Analysis of Carcass and Meat Characteristics in Breast Muscle Between Hubbard White Broilers and Xueshan Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals and Sample Collection
2.3. Hematoxylin and Eosin Staining
2.4. Physicochemical Analysis
2.5. Amino Acid Composition
2.6. Fatty Acid Composition
2.7. RNA Isolation and Real-Time PCR Verification
2.8. Statistical Analysis
3. Results
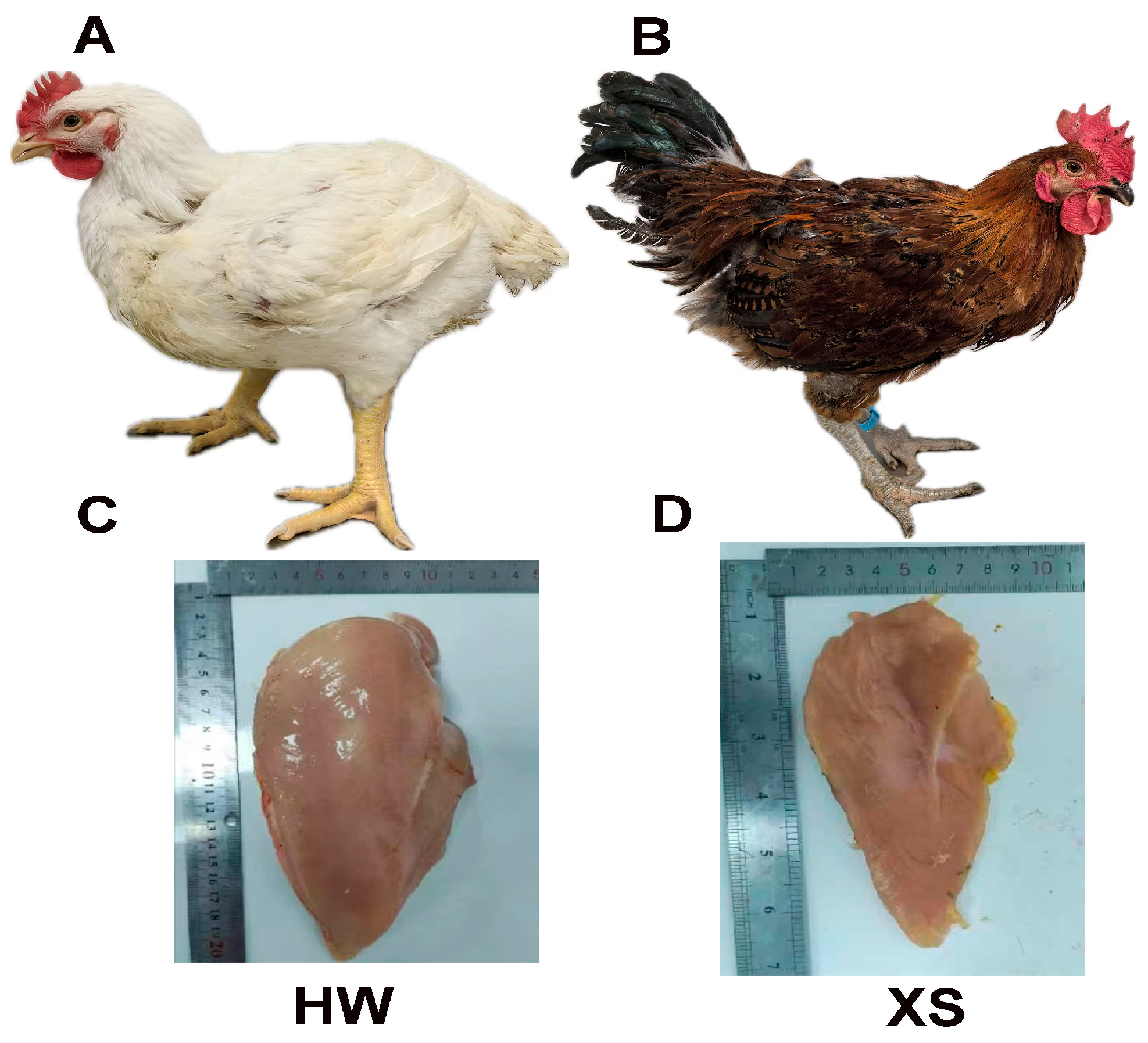
3.1. Comparison of Slaughter and Carcass Traits Between HW and XS
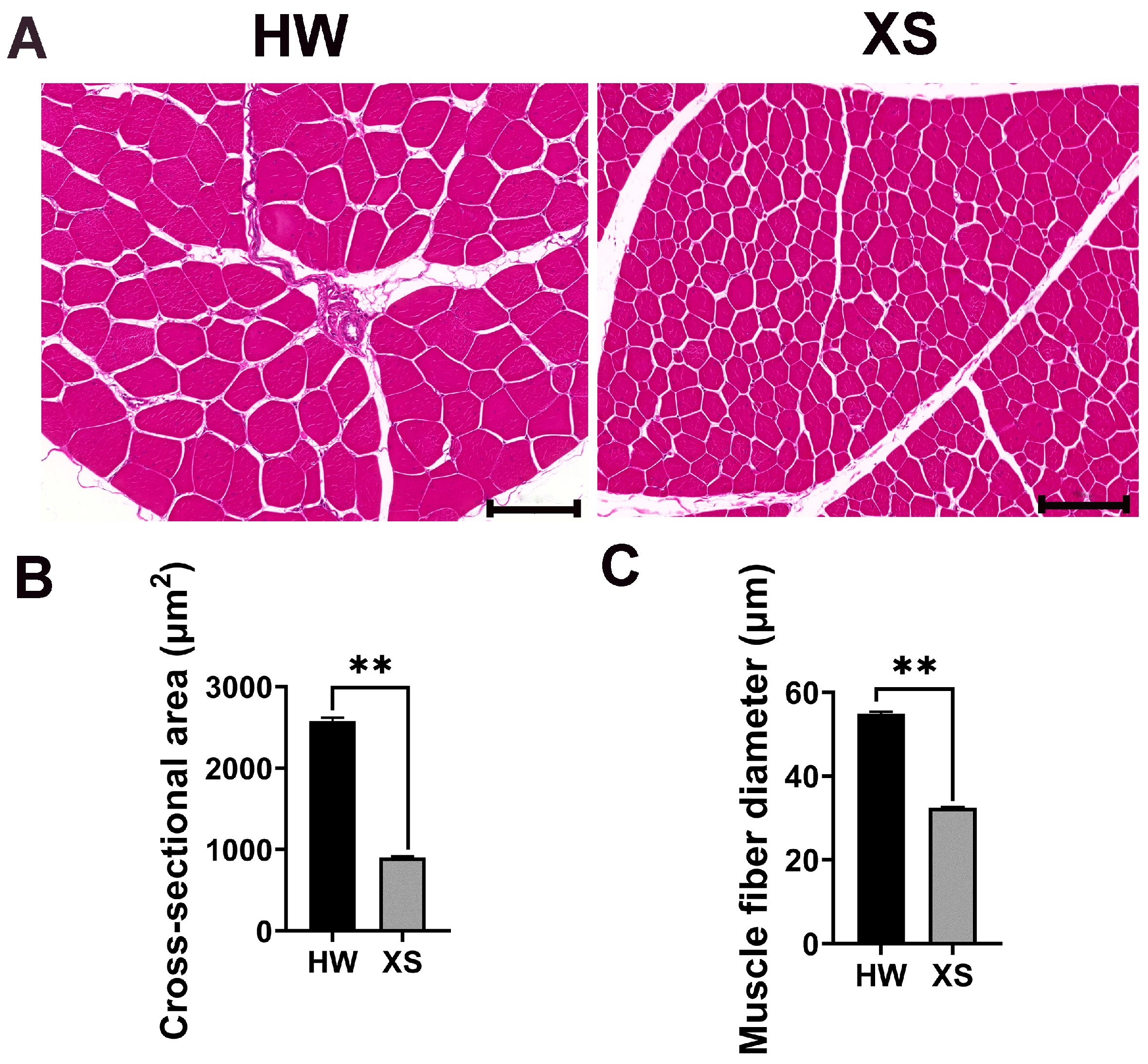
3.2. Comparison of Muscle Fiber Characteristics Between HW and XS
3.3. Comparison of Physical Characteristics Between HW and XS
3.4. Analysis of Amino Acid Profile Between HWs and XSs
3.5. Analysis of Fatty Acid Profile Between HWs and XSs
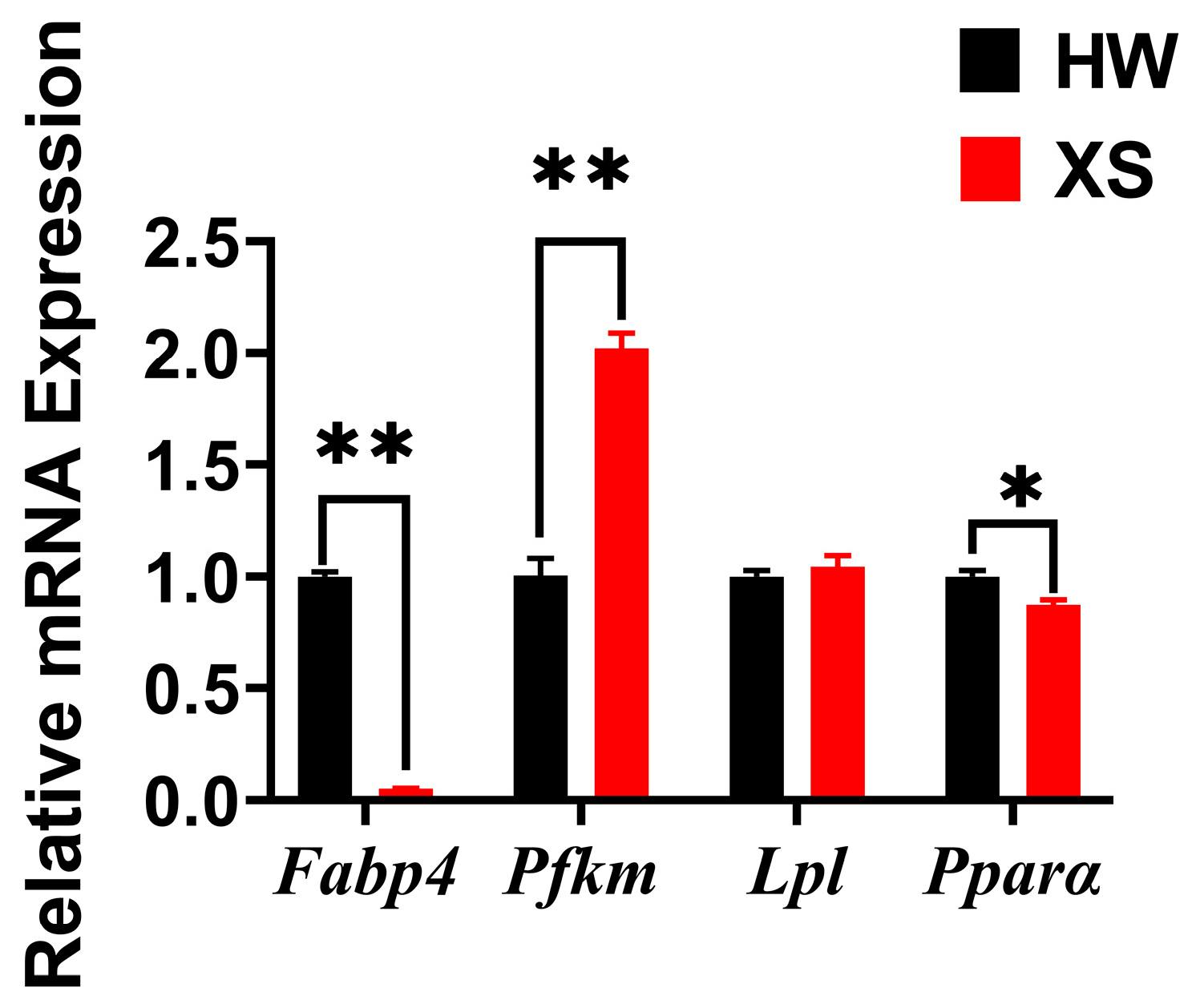
3.6. The mRNA Expression of Lipid Metabolism-Related Genes of HWs and XSs
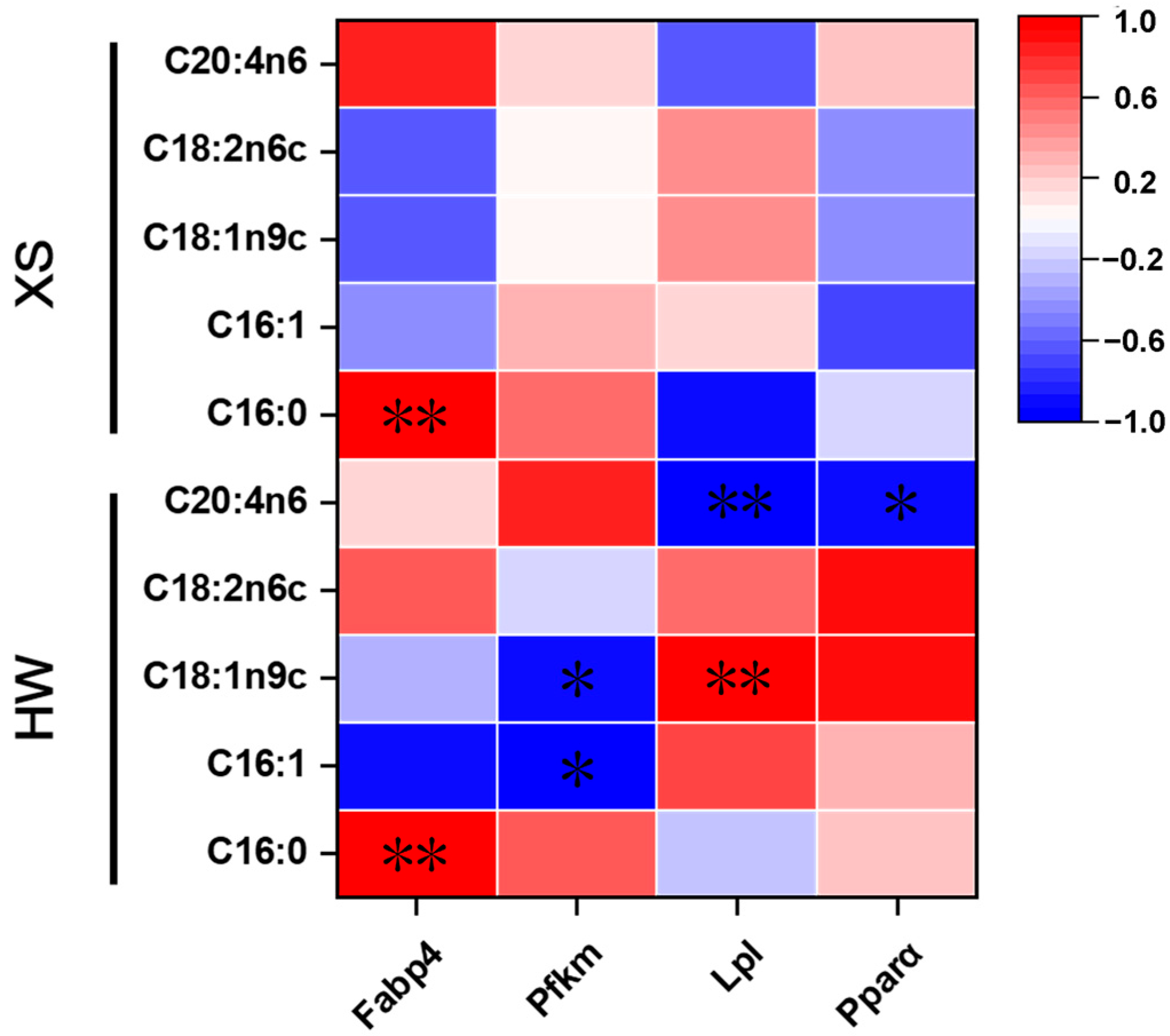
3.7. Correlation Between Gene Expression and Fatty Acid Composition
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SFA | saturated fatty acids |
| CSA | cross-sectional area |
| MFD | myofiber diameter |
| PUFA | polyunsaturated fatty acids |
| TG | triglyceride |
References
- Kearney, J. Food consumption trends and drivers. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2793–2807. [Google Scholar] [CrossRef] [PubMed]
- England, E.M.; Matarneh, S.K.; Oliver, E.M.; Apaoblaza, A.; Scheffler, T.L.; Shi, H.; Gerrard, D.E. Excess glycogen does not resolve high ultimate pH of oxidative muscle. Meat Sci. 2016, 114, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Nematbakhsh, S.; Selamat, J.; Idris, L.H.; Abdull Razis, A.F. Chicken Authentication and Discrimination via Live Weight, Body Size, Carcass Traits, and Breast Muscle Fat Content Clustering as Affected by Breed and Sex Varieties in Malaysia. Foods 2021, 10, 1575. [Google Scholar] [CrossRef] [PubMed]
