Effects of Feeding Newly Hatched Larvae on the Growth, Survival, and Growth Patterns of Kawakawa (Euthynnus affinis) Larvae and Juveniles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Experiment 1: Effects of Feeding Newly Hatched Larvae on the Growth and Survival of Kawakawa Larvae
2.2.1. Test Fish and Rearing Methods
2.2.2. Experimental Groups
2.2.3. Sampling and Proportional Measurement of Larvae and Juveniles
2.2.4. Method for Estimating Survival Rate
2.2.5. Statistical Analysis
2.3. Experiment 2: Relative Growth from Larval to Juvenile Stages in Kawakawa
2.3.1. Test Larvae and Juveniles and Their Rearing Methods
2.3.2. Sampling and Measurement of Larvae and Juveniles
2.3.3. Statistical Analysis
3. Results
3.1. Experiment 1
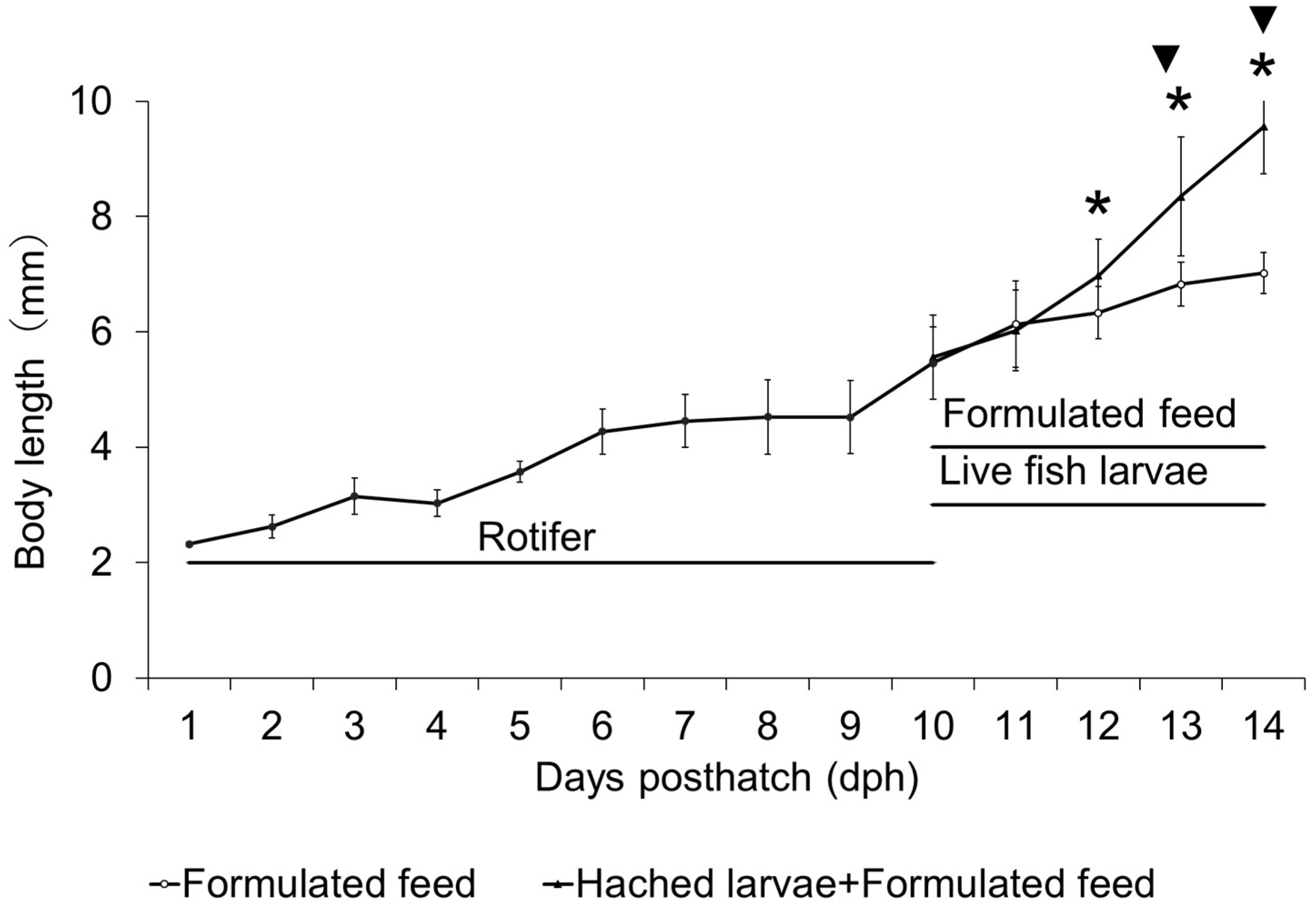
3.1.1. Absolute Growth
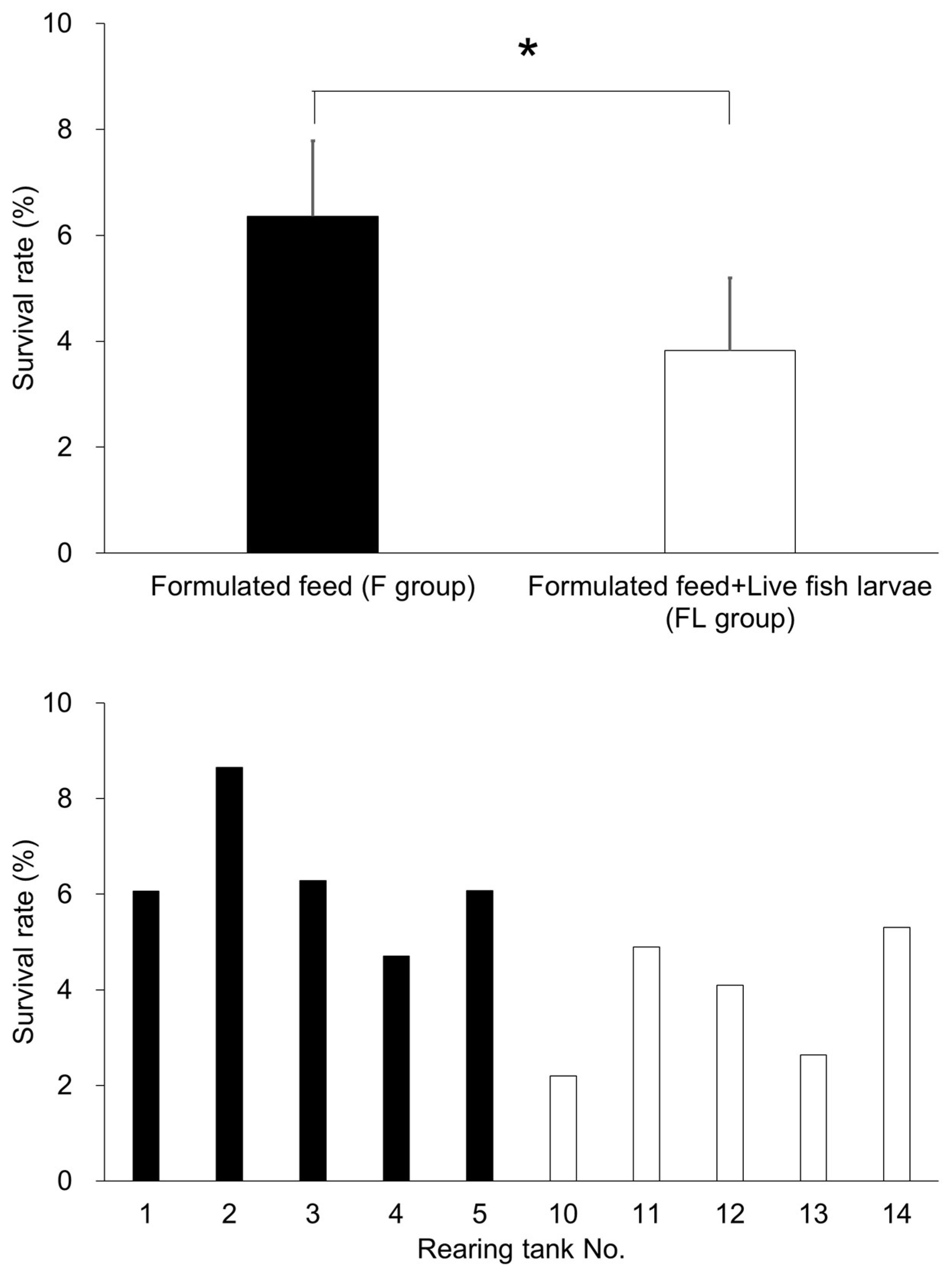
3.1.2. Survival Rate
3.2. Experiment 2: Relative Growth
3.2.1. Absolute Growth
3.2.2. Development
3.2.3. Relative Growth of Upper Jaw Length at the Onset of Newly Hatched Larvae Feeding
4. Discussion
4.1. Effects of Newly Hatched Larvae Feeding on Larval Growth and Survival of Kawakawa
