Risk Factors for the Occurrence of Cutaneous Neoplasms in Dogs: A Retrospective Study by Cytology Reports, 2019–2021
Simple Summary
Abstract
1. Introduction
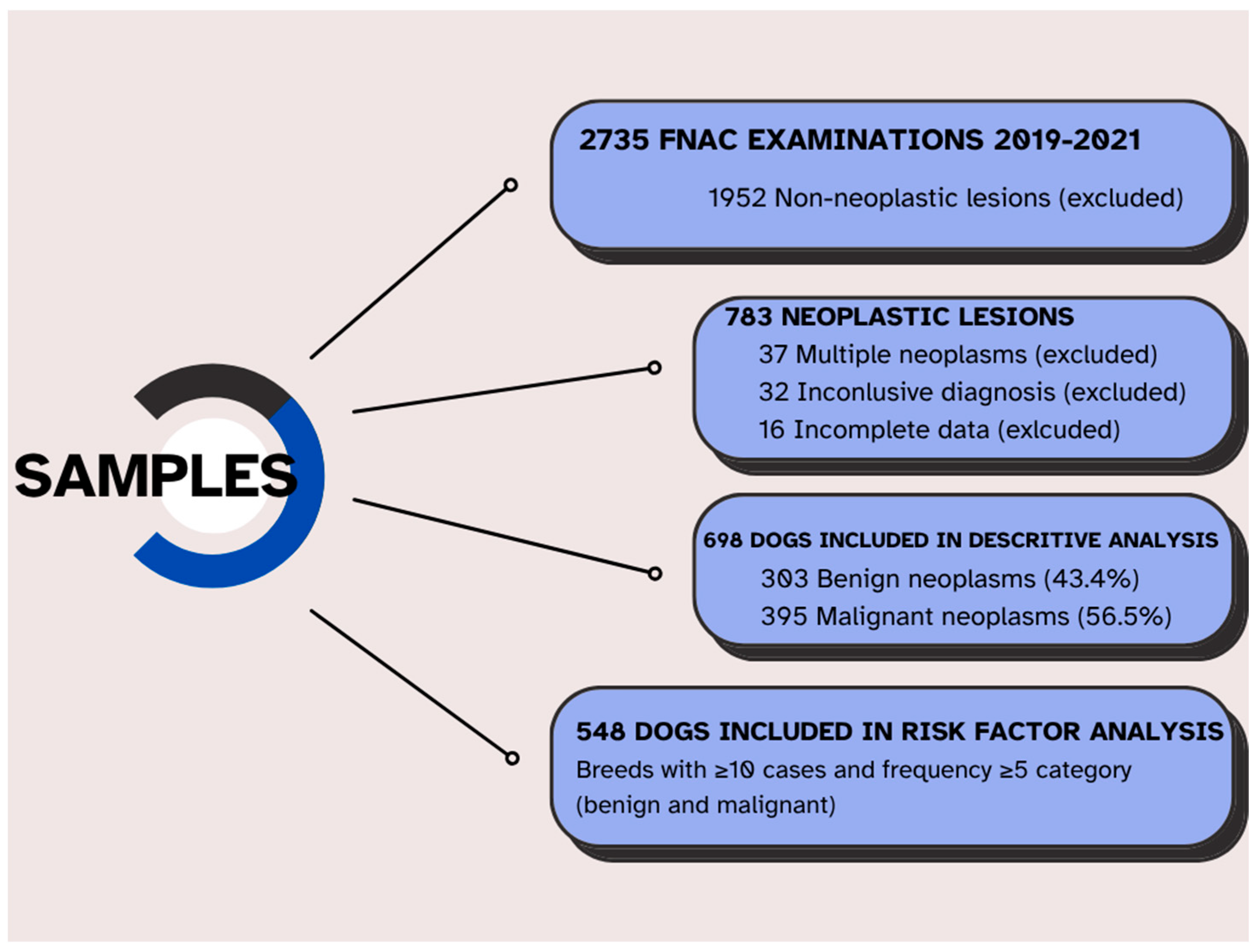
2. Materials and Methods
3. Results
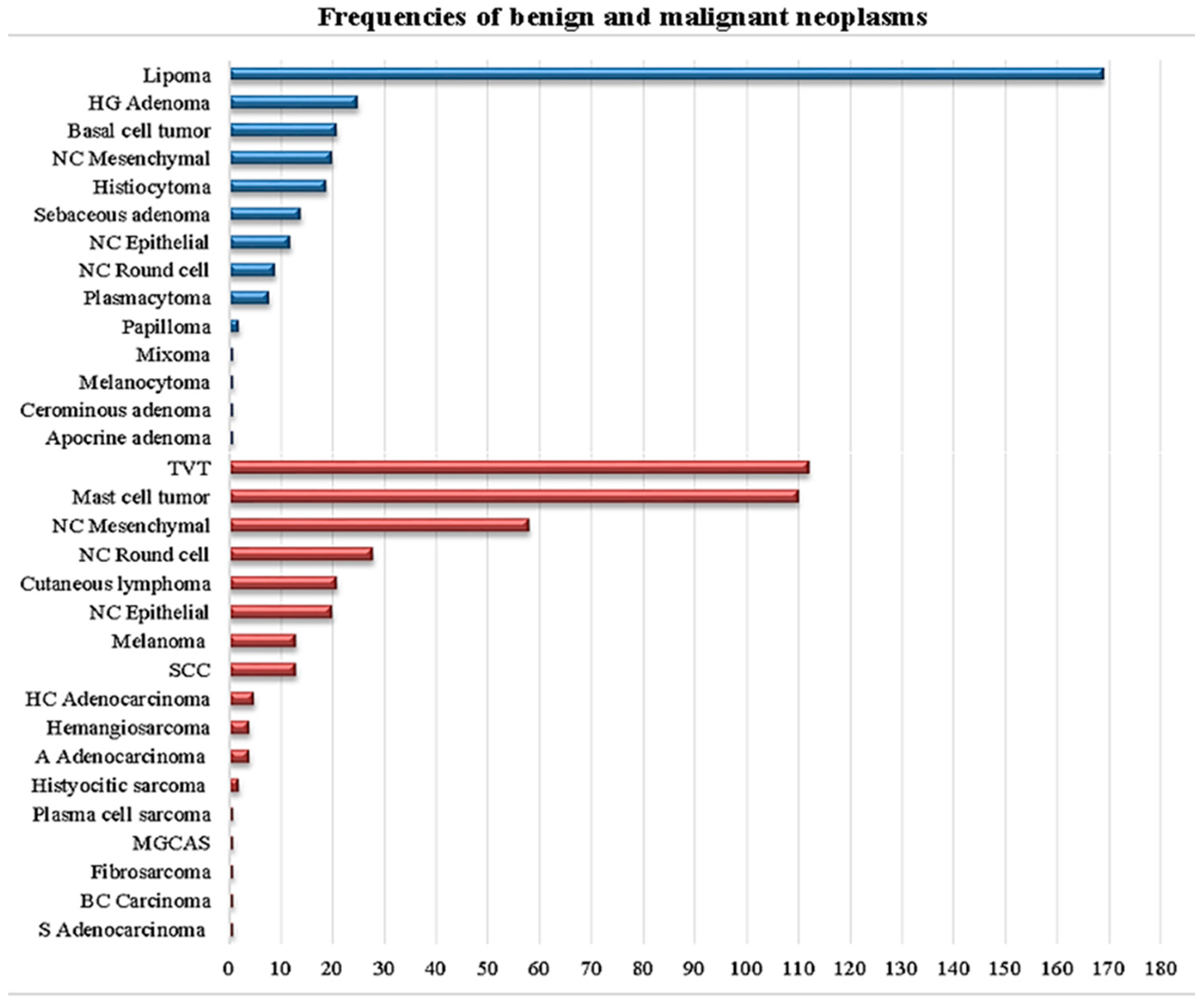
3.1. Descriptive Analysis
3.2. Risk Factors Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FNAC | Fine needle aspiration cytology |
| MCT | Mast cell tumors |
| TVT | Transmissible venereal tumor |
| OR | Odds ratio |
| CI | Confidence intervals |
References
- Kukolj, V.; Nešić, S.; Marinković, D.; Aleksić-Kovačević, S. Prevalence and distribution of canine neoplastic and non-neoplastic cutaneous lesions in Serbia: A retrospective study of 2432 cases (2011–MID 2021). Acta Vet. Beogr. 2021, 71, 403–416. [Google Scholar] [CrossRef]
- Bronden, L.B.; Flagstad, A.; Kristensen, A.T. Veterinary cancer registries in companion animal cancer: A review. Vet. Comp. Oncol. 2007, 5, 133–144. [Google Scholar] [CrossRef]
- Merlo, D.F.; Rossi, L.; Pellegrino, C.; Ceppi, M.; Cardellino, U.; Capurro, C.; Ratto, A.; Sambucco, P.L.; Sestito, V.; Tanara, G.; et al. Cancer incidence in pet dogs: Findings of the Animal Tumor Registry of Genoa, Italy. J. Vet. Intern. Med. 2008, 2, 976–984. [Google Scholar] [CrossRef]
- Baioni, E.; Scanziani, E.; Vincenti, M.C.; Leschiera, M.; Bozzetta, E.; Pezzolato, M.; Desiato, R.; Bertolini, S.; Maurella, C.; Ru, G. Estimating canine cancer incidence: Findings from a population-based tumour registry in northwestern Italy. BMC Vet. Res. 2017, 13, 203. [Google Scholar] [CrossRef]
- Landgren, O.; MacDonald, A.P.; Tani, E.; Czader, M.; Grimfors, G.; Skoog, L.; Öst, Å.; Wedelin, C.; Axdorph, U.; Svedmyr, E.; et al. A prospective comparison of fine needle aspiration cytology and histopathology in the diagnosis and classification of lymphomas. Hematol. J. 2004, 5, 69–76. [Google Scholar] [CrossRef]
- Ghisleni, G.; Roccabianca, P.; Ceruti, R.; Stefanello, D.; Bonfanti, U.; Avallone, G.; Pezzolato, M.; Lombardo, R.; Finazzi, M.; Grieco, V. Correlation between Fine-Needle Aspiration Cytology and Histopathology in the Evaluation of Cutaneous and Subcutaneous Masses from Dogs and Cats. Vet. Clin. Pathol. 2006, 35, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Simeonov, R.S. The Accuracy of Fine-Needle Aspiration Cytology in the Diagnosis of Canine Skin and Subcutaneous Masses. Comp. Clin. Path. 2012, 21, 1433–1438. [Google Scholar] [CrossRef]
- O’Neill, D.G.; Coulson, N.R.; Church, D.B.; Brodbelt, D.C. Demography and disorders of German Shepherd Dogs under primary veterinary care in the UK. Canine Genet. Epidemiol. 2017, 4, 7. [Google Scholar] [CrossRef]