- MacRae, V.E.; Mahon, M.; Gilpin, S.; Sandercock, D.A.; Mitchell, M.A. Skeletal muscle fibre growth and growth associated myopathy in the domestic chicken (Gallus domesticus). Br. Poult. Sci. 2006, 47, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.; Huo, W.; Li, Y.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Fiber characteristics and meat quality of different muscular tissues from slow- and fast-growing broilers. Poult. Sci. 2022, 101, 101537. [Google Scholar] [CrossRef] [PubMed]
- Sarsenbek, A.; Wang, T.; Zhao, J.K.; Jiang, W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013, 92, 2776–2782. [Google Scholar] [CrossRef] [PubMed]
- Devatkal, S.K.; Naveena, B.M.; Kotaiah, T. Quality, composition, and consumer evaluation of meat from slow-growing broilers relative to commercial broilers. Poult. Sci. 2019, 98, 6177–6186. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, U.; Muthukumar, M.; Haunshi, S.; Niranjan, M.; Raju, M.V.; Rama Rao, S.V.; Chatterjee, R.N. Comparative evaluation of carcass traits and meat quality in native Aseel chickens and commercial broilers. Br. Poult. Sci. 2016, 57, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Lim, A.J.; Muir, W.I.; Groves, P.J. Comparison of performance and carcass composition of a novel slow-growing crossbred broiler with fast-growing broiler for chicken meat in Australia. Poult. Sci. 2021, 100, 100966. [Google Scholar] [CrossRef] [PubMed]
- Devatkal, S.K.; Vishnuraj, M.R.; Kulkarni, V.V.; Kotaiah, T. Carcass and meat quality characterization of indigenous and improved variety of chicken genotypes. Poult. Sci. 2018, 97, 2947–2956. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Gong, Y.Z.; Wu, C.X.; Jiang, J.; Wang, Y.; Li, K. Variation of meat quality traits among five genotypes of chicken. Poult. Sci. 2009, 88, 2212–2218. [Google Scholar] [CrossRef] [PubMed]
- Mussa, N.J.; Kibonde, S.F.; Boonkum, W.; Chankitisakul, V. The Comparison between Tanzanian Indigenous (Ufipa Breed) and Commercial Broiler (Ross Chicken) Meat on the Physicochemical Characteristics, Collagen and Nucleic Acid Contents. Food Sci. Anim. Resour. 2022, 42, 833–848. [Google Scholar] [CrossRef] [PubMed]