4.1.1. Effect of Larval Feeding on Growth Acceleration
4.1.2. Poor Survival
4.2. Relative Growth of Kawakawa Larvae and Juveniles
4.2.1. Relationship Between Absolute and Relative Growth
4.2.2. Changes in Body Shape
4.2.3. Evaluation of Newly Hatched Larvae Feeding as the Feeding Strategies in Scombrid Fingerling Production Based on Their Relative Growth from the View Point of Their Strong Piscivory
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BL | Body length |
| NL | Notochord length |
| SL | Standard length |
| TL | Total length |
| PAL | Pre anal length |
| HL | Head length |
| SnL | Snout length |
| BH | Body height |
| HH | Head height |
| CPD | Caudal peduncle height |
| UJL | Upper jaw length |
| ED | Eye diameter |
References
- Williamson, G.R. Little tuna Euthynnus affinis in the Hong Kong area. Nippon Suisan Gakkaishi. 1970, 36, 9–18. [Google Scholar] [CrossRef]
- Chiou, W.; Lee, L. Migration of kawakawa Euthynnus affinis in the waters near Taiwan. Fish. Sci. 2004, 70, 746–757. [Google Scholar] [CrossRef]
- Euthynnus Affinis (Cantor, 1849). Available online: https://www.fao.org/fishery/en/aqspecies/3294/en (accessed on 13 February 2025).