- Pimentel, P.A.B.; Oliveira, C.S.F.; Horta, R.S. Epidemiological study of canine transmissible venereal tumor (CTVT) in Brazil, 2000–2020. Prev. Vet. Med. 2021, 197, 105526. [Google Scholar] [CrossRef]
- Sharkey, L.C.; Dial, S.M.; Matz, M.E. Maximizing the diagnostic value of cytology in small animal practice. Vet. Clin. N. Am. Small Anim. Pr. 2007, 37, 351–372. [Google Scholar] [CrossRef]
- Allen, S.W.; Prasse, K.W.; Mahaffey, E.A. Cytologic differentiation of benign from malignant canine mammary tumors. Vet. Pathol. 1986, 23, 649–655. [Google Scholar] [CrossRef]
- Weir, M.M.; Rosenberg, A.E.; Bell, D.A. Grading of spindle cell sarcomas in fine-needle aspiration biopsy specimens. Am. J. Clin. Pathol. 1999, 112, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Camus, M.S.; Priest, H.L.; Koehler, J.W.; Driskell, E.A.; Rakich, P.M.; Ilha, M.R.; Krimer, P.M. Cytologic criteria for mast cell tumor grading in dogs with evaluation of clinical outcome. Vet. Pathol. 2016, 53, 1117–1123. [Google Scholar] [CrossRef]
- Sapierzyński, R.; Kliczkowska-Klarowicz, K.; Jankowska, U.; Jagielski, D. Cytodiagnostics of canine lymphomas—Possibilities and limitations. Pol. J. Vet. Sci. 2016, 19, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Milovancev, M.; Townsend, K.L.; Gorman, E.; Bracha, S.; Curran, K.; Russell, D.S. Shaved margin histopathology and imprint cytology for assessment of excision in canine mast cell tumors and soft tissue sarcomas. Vet. Surg. 2017, 46, 879–885. [Google Scholar] [CrossRef]
- Dolka, I.; Czopowicz, M.; Gruk-Jurka, A.; Wojtkowska, A.; Sapierzyński, R.; Jurka, P. Diagnostic efficacy of smear cytology and Robinson’s cytological grading of canine mammary tumors with respect to histopathology, cytomorphometry, metastases and overall survival. PLoS ONE 2018, 13, e0191595. [Google Scholar] [CrossRef] [PubMed]
- Millanta, F.; Asproni, P.; Aquino, G.; Poli, A. Cytologic grading of canine and feline spindle-cell sarcomas of soft tissues and its correlation with histologic grading. Top. Companion Anim. Med. 2020, 41, 100458. [Google Scholar] [CrossRef]
- González-Chávez, M.T.; Pino, D.; Zamora, Y.; Matos, R.G. Consideraciones actuales sobre las neoplasias cutáneas en la especie canina. Rev. Salud Anim. 2020, 42, 1–19. [Google Scholar]
- Dobrin, A.A.; Militaru, M. Cytopathology and histopathology in diagnosis of malignant cutaneous and subcutaneous mesenchymal neoplasms in dogs—A review. Lucr. Ştiinţifice 2023, 56, 71–88. [Google Scholar]
- Martins, A.L.; Canadas-Sousa, A.; Mesquita, J.R.; Dias-Pereira, P.; Amorim, I.; Gärtner, F. Retrospective study of canine cutaneous tumors submitted to a diagnostic pathology laboratory in Northern Portugal (2014–2020). Canine Med. Genet. 2022, 9, 2. [Google Scholar] [CrossRef]
