhao, K.; Song, Z.B.; Zhang, Y.P.; Yang, L.D.; He, S.P. Mitogenomic perspectives on the origin of Tibetan loaches and their adaptation to high altitude. Sci. Rep. 2016, 6, 29690. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.X.; Luo, S.T.; Yao, N.; Wang, X.Y.; Song, Y.; Chen, S.G. A comprehensive analysis of Triplophysa labiata (Kessler, 1874) mitogenome and its phylogenetic implications within the Triplophysa genus. Genes 2023, 14, 1356. [Google Scholar] [CrossRef] [PubMed]
- Feng, C.G.; Zhang, Y.; Tong, C.; Zhou, B.Z.; Li, X.H.; Tang, Y.T.; Song, W.Z.; Zhao, K. A new species of Triplophysa (Cypriniformes, Nemacheilidae) from Weihe River in Gansu Province, China. Zool. Res. 2020, 41, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Sheraliev, B.; Kayumova, Y.; Peng, Z. Triplophysa daryoae, a new nemacheilid loach species (Teleostei, Nemacheilidae) from the Syr Darya River basin, Central Asia. ZooKeys 2022, 1125, 47–67. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Song, Y.; Xie, H.Y.; Zi, F.Z.; Chen, S.G.; Luo, S.T. Complete mitogenome of the Triplophysa bombifrons: Comparative analysis and phylogenetic relationships among the members of Triplophysa. Genes 2023, 14, 128. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Liu, M.; He, D. Phylogeography of Triplophysa stenura (Nemacheilidae): Responded to the mid-pleistocene climate transition in the Qinghai-Tibetan plateau. Zool. Stud. 2020, 59, e67. [Google Scholar] [PubMed]
- Xiong, H.L.; Yao, Y.H.; Wang, Z.J. Study on the structure of the digestive tract in Triplophysa bleekeri. J. Southwest China Norm. Univ. 2012, 37, 113–120. [Google Scholar]
- Wang, X.Y. Age, Growth, Reproduction and Population Discrimination of Triplophysa yarkandensis. Master’s Thesis, Tarim University, Alar, China, 2022. [Google Scholar]
- Zhao, H.; Wang, X.B.; Zhao, N.H.; Wei, J.; Shen, J.Z. Morphology and age determination of loach Triplophysa stoliczkae in Kizil River, Xinjiang Uygur Autonomous Region. Chin. J. Fish. 2021, 34, 8–15. [Google Scholar]
- Li, L.T. Study on the Age, Growth and Population Dynamics of Triplophysa orientalis in the Middle of the Yarlung Tsangpo River. Master’s Thesis, Huazhong Agricultural University, Wuhan, China, 2016. [Google Scholar]
- Zhang, X.F.; He, C.L.; Song, Z.B. Age and growth of Triplophysa markehenensis from the Markehe River in upper reaches of the Dadu River. Chin. J. Zool. 2010, 45, 11–20. [Google Scholar]
- Feng, C.G.; Tong, C.; Zhang, R.Y.; Li, G.G.; Wanghe, K.Y.; Tang, Y.T.; Zhang, C.F.; Zhao, K. Biodiversity and distribution patterns of Triplophysa species in the northeastern margin of the Tibetan Plateau. Biodivers. Sci. 2017, 25, 53–61. [Google Scholar] [CrossRef]
- Guo, Y.; Cai, L.G.; Aa, D.K.; Li, H. Study on biological characteristics of Triplophysa strauchii in Chaiwopu Lake. J. Hydroecology 2008, 28, 49–50+79. [Google Scholar][Green Version]
- Guo, Y.; Cai, L.G.; Tu, E.X.; Zhang, R.M.; Liu, K.L.; Zhang, B.P. The study on the biology of Triplophysa strauchii (Kessler) in the Sailimu Lake. Chin. J. Fish. 2002, 15, 6–11. [Google Scholar][Green Version]