- Gumułka, M.; Połtowicz, K. Comparison of carcass traits and meat quality of intensively reared geese from a Polish genetic resource flock to those of commercial hybrids. Poult. Sci. 2020, 99, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Weng, K.; Li, Y.; Huo, W.; Zhang, Y.; Cao, Z.; Zhang, Y.; Xu, Q.; Chen, G. Comparative phosphoproteomic provides insights into meat quality differences between slow- and fast-growing broilers. Food Chem. 2022, 373, 131408. [Google Scholar] [CrossRef] [PubMed]
- Kokoszyński, D.; Żochowska-Kujawska, J.; Kotowicz, M.; Sobczak, M.; Piwczyński, D.; Stęczny, K.; Majrowska, M.; Saleh, M. Carcass characteristics and selected meat quality traits from commercial broiler chickens of different origin. Anim. Sci. J. 2022, 93, e13709. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Lu, Y.; He, Z.; Yu, D.; Zhou, J.; Cao, H.; Zhang, X.; Ji, H.; Lv, K.; Yu, M. Analysis of carcass traits, meat quality, amino acid and fatty acid profiles between different duck lines. Poult. Sci. 2024, 103, 103791. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Yan, L.; Li, Y.; Jin, Y.; Zhang, J.; Che, H.; Diao, J.; Gao, Y.; He, Z.; Sun, R.; et al. Effect of season on slaughter performance, meat quality, muscle amino acid and fatty acid composition, and metabolism of pheasants (Phasianus colchicus). Anim. Sci. J. 2022, 93, e13735. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wu, C.; Li, K.; Gui, G.; Zhang, G.; Yang, H. Association of growth rate with hormone levels and myogenic gene expression profile in broilers. J. Anim. Sci. Biotechnol. 2017, 8, 43. [Google Scholar] [CrossRef] [PubMed]
- Mueller, S.; Kreuzer, M.; Siegrist, M.; Mannale, K.; Messikommer, R.E.; Gangnat, I.D.M. Carcass and meat quality of dual-purpose chickens (Lohmann Dual, Belgian Malines, Schweizerhuhn) in comparison to broiler and layer chicken types. Poult. Sci. 2018, 97, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Fanatico, A.C.; Pillai, P.B.; Hester, P.Y.; Falcone, C.; Mench, J.A.; Owens, C.M.; Emmert, J.L. Performance, livability, and