- Subapriya, S.; Pazhanivel, N.; Gokulakrishnan, M.; Nagarajan, B.; Kavitha, S.; Sumathi, D.; Vairamuthu, S. Incidence and pathology or skin tumours in dogs. PIJ 2021, 10, 620–629. [Google Scholar]
- Meirelles, A.E.; Oliveira, E.C.; Rodrigues, B.A.; Costa, G.R.; Sonne, L.; Tesser, E.S.; Driemeier, D. Prevalence of neoplasms in domestic animals diagnosed by cytological exam: 1001 cases (2008–2010). Pesqui. Vet. Bras. 2010, 30, 832–838. [Google Scholar] [CrossRef]
- Bonnett, B.N.; Egenvall, A.; Hedhammar, A.; Olson, P. Mortality in over 350,000 insured Swedish dogs from 1995–2000: I. Breed-, gender-, age- and cause-specific rates. Acta Vet. Scand. 2005, 46, 105–120. [Google Scholar] [CrossRef]
- Villamil, J.A.; Henry, C.J.; Bryan, J.N.; Ellersieck, M.; Schultz, L.; Tyler, J.W.; Hahn, A.W. Identification of the most common cutaneous neoplasms in dogs and evaluation of breed and age distributions for selected neoplasms. JAVMA 2011, 239, 960–965. [Google Scholar] [CrossRef] [PubMed]
- Blackwood, L.; Murphy, S.; Buracco, P.; De Vos, J.P.; De Fornel-Thibaud, P.; Hirschberger, J. European consensus document on mast cell tumours in dogs and cats. Vet. Comp. Oncol. 2012, 10, e1–e29. [Google Scholar] [CrossRef]
- Graf, R.; Pospischil, A.; Guscetti, F.; Meier, D.; Welle, M.; Dettwiler, M. Cutaneous tumors in Swiss dogs: Retrospective data from the Swiss Canine Cancer Registry, 2008–2013. Vet. Pathol. 2018, 55, 809–820. [Google Scholar] [CrossRef]
- Kiupel, M.; Webster, J.D.; Bailey, K.L.; Best, S.; DeLay, J.; Detrisac, C.J.; Fitzgerald, S.D.; Gamble, D.; Ginn, P.E.; Goldschmidt, M.H.; et al. Proposal of a 2-tier histologic grading system for canine cutaneous mast cell tumors to more accurately predict biological behavior. Vet. Pathol. 2011, 48, 147–155. [Google Scholar] [CrossRef]
- Warland, J.; Dobson, J. Breed predispositions in canine mast cell tumour: A single centre experience in the United Kingdom. Vet. J. 2013, 197, 496–498. [Google Scholar] [CrossRef]
- White, C.R.; Hohenhaus, A.E.; Kelsey, J.; Procter-Gray, E. Cutaneous MCTs: Associations with spay/neuter status, breed, body size, and phylogenetic cluster. J. Am. Anim. Hosp. Assoc. 2011, 47, 210–216. [Google Scholar] [CrossRef]
- Schmitt, J.; Seidler, A.; Diepgen, T.L.; Bauer, A. Occupational ultraviolet light exposure increases the risk for the development of cutaneous squamous cell carcinoma: A systematic review and meta-analysis. Br. J. Dermatol. 2011, 164, 291–307. [Google Scholar] [CrossRef]
- Hargis, A.M.; Thomassen, R.W. Animal model: Solar dermatosis (keratosis) and solar dermatosis with squamous cell carcinoma. Am. J. Pathol. 1979, 94, 193–196. [Google Scholar] [PubMed]