- Han, M.M.; Lu, J.; Wang, L.; Mahboob, S.; Al-Ghanim, K.A.; Sun, X.W. Complete mitochondrial genome of the Triplophysa bombifrons and Triplophysa strauchii. Mitochondrial DNA Part A 2016, 27, 4710–4711. [Google Scholar] [CrossRef] [PubMed]
- Friberg, N.; Dybkjaer, J.B.; Olafsson, J.S.; Gislason, G.M.; Larsen, S.E.; Lauridsen, T.L. Relationships between structure and function in streams contrasting in temperature. Freshw. Biol. 2009, 54, 2051–2068. [Google Scholar] [CrossRef]
- Jang-Liaw, N.H.; Tominaga, K.; Zhang, C.; Zhao, Y.; Nakajima, J.; Onikura, N.; Watanabe, K. Phylogeography of the Chinese false gudgeon, Abbottina rivularis, in East Asia, with special reference to the origin and artificial disturbance of Japanese populations. Ichthyol. Res. 2019, 66, 460–478. [Google Scholar] [CrossRef]
- Sun, B.; Huang, Y.; Castro, L.F.C.; Yang, S.; Huang, S.; Jin, W.; Zhou, H.; Ijiri, S.; Luo, Y.; Gao, J.; et al. The chromosome-level genome and key genes associated with mud-dwelling behavior and adaptations of hypoxia and noxious environments in loach (Misgurnus anguillicaudatus). BMC Biol. 2023, 21, 18. [Google Scholar] [CrossRef] [PubMed]
- Cano-Barbacil, C.; Haubrock, P.J.; Radinger, J. Asian Loaches: An Emerging Threat as Global Invaders. Freshw. Biol. 2025, 70, e70026. [Google Scholar] [CrossRef]
- Gardin, A.; Otero, O.; Réveillac, E.; Lafitte, A.; Valentin, X.; Lapalus, F.; Bouchon, D.; Garcia, G. Seasonality and growth in tropical freshwater ectotherm vertebrates: Results from 1-year experimentation in the African gray bichir, giraffe catfish, and the West African mud turtle. Ecol. Evol. 2023, 13, e9936. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Hu, M.H.; Wang, W.M.; Cao, L.; Yang, Y.; Lü, B.P.; Yao, R.R. Transpositional feeding rhythm of loach Misgurnus anguillicaudatus from larvae to juveniles and its ontogenesis under artificial rearing conditions. Aquac. Int. 2008, 16, 539–549. [Google Scholar] [CrossRef]
- Xie, P. Morphological Comparison and Temperature Adaptation of Phoxinus Species in China. Ph.D. Thesis, Huazhong Agricultural University, Wuhan, China, 2019. [Google Scholar]
- Zhu, S.Q. The Loaches of the Subfamily Nemacheililinae in China; Jiangsu Science and Technology Publishing House: Nanjing, China, 1989. [Google Scholar]
- Wu, Y.F.; Wu, C.Z. The Fishes of the Qinghai-Xizang Plateau; Sichuan Publishing House of Science & Technology: Chengdu, China, 1992. [Google Scholar]
- Zeng, L.; Tang, W.Q. Discussion on age determination methods for two esquamate Triplophysa fishes. Chin. J. Zool. 2010, 45, 94–103. [Google Scholar]
- Tian, N.N.; Yang, R.B.; Tan, B.Z.; Zeng, X.L.; He, L.Q.; Xu, Z.L.; Zhu, Z.; Liu, H.P.; Yang, X.F. Age, growth, and reproductive characteristics of Triplophysa stewarti in Lake Chugutso, Tibet. J. Fish. Sci. China 2022, 29, 1013–1021. [Google Scholar]
- Dinh, Q.M.; Qin, J.G.; Tran, D.D. Population and age structure of the goby Parapocryptes serperaster (Richardson, 1864; Gobiidae: Oxudercinae) in the Mekong Delta. Turk. J. Fish. Aquat. Sci. 2015, 15, 341–352. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhao, Y.; Mathers, A.; Corkum, L.D. Length frequency age estimations of American Eel recruiting to the upper St. Lawrence River and Lake Ontario. Trans. Am. Fish. Soc. 2013, 142, 333–344. [Google Scholar] [CrossRef]