- Yesaki, M. A Review of the Biology and Fisheries for Kawakawa (Euthynnus Affinis) in the Indo-Pacific Region. Available online: https://www.fao.org/4/t1817e/T1817E19.htm (accessed on 7 March 2025).
- The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation. Available online: https://www.fao.org/3/cc0461en/cc0461en.pdf. (accessed on 6 March 2025).
- Takeuchi, Y.; Yazawa, R.; Maeda, A. Future Perspective of Eastern Little Tuna Aquaculture. In Youshoku Business; Midori-Shobou: Tokyo, Japan, 2014; pp. 10–13. (In Japanese) [Google Scholar]
- Imayoshi, Y.; Nomoto, S.; Nibe, H.; Kubo, M. Cultivation Trial for the Utilization of Valuable Fish Species (Kawakawa Seedling Rearing Trial). In Kagoshima Prefectural Fisheries Technology Development Center Business Report; Kagoshima Prefectural Fisheries Technology Development Center: Kagoshima City, Japan, 2017. (In Japanese) [Google Scholar]
- Development of Aquaculture Technology for Eastern Little Tuna (Kawakawa). Available online: https://www.cri.ehime-u.ac.jp/researches/research-4661/ (accessed on 20 May 2025).
- Ehime Prefecture. Hime Suma (Ehime Prefecture Farmed Kawakawa). Available online: https://www.pref.ehime.jp/h37100/suisan_okoku_ehime/fish_suma.html (accessed on 20 May 2025).
- Wakayama Prefecture. History of Research on Suma (Eastern Little Tuna). Available online: https://www.pref.wakayama.lg.jp/prefg/071001/sub2_3suma.html (accessed on 20 May 2025).
- Yazawa, R.; Takeuchi, Y.; Satoh, K.; Machida, Y.; Amezawa, K.; Kabeya, N.; Shimada, Y.; Yoshizaki, G. Eastern little tuna, Euthynnus affinis (Cantor, 1849) mature and reproduce within 1 year of rearing in land-based tanks. Aquac. Res. 2016, 47, 3800–3810. [Google Scholar] [CrossRef]
- Harada, T.; Murata, O.; Oda, S. Rearing and morphological changes of yellowfin tuna larvae and juveniles. Mem. Fac. Agr. Kinki Univ. 1980, 13, 33–36, (In Japanese with English abstract). [Google Scholar]
- Kaji, T.; Tanaka, M.; Takahashi, Y.; Oka, M.; Ishibashi, N. Preliminary observations on development of Pacific bluefin tuna Thunnus thynnus (Scombridae) larvae reared in the laboratory, with special reference to the digestive system. Mar. Freshw. Res. 1996, 47, 261–269. [Google Scholar] [CrossRef]
- Miyashita, S.; Kato, K.; Sawada, Y.; Murata, O.; Ishitani, Y.; Shimizu, K.; Yamamoto, S.; Kumai, H. Development of digestive system and digestive enzyme activities of larval and juvenile bluefin tuna, Thunnus thynnus, reared in the laboratory. Aquac. Sci. 1998, 46, 111–120. [Google Scholar] [CrossRef]
- Miyashita, S.; Sawada, Y.; Okada, T.; Murata, O.; Kumai, H. Morphological development and growth of laboratory-reared larval and juvenile Thunnus thynnus (Pisces: Scombridae). Fish. Bull. 2001, 99, 601–616. [Google Scholar]
- Metrio, G.; Bridges, C.; Mylonas, C.; Caggiano, M.; Deflorio, M.; Santamaria, N.; Zupa, R.; Pousis, C.; Vassallo-Agius, R.; Gordin, H.; et al. Spawning induction and large-scale collection of fertilized eggs in captive Atlantic bluefin tuna (Thunnus thynnus L.) and the first larval rearing efforts. J. Appl. Ichthyol. 2010, 26, 596–609. [Google Scholar] [CrossRef]
- Chen, B.; Hutchinson, W.; Foster, C. Southern bluefin tuna captive breeding in Australia. In Advances in Tuna Aquaculture; Bennetti, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 233–252. [Google Scholar] [CrossRef]
- Harada, T.; Mizuno, K.; Murata, O.; Miyashita, S.; Huruya, H. Artificial hatching and larvae rearing of yellowfin tuna. Mem. Fac. Agr. Kinki Univ. 1971, 4, 145, (In Japanese with English abstract). [Google Scholar]
- Harada, T. Report on experimental tuna culture. Fish. Inst. Kinki Univ. 1980, 30. [Google Scholar]