carcass yield of slow- and fast-growing chicken genotypes fed low-nutrient or standard diets and raised indoors or with outdoor access. Poult. Sci. 2008, 87, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Xue, M.; Tang, Y.; Zhang, L.; Hu, P.; Hu, Y.; Cai, D.; Luo, X.; Sun, M.A. Effects of Zinc Source and Level on the Intestinal Immunity of Xueshan Chickens under Heat Stress. Animals 2023, 13, 3025. [Google Scholar] [CrossRef] [PubMed]
- Haunshi, S.; Devatkal, S.; Prince, L.L.L.; Ullengala, R.; Ramasamy, K.; Chatterjee, R. Carcass Characteristics, Meat Quality and Nutritional Composition of Kadaknath, a Native Chicken Breed of India. Foods 2022, 11, 3603. [Google Scholar] [CrossRef] [PubMed]
- Wattanachant, S.; Benjakul, S.; Ledward, D.A. Composition, color, and texture of Thai indigenous and broiler chicken muscles. Poult. Sci. 2004, 83, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of broiler chicken meat quality and factors affecting them: A review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Choo, Y.K.; Kwon, H.J.; Oh, S.T.; Um, J.S.; Kim, B.G.; Kang, C.W.; Lee, S.K.; An, B.K. Comparison of growth performance, carcass characteristics and meat quality of korean local chickens and silky fowl. Asian-Australas. J. Anim. Sci. 2014, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.N.; Iwamoto, H.; Shiba, N.; Miyachi, H.; Tabata, S.; Nishimura, S. Developmental states of the collagen content, distribution and architecture in the pectoralis, iliotibialis lateralis and puboischiofemoralis muscles of male Red Cornish x New Hampshire and normal broilers. Br. Poult. Sci. 2004, 45, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Huo, W.; Weng, K.; Gu, T.; Zhang, Y.; Zhang, Y.; Chen, G.; Xu, Q. Effect of muscle fiber characteristics on meat quality in fast- and slow-growing ducks. Poult. Sci. 2021, 100, 101264. [Google Scholar] [CrossRef] [PubMed]