- Goldschmidt, M.H.; Shofer, F.S. Skin Tumors of the Dog and Cat; Pergamon Press: Oxford, UK, 1992. [Google Scholar]
- Hayes, H.M., Jr.; Hoover, R.; Tarone, R.E. Bladder cancer in pet dogs: A sentinel for environmental cancer? Am. J. Epidemiol. 1981, 114, 229–233. [Google Scholar] [CrossRef]
- Reif, J.S.; Lower, K.S.; Ogilvie, G.K. Residential exposure to magnetic fields and risk of canine lymphoma. Am. J. Epidemiol. 1995, 141, 352–359. [Google Scholar] [CrossRef]
- Rothwell, T.L.; Howlett, C.R.; Middleton, D.J.; Griffiths, D.A.; Duff, B.C. Skin neoplasms of dogs in Sidney. Aust. Vet. J. 1987, 64, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Mukaratirwa, S.; Chipunza, J.; Chitanga, S.; Chimonyo, M.; Bhebhe, E. Canine cutaneous neoplasms: Prevalence and influence of age, sex and site on the presence and potential malignancy of cutaneous neoplasms in dogs from Zimbabwe. J. South Afr. Vet. Assoc. 2005, 76, 59–62. [Google Scholar] [CrossRef]
- Fajardo, R.; Alpízar, A.; Pérez, L.S.; Martínez, J.S.; Córdova, E. Prevalence of tumors in dogs from the municipality of Toluca, Mexico, from 2002 to 2008. Arch. Med. Vet. 2013, 45, 305–309. [Google Scholar] [CrossRef]
- García, E.; Alpízar, A.; Fajardo, R.; Córdova, D.; Pérez, L.; Martínez, S. Epidemiology of tumors in dogs in the capital of the state of Mexico from 2002–2016. Arq. Bras. Med. Vet. Zootec. 2019, 71, 1085–1092. [Google Scholar] [CrossRef]
- Hassan, B.B.; Al-Mokaddem, A.K.; Abdelrahman, H.A.; Samir, A.; Mousa, M.R. Cutaneous tumors in dogs: A retrospective epidemiological and histological study of 112 cases. Adv. Anim. Vet. Sci. 2022, 10, 170–182. [Google Scholar] [CrossRef]
- Pakhrin, B.; Kang, M.S.; Bae, I.H.; Park, M.S.; Jee, H.; You, M.H.; Kim, J.H.; Yoon, B.I.; Choi, Y.K.; Kim, D.Y. Retrospective study of canine cutaneous tumors in Korea. J. Vet. Sci. 2007, 8, 229–236. [Google Scholar] [CrossRef]
- Souza, T.M.; Fighera, R.A.; Irigoyen, L.F.; Barros, C.S.L. Estudo retrospectivo de 761 tumores cutâneos em cães. Ciênc. Rural. 2006, 36, 555–560. [Google Scholar] [CrossRef]
- Strakova, A.; Murchison, E.P. The changing global distribution and prevalence of canine transmissible venereal tumour. BMC Vet. Res. 2014, 10, 168. [Google Scholar] [CrossRef] [PubMed]
- Ortega-Pacheco, A.; Segura-Correa, J.C.; Jimenez-Coello, M.; Forsberg, C.L. Reproductive patterns and reproductive pathologies of stray bitches in the tropics. Theriogenology 2007, 67, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Calderón, L.G.; Fonseca-Alves, C.E.; Kobayashi, P.E.; Carvalho, M.; Drigo, S.A.; de Oliveira Vasconcelos, R.; Laufer-Amorim, R. Alterations in PTEN, MDM2, TP53 and AR protein and gene expression are associated with canine prostate carcinogenesis. Res. Vet. Sci. 2016, 106, 56–61. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef]