- Kaji, T.; Tanaka, M.; Oka, M.; Takeuchi, H.; Ohsumi, S.; Teruya, K.; Hirokawa, J. Growth and morphological development of laboratory-reared yellowfin tuna Thunnus albacares larvae and early juveniles, with special emphasis on the digestive system. Fish. Sci. 1999, 65, 700–707. [Google Scholar] [CrossRef]
- Margulies, D.; Suter, J.; Hunt, S.; Olson, R.J.; Scholey, V.P.; Wexeler, J.B.; Nakazawa, A. Spawning and early development of captive yellowfin tuna (Thunnus albacares). Fish. Bull. 2007, 105, 249–265. [Google Scholar]
- Kaji, T.; Kodama, M.; Arai, H.; Tagawa, M.; Tanaka, M. Precocious development of the digestive system in relation to early appearance of piscivory in striped bonito Sarda orientalis larvae. Fish. Sci. 2002, 68, 1212–1218. [Google Scholar] [CrossRef]
- Reglero, P.; Ortega, A.; Blanco, E.; Fiksen, Ø.; Viguri, F.J.; de la Gándara, F.; Seoka, M.; Folkvord, A. Size-related differences in growth and survival in piscivorous fish larvae fed different prey types. Aquaculture 2014, 433, 94–101. [Google Scholar] [CrossRef]
- Harada, T.; Murata, O.; Miyashita, S. On the artificial fertilization and rearing of larvae in bonito. Mem. Fac. Agr. Kinki Univ. 1974, 7, 1–4, (In Japanese with English abstract). [Google Scholar]
- Shimizu, H.; Arai, H.; Takeuchi, T. Allometry and Development of Caudal Skeleton of Hatchery-reared Striped Bonito Sarda orientalis. Bull. Seikai Natl. Fish. Res. Inst. 1998, 76, 11–18. [Google Scholar]
- Tanaka, M.; Kawai, S.; Seikai, T.; Burke, J.S. Development of the digestive organ system In Japanese Spanish mackerel Scomberomorus niphonius larvae. Mar. Biol. 1996, 126, 533–542. [Google Scholar] [CrossRef]
- Harada, T.; Murata, O.; Huruya, H. Artificial hatching and larvae rearing of skipjack tuna. Mem. Fac. Agr. Kinki Univ. 1973, 6, 113, (In Japanese with English abstract). [Google Scholar]
- Harada, T.; Murata, O.; Furuya, H. Artificial hatching and larvae rearing of bullet mackerel. Mem. Fac. Agric. Kinki Univ. 1973, 6, 113–116. [Google Scholar]
- Aoyama, M.; Kurata, Y.; Mukai, Y. Morphological development and growth of laboratory-reared larval chub mackerel Scomber japonicus. Fish. Bull. 2001, 99, 616–627. [Google Scholar]
- Mendiola, D.; Alvarez, P.; Cotano, U.; Martínez de Murguía, A. Early development and growth of the laboratory reared north-east Atlantic mackerel Scomber scombrus L. J. Fish. Biol. 2007, 70, 911–933. [Google Scholar] [CrossRef]
- Sawada, Y.; Okada, T.; Miyashita, S.; Murata, O.; Kumai, H. Completion of the Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel) life cycle. Aquac. Res. 2005, 36, 413–421. [Google Scholar] [CrossRef]
- Betancor, M.; Ortega, A.; Gándara, F.; Tocher, D.; Mourente, G. Lipid metabolism-related gene expression pattern of Atlantic bluefin tuna (Thunnus thynnus L.) larvae fed on live prey. Fish. Physiol. Biochem. 2017, 43, 493–516. [Google Scholar] [CrossRef]
- Manabe, R. Development of aquaculture technologies for the kawakawa Euthynnus affinis. Nippon Suisan Gakkaishi. 2019, 85, 200. (In Japanese) [Google Scholar] [CrossRef]
- Shiraishi, T.; Katou, F.; Takeuchi, Y.; Yazawa, R.; Higashi, T. Establishment of kawakawa aquaculture technology in Wakayama Prefecture, spawning and fingerling production, and shipment to the market. Mon. Aquac. Bus. 2017, 54, 37–40. (In Japanese) [Google Scholar]
- Ohshimo, S.; Matsuzaki, K.; Fujinami, Y.; Kodama, T. Biology of kawakawa Euthynnus affinis in the East China Sea: Growth, reproduction, and stable isotope ratios. Reg. Stud. Mar. Sci. 2024, 69, 103346. [Google Scholar] [CrossRef]