- Pauly, D. Fish Population Dynamics in Tropical Waters: A Manual for Use with Programmable Calculators; WorldFish: Penang, Malaysia, 1984; Volume 8. [Google Scholar]
- Liang, W.T. The Biology Research About Several Species of Triplophysa. Master’s Thesis, Wuhan Polytechnic University, Wuhan, China, 2014. [Google Scholar]
- Maciel, T.R.; Vaz-dos-Santos, A.M.; Barradas, J.R.d.S.; Vianna, M. Sexual dimorphism in the catfish Genidens genidens (Siluriformes: Ariidae) based on otolith morphometry and relative growth. Neotrop. Ichthyol. 2019, 17, e180101. [Google Scholar] [CrossRef]
- Wang, Z.W.; Hao, H.M.; Wei, J.; Wu, H.; Hamid, S.M.; Lv, R.X.; Lu, H.L.; Nie, Z.L. Morphology, Age, and Growth of Triplophysa strauchii in Sayram Lake, Xinjiang, China. Animals 2025, 15, 1039. [Google Scholar] [CrossRef] [PubMed]
- Von Bertalanffy, L. Quantitative laws in metabolism and growth. Q. Rev. Biol. 1957, 32, 217–231. [Google Scholar] [CrossRef] [PubMed]
- Ricker, W.E. Computation and Interpretation of Biological Statistics of Fish Populations (Fisheries Research Board of Canada Bulletin); The Blackburn Press: Caldwell, NJ, USA, 1975; Volume 191, pp. 1–382. [Google Scholar]
- Gompertz, B. On the nature of the function expressive of the law of human mortality and on a new mode of determining the value of life contingencies. Philos. Trans. R. Soc. 1825, 115, 513–583. [Google Scholar]
- Araya, P.R.; Agostinho, A.A.; Bechara, J.A. The influence of dam construction on a population of Leporinus obtusidens (Valenciennes, 1847) (Pisces, Anostomidae) in the Yacyreta Reservoir (Argentina). Fish. Res. 2005, 74, 198–209. [Google Scholar] [CrossRef]
- Quinn, T.P.; Wetzel, L.; Bishop, S.; Overberg, K.; Rogers, D.E. Influence of breeding habitat on bear predation and age at maturity and sexual dimorphism of sockeye salmon populations. Can. J. Zool. 2001, 79, 1782–1793. [Google Scholar] [CrossRef]
- Michaletz, P. Variation in characteristics among gizzard shad populations: The role of impoundment size and productivity. Fish. Manag. Ecol. 2017, 24, 361–371. [Google Scholar] [CrossRef]
- He, J.Y.; Wu, Z.Q.; Huang, L.L.; Sun, Y.Y.; Wang, D.J.; Lin, Y.; He, A.Y.; Feng, J.; Liu, H. Age, growth, reproduction and status of resource development of Ptychidio jordani, a critically endangered freshwater fish in the Hongshui River, China. J. Fish Biol. 2024, 104, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.X. Ichthyology; China Agriculture Press: Beijing, China, 2010. [Google Scholar]
- Verreycken, H.; Van Thuyne, G.; Belpaire, C. Length–weight relationships of 40 freshwater fish species from two decades of monitoring in Flanders (Belgium). J. Appl. Ichthyol. 2011, 27, 1416–1421. [Google Scholar] [CrossRef]
- Magloo, A.H.; Bhat, F.A.; Shah, T.H.; Wanjari, R.N.; Bazaz, A.I.; Mir, S.A.; Gul, S.; Sidiq, J. Length-weight relationship and condition factor of an indigenous fish, Triplophysa marmorata (Heckel, 1838), from Kashmir Valley. Int. J. Vet. Sci. Anim. Husb. 2024, 9, 766–770. [Google Scholar]