- Mochizuki, H.; Kennedy, K.; Shapiro, S.G.; Breen, M. BRAF mutations in canine cancers. PLoS ONE 2015, 10, e0129534. [Google Scholar] [CrossRef]
- Sledge, D.G.; Webster, J.; Kiupel, M. Canine cutaneous mast cell tumors: A combined clinical and pathologic approach to diagnosis, prognosis, and treatment selection. Vet. J. 2016, 215, 43–54. [Google Scholar] [CrossRef]
- Pang, L.Y.; Argyle, D.J. The evolving cancer cell: Molecular characterization of canine cancer. In BSAVA Manual of Canine and Feline Oncology, 3rd ed.; Dobson, J.M., Lascelles, B.D.X., Eds.; British Small Animal Veterinary Association: Gloucester, UK, 2011; pp. 11–28. [Google Scholar]
- García-Cueto, O.R.; Santillán-Espinoza, M.T.; Martínez-López, M.; Tejeda-Martínez, A. Extreme temperature scenarios in Mexicali, Mexico under climate change conditions. Atmósfera 2013, 26, 509–520. [Google Scholar] [CrossRef]
- Ortega-Pacheco, A.; Jiménez-Coello, M. Debate for and against euthanasia in the control of dog populations. In Euthanasia—The "Good Death" Controversy in Humans and Animals; Kure, J., Ed.; InTech: Rijeka, Croatia, 2011; pp. 233–244. [Google Scholar] [CrossRef]
- Valenciano, A.C.; Cowell, R.L. Cowell and Tyler’s Diagnostic Cytology and Hematology of the Dog and Cat, 5th ed.; Elsevier: St. Louis, MO, USA, 2019. [Google Scholar]
- Chikweto, A.; McNeil, P.; Bhaiyat, M.I.; Stone, D.; Sharma, R.N. Neoplastic and nonneoplastic cutaneous tumors of dogs in Grenada, West Indies. ISRN Vet. Sci. 2011, 2011, 416435. [Google Scholar] [CrossRef]
- Nikula, K.J.; Benjamin, S.A.; Angleton, G.M.; Saunders, W.J.; Lee, A.C. Ultraviolet radiation, solar dermatosis, and cutaneous neoplasia in beagle dogs. Radiat. Res. 1992, 129, 11–18. [Google Scholar] [CrossRef]
- Megquier, K.; Turner-Maier, J.; Swofford, S.; Kim, J.H.; Sarver, A.L.; Wang, C.; Sakthikumar, S.; Johnson, J.; Koltookian, M.; Lewellen, M.; et al. Comparative genomics reveals shared mutational landscape in canine hemangiosarcoma and human angiosarcoma. Mol. Cancer Res. 2020, 18, 241–2421. [Google Scholar] [CrossRef]
- Dhein, E.S.; Heikklä, U.; Oevermann, A.; Blatter, S.; Meier, D.; Hartnack, S.; Guscetti, F. Incidence rates of the most common canine tumors based on data from the Swiss Canine Cancer Registry (2008 to 2020). PLoS ONE 2024, 19, e0302231. [Google Scholar] [CrossRef]