- Kamal, R.; Chandran, P.C.; Dey, A.; Sarma, K.; Padhi, M.K.; Giri, S.C.; Bhatt, B.P. Status of Indigenous duck and duck production system of India—A review. Trop. Anim. Health Prod. 2022, 55, 15. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Cao, S.; Yang, L.; Li, Z. Flavor formation based on lipid in meat and meat products: A review. J. Food Biochem. 2022, 46, e14439. [Google Scholar] [CrossRef] [PubMed]
- Chaiwang, N.; Marupanthorn, K.; Krutthai, N.; Wattanakul, W.; Jaturasitha, S.; Arjin, C.; Sringarm, K.; Setthaya, P. Assessment of nucleic acid content, amino acid profile, carcass, and meat quality of Thai native chicken. Poult. Sci. 2023, 102, 103067. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.D.; Enser, M.; Fisher, A.V.; Nute, G.R.; Richardson, R.I.; Sheard, P.R. Manipulating meat quality and composition. Proc. Nutr. Soc. 1999, 58, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Benet, I.; Guàrdia, M.D.; Ibañez, C.; Solà, J.; Arnau, J.; Roura, E. Low intramuscular fat (but high in PUFA) content in cooked cured pork ham decreased Maillard reaction volatiles and pleasing aroma attributes. Food Chem. 2016, 196, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H. Association Between Arachidonic Acid and Chicken Meat and Egg Flavor, and Their Genetic Regulation. J. Poult. Sci. 2018, 55, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Functional Roles of Fatty Acids and Their Effects on Human Health. J. Parenter. Enter. Nutr. 2015, 39, 18S–32S. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guo, X.; Deng, Y.; Cheng, L.; Hu, S.; Liu, H.; Hu, J.; Hu, B.; Li, L.; He, H.; et al. Effects of different rearing systems on intramuscular fat content, fatty acid composition, and lipid metabolism-related genes expression in breast and thigh muscles of Nonghua ducks. Poult. Sci. 2020, 99, 4832–4844. [Google Scholar] [CrossRef] [PubMed]
- Eckel, R.H. Lipoprotein lipase. A multifunctional enzyme relevant to common metabolic diseases. N. Engl. J. Med. 1989, 320, 1060–1068. [Google Scholar] [PubMed]
- Wagner, N.; Wagner, K.D. The Role of PPARs in Disease. Cells 2020, 9, 2367. [Google Scholar] [CrossRef] [PubMed]