- Rennie, M.D.; Purchase, C.F.; Lester, N.; Collins, N.C.; Shuter, B.J.; Abrams, P.A. Lazy males? Bioenergetic differences in energy acquisition and metabolism help to explain sexual size dimorphism in percids. J. Anim. Ecol. 2008, 77, 916–926. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.Q.; Li, Y.H.; Wei, J.; Nie, Z.L.; Yang, Z.J. Relationship of body mass, body length, and fatness of Schizothorax eurystomus. Acta Agric. Boreali-Occident. Sin. 2019, 28, 1380–1386. [Google Scholar]
- Șerban, D.A.; Barbacariu, C.A.; Burducea, M.; Ivancia, M.; Creangă, Ș. Comparative Analysis of Growth Performance, Morphological Development, and Physiological Condition in Three Romanian Cyprinus carpio Varieties and Koi: Implications for Aquaculture. Life 2024, 14, 1471. [Google Scholar] [CrossRef] [PubMed]
- Gamito, S. Growth models and their use in ecological modelling: An application to a fish population. Ecol. Model. 1998, 113, 83–94. [Google Scholar] [CrossRef]
- Murphy, M.D.; Taylor, R.G. Age, growth, and mortality of spotted seatrout in Florida waters. Trans. Am. Fish. Soc. 1994, 123, 482–497. [Google Scholar] [CrossRef]
- Lin, Y.J.; Tzeng, W.N. Modelling the growth of Japanese eel Anguilla japonica in the lower reach of the Kao-Ping River, southern Taiwan: An information theory approach. J. Fish Biol. 2009, 75, 100–112. [Google Scholar] [CrossRef] [PubMed]
- Dippold, D.A.; Leaf, R.T.; Hendon, J.R.; Franks, J.S. Estimation of the length-at-age relationship of Mississippi’s Spotted Seatrout. Trans. Am. Fish. Soc. 2016, 145, 295–304. [Google Scholar] [CrossRef]
- Branstetter, S. Biological Parameters of the Sharks of the Northwestern Gylf of Mexico in Relation to Their Potential as a Commercial Fishery Resource. Ph.D. Thesis, Texas A&M University, College Station, TX, USA, 1986. [Google Scholar]
- Cox, R.M.; Parker, E.U.; Cheney, D.M.; Liebl, A.L.; Martin, L.B.; Calsbeek, R. Experimental evidence for physiological costs underlying the trade-off between reproduction and survival. Funct. Ecol. 2010, 24, 1262–1269. [Google Scholar] [CrossRef]

| Growth Model | Equation | Parameter Description | Reference |
|---|---|---|---|
| Von Bertalanffy | L(t) = standard length at age t L∞ = theoretical asymptotic length t0 = age at zero length k = growth parameter | [58] | |
| Logistic | tIP = growth inflection point | [59] | |
| Gompertz | G = instantaneous growth rate coefficient at age tIP L0 = the length at t0 | [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, Y.-H.; Gao, W.-Z.; Li, Y.; Shi, L. Effects of Habitat Differences and Invasive Species Competition on Age and Growth of Triplophysa strauchii. Animals 2025, 15, 2128. https://doi.org/10.3390/ani15142128
Meng Y-H, Gao W-Z, Li Y, Shi L. Effects of Habitat Differences and Invasive Species Competition on Age and Growth of Triplophysa strauchii. Animals. 2025; 15(14):2128. https://doi.org/10.3390/ani15142128
Chicago/Turabian StyleMeng, Ya-Han, Wei-Zhen Gao, Yan Li, and Lei Shi. 2025. "Effects of Habitat Differences and Invasive Species Competition on Age and Growth of Triplophysa strauchii" Animals 15, no. 14: 2128. https://doi.org/10.3390/ani15142128
APA StyleMeng, Y.-H., Gao, W.-Z., Li, Y., & Shi, L. (2025). Effects of Habitat Differences and Invasive Species Competition on Age and Growth of Triplophysa strauchii. Animals, 15(14), 2128. https://doi.org/10.3390/ani15142128