- Vozdova, M.; Kubickova, S.; Fictum, P.; Sliva, J.; Rubes, J. Recurrent gene mutations detected in canine mast cell tumours by next generation sequencing. Vet. Comp. Oncol. 2020, 18, 509–518. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Grassinger, J.M.; Floren, A.; Törner, K.; Beitzinger, C.; Loesenbeck, G.; Müller, T. Tumour incidence in dogs in Germany: A retrospective analysis of 109,616 histopathological diagnoses (2014–2019). J. Comp. Pathol. 2022, 198, 33–55. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.L.; Bryan, M.E.; Atkinson, E.S.; Keeler, M.R.; Hahn, A.W.; Bryan, J.N. Neutering is associated with developing hemangiosarcoma in dogs in the Veterinary Medical Database: An age and time-period matched case-control study (1964–2003). Can. J. Vet. Res. 2020, 84, 106–114. [Google Scholar]
- Zink, M.C.; Farhoody, P.; Elser, S.E.; Ruffini, L.D.; Gibbons, T.A.; Rieger, R.H. Evaluation of the risk and age of onset of cancer and behavioral disorders in gonadectomized Vizslas. JAVMA 2014, 244, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Shoop, S.J.W.; Marlow, S.; Church, D.B.; English, K.; McGreevy, P.D.; Stell Thomson, P.C.; O’Neill, D.G.; Brodbelt, D.C. Prevalence and risk factors for mast cell tumours in dogs in England. CAGH 2015, 2, 1. [Google Scholar] [CrossRef]
- Egenvall, A.; Bonnett, B.N.; Öhagen, P.; Olson, P.; Hedhammar, Å.; von Euler, H. Incidence of and survival after mammary tumors in a population of over 80,000 insured female dogs in Sweden from 1995 to 2002. Prev. Vet. Med. 2005, 69, 109–127. [Google Scholar] [CrossRef]
- Torres de la Riva, G.; Hart, B.L.; Farver, T.B.; Oberbauer, A.M.; Messam, L.L.M.; Willits, N.H.; Oberbauer, A.M.; Hart, L.A. Neutering dogs: Effects on joint disorders and cancers in golden retrievers. PLoS ONE 2013, 8, e55937. [Google Scholar] [CrossRef]
- Maniscalco, L.; Olimpo, M.; Parisi, L.; Buracco, P.; Mazzone, E.; Martinelli, G.; Martano, M.; Lussich, S.; Morello, E. A novel scoring system proposal to guide treatment of dogs with hepatoid gland tumors. Front. Vet. Sci. 2025, 12, 1451. [Google Scholar] [CrossRef]
- Petterino, C.; Martini, M.; Castagnaro, M. Immunohistochemical Detection of growth hormone (GH) in canine hepatoid gland tumors. J. Vet. Med. Sci. 2004, 66, 569–572. [Google Scholar] [CrossRef]
- Aupperle-Lellbach, H.; Heidrich, D.; Kehl, A.; Conrad, D.; Brockman, M.; Törner, K.; Beitzinger, C.; Müller, T. KITLG copy number germline variations in Schnauzer breeds and their relevance in digital squamous cell carcinoma in black giant Schnauzers. Vet. Sci. 2023, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Gies, P.; van Deventer, E.; Green, A.C.; Sinclair, C.; Tinker, R. Review of the global solar UV index 2015 workshop report. Health Phys. 2018, 114, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Szivek, A.; Burns, R.E.; Gericota, B.; Affolter, V.K.; Verstraete, F.J.M.; Kass, P.H. Clinical outcome of dogs with cutaneous hemangiosarcoma treated with surgery alone: 104 cases (2008–2019). Vet. Comp. Oncol. 