- Bougarne, N.; Weyers, B.; Desmet, S.J.; Deckers, J.; Ray, D.W.; Staels, B.; De Bosscher, K. Molecular Actions of PPARα in Lipid Metabolism and Inflammation. Endocr. Rev. 2018, 39, 760–802. [Google Scholar] [CrossRef] [PubMed]
- Garin-Shkolnik, T.; Rudich, A.; Hotamisligil, G.S.; Rubinstein, M. FABP4 attenuates PPARγ and adipogenesis and is inversely correlated with PPARγ in adipose tissues. Diabetes 2014, 63, 900–911. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Qin, L.; Feng, Y.; Zheng, R.; Deng, C.; Xiong, Y.; Zuo, B. Molecular characterization, expression profile, and association study with meat quality traits of porcine PFKM gene. Appl. Biochem. Biotechnol. 2014, 173, 1640–1651. [Google Scholar] [CrossRef] [PubMed]
- Uyeda, K. Phosphofructokinase. Adv. Enzymol. Relat. Areas Mol. Biol. 1979, 48, 193–244. [Google Scholar] [PubMed]
| Gene | GenBank Accession | Primer Sequence (5′-3′) | Product Size (bp) |
|---|---|---|---|
| Fabp4 | NM_204290.2 | F:GATGAGACCACAGCAGATGACAG | 120 |
| R:ATCCACCACTTTCCTCTTGATAACAG | |||
| Pfkm | XM_046904942.1 | F:TGTGACAACAGCGATGAATGAGAG | 117 |
| R:GTGGAGGGTGGGCGGATG | |||
| Pparα | NM_001001464.1 | F:ACCAGCATCCAGTCCTTCATCC | 112 |
| R: TGAGGCTTTATCCCCACAAATTCTAC | |||
| Lpl | NM_205282.2 | F:ACGGTGACAGGAATGTATGAAAGC | 99 |
| R:AACCAGCCAGTCCACAACAATG | |||
| β-actin | NM_205518.2 | F:CAGCCATGTATGTAGCCATCCAG | 84 |
| R:CATCACCAGAGTCCATCACAATACC |
| Items | HW | XS | p Value |
|---|---|---|---|
| Live body weight (g) | 3016.10 ± 37.32 A | 1668.82 ± 70.71 B | 0.000 |
| Slaughter weight (g) | 2826.69 ± 32.71 A | 1535.53 ± 63.45 B | 0.000 |
| Semi-eviscerated weight (g) | 2586.77 ± 34.71 A | 1357.61 ± 58.38 B | 0.000 |
| Eviscerated weight (g) | 2154.81 ± 34.03 A | 1002.17 ± 40.31 B | 0.000 |
| Wing weight (g) | 219.49 ± 5.17 A | 142.92 ± 6.71 B | 0.000 |
| Breast muscle weight (g) | 730.13 ± 20.27 A | 179.46 ± 8.28 B | 0.000 |
| Thigh muscle weight (g) | 487.25 ± 12.29 A | 259.16 ± 15.47 B | 0.000 |
| Abdominal fat weight (g) | 23.65 ± 2.10 b | 35.10 ± 4.79 a | 0.037 |