- Etienne, B.; Patrick, K.; Charles, M. Effect of stocking density on the dynamics of cannibalism in sibling larvae of Perca fluviatilis under controlled conditions. Aquaculture 2003, 219, 241–255. [Google Scholar] [CrossRef]
- Takeda, T.; Okada, T.; Ishibashi, Y. Effects of stocking density and rearing factors on aggressive behaviour and cannibalism in the Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel) larvae. Aquac. Sci. 2022, 72, 103–113. [Google Scholar] [CrossRef]
- Sabate, F.D.L.; Sakakura, Y.; Tanaka, Y.; Kumon, K.; Nikaido, H.; Eba, T.; Nishi, A.; Shiozawa, S.; Hagiwara, A.; Masuma, S. Onset and development of cannibalistic and schooling behavior in the early life stages of Pacific bluefin tuna Thunnus orientalis. Aquaculture 2010, 301, 16–21. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Miki, T.; Sawada, Y.; Kurata, M. Effects of feeding conditions and size differences on aggressive behaviour and cannibalism in the Pacific bluefin tuna Thunnus orientalis (Temminck and Schlegel) larvae. Aquac. Res. 2013, 45, 45–53. [Google Scholar] [CrossRef]
- Kawabe, K.; Nakano, T.; Murai, M.; Takashima, F. Relative growth of larval and juvenile striped jack, Pseudocaranx dentex. Suisanzoshoku 1992, 40, 253–259, (In Japanese with English abstract). [Google Scholar]
- Osse, J.W.M.; van den Boogaart, J.G.M. Fish larvae, development, allometric growth, and the aquatic environment. ICES. Mar. Sci. Symp. 1995, 201, 21–34. [Google Scholar] [CrossRef]
- Masuda, R.; Tsukamoto, K. Morphological development in relation to phototaxis and rheotaxis in the striped jack, Pseudocaranx dentex. Mar. Freshw. Behav. Physiol. 1996, 28, 75–90. [Google Scholar] [CrossRef]
- Huang, Y.F.; Song, B.L.; Deng, T.H.; Wang, Q.; Shen, Q.; Liu, L.G. Ontogenetic development, allometric growth patterns, and daily increment validation of larvae and juvenile Culter alburnus. Environ. Biol. Fish. 2021, 104, 1593–1610. [Google Scholar] [CrossRef]
- Hattori, N. Studies on seedling production of chub mackerel from larvae to juvenile stage. Bull. Aquac. Res. Inst. Kindai Univ. 2023, 23, 28–127. [Google Scholar]
- Kanda, Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013, 48, 452–458. [Google Scholar] [CrossRef]
- Kendall, A.W., Jr.; Ahlstrom, E.H.; Moser, H.G. Early life history stages of fishes and their characters. In Ontogeny and Systematics of Fishes; Moser, H.G., Richardson, W.J., Cohen, D.M., Fahay, M.P., Kendall, A.W., Jr., Eds.; American Society of Ichthyologists and Herpetologists: Lawrence, KS, USA, 1984; pp. 11–22. [Google Scholar]
- Seoka, M.; Kurata, M.; Hatanaka, Y.; Biswas, A.K.; Ji, S.C.; Kumai, H. Possible nutrients in Artemia affecting the larval growth of Pacific bluefin tuna Thunnus orientalis. Aquac. Sci. 2007, 55, 55–64. [Google Scholar] [CrossRef]
- Seoka, M.; Kurata, M.; Tamagawa, R.; Biswas, A.K.; Biswas, B.K.; Yong, A.S.K.; Kim, Y.S.; Ji, S.C.; Takii, K.; Kumai, H. Dietary supplementation of salmon roe phospholipid enhances the growth and survival of Pacific bluefin tuna Thunnus orientalis larvae and juveniles. Aquaculture 2008, 275, 225–234. [Google Scholar] [CrossRef]
- Betancor, M.; Ortega, A.; Gándara, F.; Varela, J.; Tocher, D.; Mourente, G. Evaluation of different feeding protocols for larvae of Atlantic bluefin tuna (Thunnus thynnus L.). Aquaculture 2019, 505, 523–538. [Google Scholar] [CrossRef]
- Takeuchi, T. Studies on the Requirement and Nutritional Role of Marine Fish Larvae for EPA and DHA. KAKEN Annual Research Report 1991, Project No. 02660208. Available online: https://kaken.nii.ac.jp/en/report/KAKENHI-PROJECT-02660208/026602081991jisseki/ (accessed on 22 May 2025).