2011, 10, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Mello, C.B.E.; Engelmann, A.M.; Kommers, G.D.; Flores, M.M.; Fighera, R.A.; Rodrigues, B.R.; Lamego, E.C.; da Silva, C.B.; Bueno, A.; de Andrade, C.M. Fine needle aspiration cytology: High accuracy in diagnosing cutaneous and subcutaneous neoplasms in dogs. Comp. Clin. Pathol. 2023, 32, 155–164. [Google Scholar] [CrossRef]
- Rinaldi, V.; Crisi, P.E.; Vignoli, M.; Pierini, A.; Terragni, R.; Cabibbo, E.; Boari, A.; Finotello, R. The Role of Fine Needle Aspiration of Liver and Spleen in the Staging of Low-Grade Canine Cutaneous Mast Cell Tumor. Vet. Sci. 2022, 9, 473. [Google Scholar] [CrossRef]
- Dank, G.; Buber, T.; Rice, A.; Kraicer, N.; Hanael, E.; Shasha, T.; Aviram, G.; Yehudayoff, A.; Kent, M.S. Training and validation of a novel non-invasive imaging system for ruling out malignancy in canine subcutaneous and cutaneous masses using machine learning in 664 masses. Front. Vet. Sci. 2023, 10, 1164438. [Google Scholar] [CrossRef]
| Breed | Freq. | Breed | Freq. | Breed | Freq. |
|---|---|---|---|---|---|
| Akita | 1 | Fila Brasileiro | 1 | Poodle | 38 |
| Belgian Shepherd | 3 | French Bulldog | 4 | Pug | 13 |
| Boston Terrier | 4 | German Shepherd | 14 | Portuguese Water Dog | 1 |
| Bull Terrier | 2 | Golden Retriever | 5 | Rat Terrier | 1 |
| Basset Hound | 7 | Greyhound | 1 | Rottweiler | 6 |
| Beagle | 7 | Great Dane | 4 | Saint Bernard | 2 |
| Boxer | 21 | Jack Russell Terrier | 2 | Schnauzer | 56 |
| Chihuahua | 53 | Labrador Retriever | 36 | Shar Pei | 3 |
| Chow Chow | 2 | Malinois B. Shepherd | 1 | Shih Tzu | 10 |
| Cocker Spaniel | 7 | Maltese | 6 | Siberian Husky | 9 |
| Corgi | 1 | Miniature Pinscher | 1 | Springer Spaniel | 2 |
| Dachshund | 11 | Mixed-breed dogs | 247 | Swiss Shepherd | 1 |
| Doberman | 4 | Old English Sheepdog | 1 | Weimaraner | 1 |
| Dogo Argentino | 2 | Pekingese | 1 | Xoloitzcuintle | 1 |
| Dogue of Bordeaux | 1 | Pitbull Terrier | 94 | Yorkshire Terrier | 4 |
| English Bulldog | 4 | Pointer | 2 | Total | 698 |
| Breed | Benign | Malignant | Frequency (%) |
|---|---|---|---|
| Chihuahua | 26 | 27 | 53 (9.67) |
| German Shepherd | 7 | 7 | 14 (2.55) |
| Labrador Retriever | 22 | 14 | 36 (6.56) |
| Mixed-breed dogs | 86 | 161 | 247 (45.07) |
| Pitbull Terrier | 22 | 72 | 94 (17.15) |
| Poodle | 24 | 14 | 38 (6.93) |
| Schnauzer | 43 | 13 | 56 (10.21) |
| Shih Tzu | 5 | 5 | 10 (1.82) |
| Total | 235 | 313 | 548 |