| Semi-eviscerated yield (%) | 85.75 ± 0.27 A | 81.69 ± 0.42 B | 0.000 |
| Eviscerated yield (%) | 71.46 ± 0.79 A | 61.34 ± 0.62 B | 0.000 |
| Thigh muscles in carcass (%) | 22.65 ± 0.57 B | 25.93 ± 0.67 A | 0.001 |
| Breast muscles in carcass (%) | 33.94 ± 0.96 A | 17.60 ± 0.28 B | 0.000 |
| Items | HW | XS | p Value |
|---|---|---|---|
| pH | 6.52 ± 0.04 A | 6.06 ± 0.06 B | 0.000 |
| pH 24 h | 6.47 ± 0.05 A | 5.97 ± 0.04 B | 0.000 |
| L* value 24 h | 27.57 ± 1.61 B | 45.59 ± 1.39 A | 0.000 |
| a* value 24 h | 2.85 ± 0.55 B | 6.29 ± 0.40 A | 0.000 |
| b* value24h | 13.01 ± 0.66 B | 16.68 ± 0.73 A | 0.000 |
| Water-holding capacity (%) | 33.36 ± 2.52 | 32.01 ± 2.90 | 0.726 |
| Thermal loss (%) | 29.01 ± 0.69 A | 22.78 ± 0.97 B | 0.000 |
| Warner–Bratzler shear force (N) | 31.10 ± 4.82 A | 15.48 ± 2.03 B | 0.000 |
| Drip loss (%) | 2.20 ± 0.18 B | 7.61 ± 0.47 A | 0.000 |
| Items | HW | XS | p Value |
|---|---|---|---|
| Non-essential Amino Acid (NEAA) | |||
| Glutamic acid (Glu) * | 3.40 ± 0.10 | 3.31 ± 0.25 | 0.733 |
| Aspartic acid (Asp) * | 2.16 ± 0.07 | 2.15 ± 0.17 | 0.961 |
| Arginine (Arg) | 1.48 ± 0.05 | 1.50 ± 0.12 | 0.853 |
| Alanine (Ala) * | 1.39 ± 0.05 | 1.37 ± 0.11 | 0.847 |
| Glycine (Gly) * | 1.02 ± 0.03 | 1.09 ± 0.10 | 0.525 |
| Serine (Ser) | 0.87 ± 0.03 | 0.84 ± 0.06 | 0.735 |
| Essential Amino Acid (EAA) | |||
| Lysine (Lys) | 2.12 ± 0.06 | 2.12 ± 0.16 | 0.974 |
| Leucine (Leu) | 1.88 ± 0.06 | 1.90 ± 0.15 | 0.909 |
| Valine (Val) | 1.21 ± 0.04 | 1.23 ± 0.10 | 0.852 |
| Isoleucine (Ile) | 1.16 ± 0.04 | 1.17 ± 0.09 | 0.867 |
| Phenylalanine (Phe) * | 0.92 ± 0.03 | 0.95 ± 0.07 | 0.683 |
| Tyrosine (Tyr) * | 0.79 ± 0.02 | 0.79 ± 0.06 | 0.971 |
| Threonine (Thr) | 1.02 ± 0.03 | 1.01 ± 0.08 | 0.835 |
| Histidine (His) | 0.89 ± 0.04 | 0.95 ± 0.07 | 0.482 |
| Methionine (Met) | 0.62 ± 0.02 | 0.62 ± 0.05 | 0.986 |
| Σ EAA | 11.31 ± 0.27 | 11.13 ± 0.21 | 0.615 |
| Σ FAA | 10.35 ± 0.49 b | 11.92 ± 0.52 a | 0.045 |
| Fatty Acids | HW | XS | p Value |
|---|---|---|---|
| C14:0 | 0.13 ± 0.03 B | 0.37 ± 0.03 A | 0.000 |