- Takeuchi, T. Nutritional studies on improvement of health and quality of marine aquatic animals larvae. Nippon Suisan Gakkaishi 2009, 75, 623–635. [Google Scholar] [CrossRef][Green Version]
- Biswas, A.; Nozaki, J.; Kurata, M.; Takii, K.; Kumai, H.; Seoka, M. Effect of Artemia enrichment on the growth and survival of Pacific bluefin tuna Thunnus orientalis (Temminck & Schlegel) larvae. Aquac. Res. 2006, 37, 1662–1670. [Google Scholar] [CrossRef]
- Seoka, M.; Kurata, M.; Kumai, H. Effect of docosahexaenoic acid enrichment in Artemia on growth of Pacific bluefin tuna Thunnus orientalis larvae. Aquaculture 2007, 270, 193–199. [Google Scholar] [CrossRef]
- Koven, W.; Nixon, O.; Allon, G.; Gaon, A.; El Sadin, S.; Falcon, J.; Besseau, L.; Escande, M.; Agius, R.V.; Gordin, H.; et al. The effect of dietary DHA and taurine on rotifer capture success, growth, survival and vision in the larvae of Atlantic bluefin tuna (Thunnus thynnus). Aquaculture 2018, 482, 137–145. [Google Scholar] [CrossRef]
- Koven, W.; Yanowski, E.; Gardner, L.; Nixon, O.; Block, B. Docosahexaenoic acid (DHA) is a driving force regulating gene expression in bluefin tuna (Thunnus thynnus) larvae development. Sci. Rep. 2024, 14, 23191. [Google Scholar] [CrossRef]
- Margulies, D.; Scholey, V.P.; Wexler, J.B.; Stein, M.S. Research on the reproductive biology and early life history of yellowfin tuna Thunnus albacares in Panama. In Advances in Tuna Aquaculture: From Hatchery to Market; Benetti, D., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 77–114. [Google Scholar] [CrossRef]
- Kestemont, P.; Jourdan, S.; Houbart, M.; Mélard, C.; Paspatis, M.; Fontaine, P.; Cuvier, A.; Kentouri, M.; Baras, E. Size heterogeneity, cannibalism and competition in cultured predatory fish larvae: Biotic and abiotic influences. Aquaculture 2003, 227, 333–356. [Google Scholar] [CrossRef]
- Dou, S.; Seikai, T.; Tsukamoto, K. Cannibalism In Japanese flounder juveniles, Paralichthys olivaceus, reared under controlled conditions. Aquaculture 2000, 182, 149–159. [Google Scholar] [CrossRef]
- Li, Y.-Q.; Sun, X. Agonistic behaviors of aquatic animals. Zool. Res. 2013, 34, 214–220. [Google Scholar]
- Adamek, J.; Kamler, E.; Epler, P. Uniform maternal age/size and light restrictions mitigate cannibalism in Clarias gariepinus larvae and juveniles reared under production-like controlled conditions. Aquac. Eng. 2011, 45, 13–19. [Google Scholar] [CrossRef]
- Pienaar, A.G. A Study of Coeval Sibling Cannibalism in Larval and Juvenile Fishes and Its Control Under Culture Conditions. Master’s Thesis, Rhodes University, Grahamstown, South Africa, 1990. [Google Scholar]
- Li, C.X. Observation on the turbidity of water and the phenomenon of cannibalism of black seabream. Mar. Fish. 1992, 2, 58–59. [Google Scholar]
- Liao, I.C.; Chang, E.Y. Role of sensory mechanisms in predatory feeding behavior of juvenile red drum Sciaenops ocellatus. Fish. Sci. 2003, 69, 317–322. [Google Scholar] [CrossRef]
- Leu, M.Y.; Tai, K.Y.; Meng, P.J.; Tang, C.H.; Wang, P.H.; Tew, K.S. Embryonic, larval and juvenile development of the longfin batfish, Platax teira (Forsskål, 1775) under controlled conditions with special regard to mitigate cannibalism for larviculture. Aquaculture 2018, 493, 204–213. [Google Scholar] [CrossRef]