| Variable | n (%) | Adjusted Odds Ratio | 95% CI | p-Value |
|---|---|---|---|---|
| Age group (years) | ||||
| 0–4 years (reference) | 99 (18.06) | 1.00 | — | — |
| 5–8 years | 181 (33.02) | 0.614 | 0.353–1.067 | 0.1366 |
| 9–12 years | 193 (35.21) | 0.241 | 0.141–0.415 | 0.0025 * |
| 13–18 years | 75 (13.68) | 0.295 | 0-155–0563 | 0.1256 |
| Sex and Reproductive Status | ||||
| Neutered male (reference) | 91 (16.60) | 1.00 | — | — |
| Intact female | 148 (27.00) | 2.499 | 1.462–4.271 | 0.0042 * |
| Spayed female | 147 (26.82) | 1.365 | 0.807–2.308 | 0.2492 |
| Intact male | 162 (29.56) | 2.004 | 1.190–3.372 | 0.1387 |
| Breed | ||||
| Mixed breed (reference) | 247 (45.07) | 1.00 | — | — |
| Chihuahua | 53 (9.67) | 0.555 | 0.305–1.010 | 0.7705 |
| Labrador Retriever | 36 (6.56) | 0.340 | 0.166–0.698 | 0.2079 |
| German Sheperd | 14 (2.55) | 0.534 | 0.181–1.573 | 0.9304 |
| American Pit Bull Terrier | 94 (17.15) | 1.748 | 1.014–3.013 | <0.0001 * |
| Poodle | 38 (6.93) | 0.312 | 0.153–0.633 | 0.1217 |
| Schnauzer | 56 (10.21) | 0.161 | 0.082–0.317 | 0.0004 * |
| Shih Tzu | 10 (1.82) | 0.534 | 0.150–1.896 | 0.9405 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Reynoso, I.C.; Flores-Dueñas, C.A.; Castro-del Campo, N.; Jácome-Ibarra, M.; Herrera-Ramírez, J.C.; Gómez-Gómez, S.D.; Rodríguez-Gaxiola, M.Á.; Gaxiola-Camacho, S.M. Risk Factors for the Occurrence of Cutaneous Neoplasms in Dogs: A Retrospective Study by Cytology Reports, 2019–2021. Animals 2025, 15, 2069. https://doi.org/10.3390/ani15142069
García-Reynoso IC, Flores-Dueñas CA, Castro-del Campo N, Jácome-Ibarra M, Herrera-Ramírez JC, Gómez-Gómez SD, Rodríguez-Gaxiola MÁ, Gaxiola-Camacho SM. Risk Factors for the Occurrence of Cutaneous Neoplasms in Dogs: A Retrospective Study by Cytology Reports, 2019–2021. Animals. 2025; 15(14):2069. https://doi.org/10.3390/ani15142069
Chicago/Turabian StyleGarcía-Reynoso, Issa Carolina, Cesar Augusto Flores-Dueñas, Nohemí Castro-del Campo, Mariana Jácome-Ibarra, José Carlomán Herrera-Ramírez, Sergio Daniel Gómez-Gómez, Miguel Ángel Rodríguez-Gaxiola, and Soila Maribel Gaxiola-Camacho. 2025. "Risk Factors for the Occurrence of Cutaneous Neoplasms in Dogs: A Retrospective Study by Cytology Reports, 2019–2021" Animals 15, no. 14: 2069. https://doi.org/10.3390/ani15142069
APA StyleGarcía-Reynoso, I. C., Flores-Dueñas, C. A., Castro-del Campo, N., Jácome-Ibarra, M., Herrera-Ramírez, J. C., Gómez-Gómez, S. D., Rodríguez-Gaxiola, M. Á., & Gaxiola-Camacho, S. M. (2025). Risk Factors for the Occurrence of Cutaneous Neoplasms in Dogs: A Retrospective Study by Cytology Reports, 2019–2021. Animals, 15(14), 2069. https://doi.org/10.3390/ani15142069