| C16:0 | 21.94 ± 0.14 B | 24.68 ± 0.33 A | 0.000 |
| C17:0 | 0.19 ± 0.01 a | 0.14 ± 0.02 b | 0.015 |
| C18:0 | 9.46 ± 0.44 | 10.19 ± 0.50 | 0.219 |
| C20:0 | 0.26 ± 0.12 | 0.14 ± 0.01 | 0.388 |
| Σ SFAΣ | 32.16 ± 0.56 B | 35.49 ± 0.61 A | 0.001 |
| C16:1 | 2.89 ± 0.17 | 2.81 ± 0.40 | 0.850 |
| C17:1 | 0.39 ± 0.05 B | 0.69 ± 0.08 A | 0.007 |
| C18:1n9t | 0.18 ± 0.01 A | 0.11 ± 0.01 B | 0.000 |
| C18:1n9C | 33.05 ± 0.55 A | 27.14 ± 0.99 B | 0.000 |
| Σ MUFA | 36.52 ± 0.66 A | 30.68 ± 1.28 B | 0.001 |
| C18:2n6C | 23.62 ± 0.46 A | 21.30 ± 0.43 B | 0.002 |
| C18:2nt | 0.09 ± 0.01 | 0.14 ± 0.02 | 0.075 |
| C20:2 | 0.96 ± 0.18 A | 0.36 ± 0.03 B | 0.008 |
| C20:3n6 | 0.64 ± 0.07 | 0.69 ± 0.06 | 0.581 |
| C20:4n6 | 4.27 ± 0.53 B | 9.15 ± 1.07 A | 0.001 |
| C22:6n3 | 0.19 ± 0.04 b | 0.43 ± 0.09 a | 0.036 |
| Σ PUFA | 29.42 ± 0.35 b | 32.04 ± 0.94 a | 0.018 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, F.; Zhang, X.; Yu, J.; Yuan, J.; Zhang, Y.; He, H.; Ma, Q.; Lu, Y.; Xiang, X.; Yu, M. Analysis of Carcass and Meat Characteristics in Breast Muscle Between Hubbard White Broilers and Xueshan Chickens. Animals 2025, 15, 2099. https://doi.org/10.3390/ani15142099
Li F, Zhang X, Yu J, Yuan J, Zhang Y, He H, Ma Q, Lu Y, Xiang X, Yu M. Analysis of Carcass and Meat Characteristics in Breast Muscle Between Hubbard White Broilers and Xueshan Chickens. Animals. 2025; 15(14):2099. https://doi.org/10.3390/ani15142099
Chicago/Turabian StyleLi, Fan, Xingyu Zhang, Jiajia Yu, Jiaxue Yuan, Yuanfeng Zhang, Huiting He, Qing Ma, Yinglin Lu, Xiaoe Xiang, and Minli Yu. 2025. "Analysis of Carcass and Meat Characteristics in Breast Muscle Between Hubbard White Broilers and Xueshan Chickens" Animals 15, no. 14: 2099. https://doi.org/10.3390/ani15142099
APA StyleLi, F., Zhang, X., Yu, J., Yuan, J., Zhang, Y., He, H., Ma, Q., Lu, Y., Xiang, X., & Yu, M. (2025). Analysis of Carcass and Meat Characteristics in Breast Muscle Between Hubbard White Broilers and Xueshan Chickens. Animals, 15(14), 2099. https://doi.org/10.3390/ani15142099