- Baras, E.; Slembrouck, J.; Cochet, C.; Caruso, D.; Legendre, M. Morphological factors behind the early mortality of cultured larvae of the Asian catfish, Pangasianodon hypophthalmus. Aquaculture 2010, 298, 211–219. [Google Scholar] [CrossRef]
- Duy Khoa, T.N.; Viliame, W.; Honda, A.; Shiozaki, K.; Kotani, T. An integrative description of the digestive system morphology and function of Japanese flounder (Paralichthys olivaceus) during early ontogenetic development. Aquaculture 2021, 531, 735855. [Google Scholar] [CrossRef]
- Murashita, K.; Matsunari, H.; Kumon, K.; Tanaka, Y.; Shiozawa, S.; Furuita, H.; Oku, H.; Yamamoto, T. Characterization and ontogenetic development of digestive enzymes in Pacific bluefin tuna Thunnus orientalis larvae. Fish. Physiol. Biochem. 2014, 40, 1741–1755. [Google Scholar] [CrossRef]
- Yúfera, M.; Ortiz-Delgado, J.B.; Hoffman, T.; Siguero, I.; Urup, B.; Sarasquete, C. Organogenesis of digestive system, visual system and other structures in Atlantic bluefin tuna (Thunnus thynnus) larvae reared with copepods in mesocosm system. Aquaculture 2014, 426–427, 126–137. [Google Scholar] [CrossRef]
- Sugino, H.; Kashito, Y.; Oda, T. General experiment on seedling production of the Japanese Spanish mackerel Scomberomorus niphonius. Bull. Fish. Exp. Sta. Okayama Pref. 2005, 20, 49–53. (In Japanese) [Google Scholar]
- Tsujimura, H. Seed production of Japanese Spanish mackerel (Scomberomorus niphonius): A case in Osaka Prefecture. Bull. Res. Inst. Environ. Agr. Fish. Osaka Pref. Govern. 2012, 5, 4. (In Japanese) [Google Scholar]
- Shimizu, H.; Shiozawa, S. Allometry and development of caudal skeleton of hatchery-reared yellowfin tuna Thunnus albacares. Bull. Fish. Res. Agen. 2004, 10, 1–7, (In Japanese with English abstract). [Google Scholar]
- Collette, B.B. Coryphaenidae. In FAO Species Identification Sheets for Fishery Purposes. Western Indian Ocean (Fishing Area 51); Fischer, W., Bianchi, G., Eds.; FAO: Rome, Italy, 1984; Volume 2. [Google Scholar]
- Shirota, A. Studies on the mouth size of fish larvae-II specific characteristics of the upper jaw length. Nippon Suisan Gakkaishi 1978, 44, 1171–1177, (In Japanese with English abstract). [Google Scholar] [CrossRef][Green Version]
- Umeki, K.; Imai, T.; Goshi, M.; Kojima, T.; Akiyama, N. Changes in size of possible to feed food organisms and food granules with growth of ayu Plecoglossus altivelis altivelis larvae and juveniles. Aquacult. Sci. 2008, 56, 469–478. [Google Scholar] [CrossRef]
- Iwasaki, T.; Ide, K.; Sato, J.; Hamasaki, K. Feeding selectivity of rotifers and Artemia nauplii in juvenile Epinephelus bruneus and Epinephelus septemfasciatus. Nippon Suisan Gakkaishi 2016, 82, 119–127. [Google Scholar] [CrossRef][Green Version]
- Kim, D.G.; Seong, G.C.; Kang, D.Y.; Jin, S.; Soh, H.Y.; Baeck, G.W. Feeding habits of chub mackerel, Scomber japonicus (Houttuyn, 1782) in the South Sea of Korea. Iran. J. Fish. Sci. 2023, 22, 352–367. [Google Scholar] [CrossRef]
- Richards, W.J. Comments on the identification of larval scombrid fishes. Middle Atl. Coast. Fish. Cent. Tech. Publ. 1973, 1, 20–27. [Google Scholar]
- Matsumoto, W.M. Morphology and distribution of larval wahoo Acanthocybium solandri (Cuvier) in the central Pacific Ocean. Fish. Bull. 1967, 66, 299–322. [Google Scholar]



| Allometry (y = a + bx; y: Length Body Parts, x: Body Length) | ||||
|---|---|---|---|---|
| Body Part | Range of Body Length | a | b | Correlation Coefficient (R2) |
| Total length | BL < 3 | −30.43 | 114.54 | 0.97 |
| 3 ≤ BL < 10 | −55.48 | 119.12 | 0.99 | |
| 10 ≤ BL < 30 | −38.49 | 117.67 | 0.99 | |
| Preanal length | BL < 3 | 63.23 | −9.70 | 0.27 |
| 3 ≤ BL < 10 | 22.02 | 4.15 | 0.82 | |
| 10 ≤ BL < 30 | 56.78 | 0.55 | 0.44 | |
| 30 ≤ BL | 75.11 | 0.13 | 0.09 | |
| Head length | 3 ≤ BL < 10 | 7.00 | 4.67 | 0.80 |
| 10 ≤ BL < 30 | 44.65 | −0.27 | 0.80 | |
| 30 ≤ BL | 41.01 | −0.17 | 0.37 | |
| Snout length | 3 ≤ BL < 10 | −0.90 | 2.50 | 0.89 |
| 10 ≤ BL < 30 | 17.75 | −0.12 | 0.31 | |
| 30 ≤ BL | 16.62 | −0.10 | 0.40 | |
| Body height | BL < 3 | 24.98 | −3.73 | 0.19 |
| 3 ≤ BL < 10 | 9.04 | 2.62 | 0.79 | |
| 10 ≤ BL < 30 | 33.05 | −0.44 | 0.79 | |
| Head height | 3 ≤ BL < 10 | 9.37 | 2.95 | 0.78 |
| 10 ≤ BL < 30 | 31.64 | −0.29 | 0.75 | |
| 30 ≤ BL | 26.46 | −0.14 | 0.40 | |
| Caudal peduncle depth | BL < 3 | 1.49 | 0.43 | 0.03 |
| 3 ≤ BL < 10 | 0.41 | 0.90 | 0.74 | |
| 10 ≤ BL < 30 | 8.08 | −0.17 | 0.87 | |
| 30 ≤ BL | 4.54 | −0.04 | 0.67 | |
| Upper jaw length | 10 ≤ BL < 30 | 29.45 | −0.35 | 0.42 |
| 30 < BL | 22.09 | −0.11 | 0.40 | |
| Eye diameter | 3 ≤ BL < 10 | 6.08 | 0.73 | 0.63 |
| 10 ≤ BL < 30 | 15.08 | −0.19 | 0.65 | |
| Species | Body Length (mm) | Upper Jaw Length (mm) | UJL/BL (%) | Reference |
|---|---|---|---|---|
| Chub mackerel | 6.0 | 0.8 | 13.3 | 473 |
| Striped bonito | 8.0–10.0 | 2.5 | 25.0 | 25 |
| Kawakawa | 11.2 | 3.0 | 26.8 | This study |
| Yellowfin tuna | 17.0 | 3.4 | 20.0 | 74 |
| Pacific bluefin tuna | 18.0 | 3.6 | 20.0 | 15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuruki, L.; Miyashima, A.; Agawa, Y.; Sawada, Y. Effects of Feeding Newly Hatched Larvae on the Growth, Survival, and Growth Patterns of Kawakawa (Euthynnus affinis) Larvae and Juveniles. Animals 2025, 15, 1997. https://doi.org/10.3390/ani15131997
Nuruki L, Miyashima A, Agawa Y, Sawada Y. Effects of Feeding Newly Hatched Larvae on the Growth, Survival, and Growth Patterns of Kawakawa (Euthynnus affinis) Larvae and Juveniles. Animals. 2025; 15(13):1997. https://doi.org/10.3390/ani15131997
Chicago/Turabian StyleNuruki, Lynn, Aki Miyashima, Yasuo Agawa, and Yoshifumi Sawada. 2025. "Effects of Feeding Newly Hatched Larvae on the Growth, Survival, and Growth Patterns of Kawakawa (Euthynnus affinis) Larvae and Juveniles" Animals 15, no. 13: 1997. https://doi.org/10.3390/ani15131997
APA StyleNuruki, L., Miyashima, A., Agawa, Y., & Sawada, Y. (2025). Effects of Feeding Newly Hatched Larvae on the Growth, Survival, and Growth Patterns of Kawakawa (Euthynnus affinis) Larvae and Juveniles. Animals, 15(13), 1997. https://doi.org/10.3390/ani15131997





