Effect of Bacillus subtilis DSM 32315 in Diets on Performance and Gut Integrity of Post-Weaning Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Experimental Design
2.2. Measurements and Collected Data
2.3. Concentration of Escherichia coli in Feces
2.4. Sugar Absorption Test (SAT)
2.5. Calculations and Statistical Analyses
3. Results
3.1. Performance
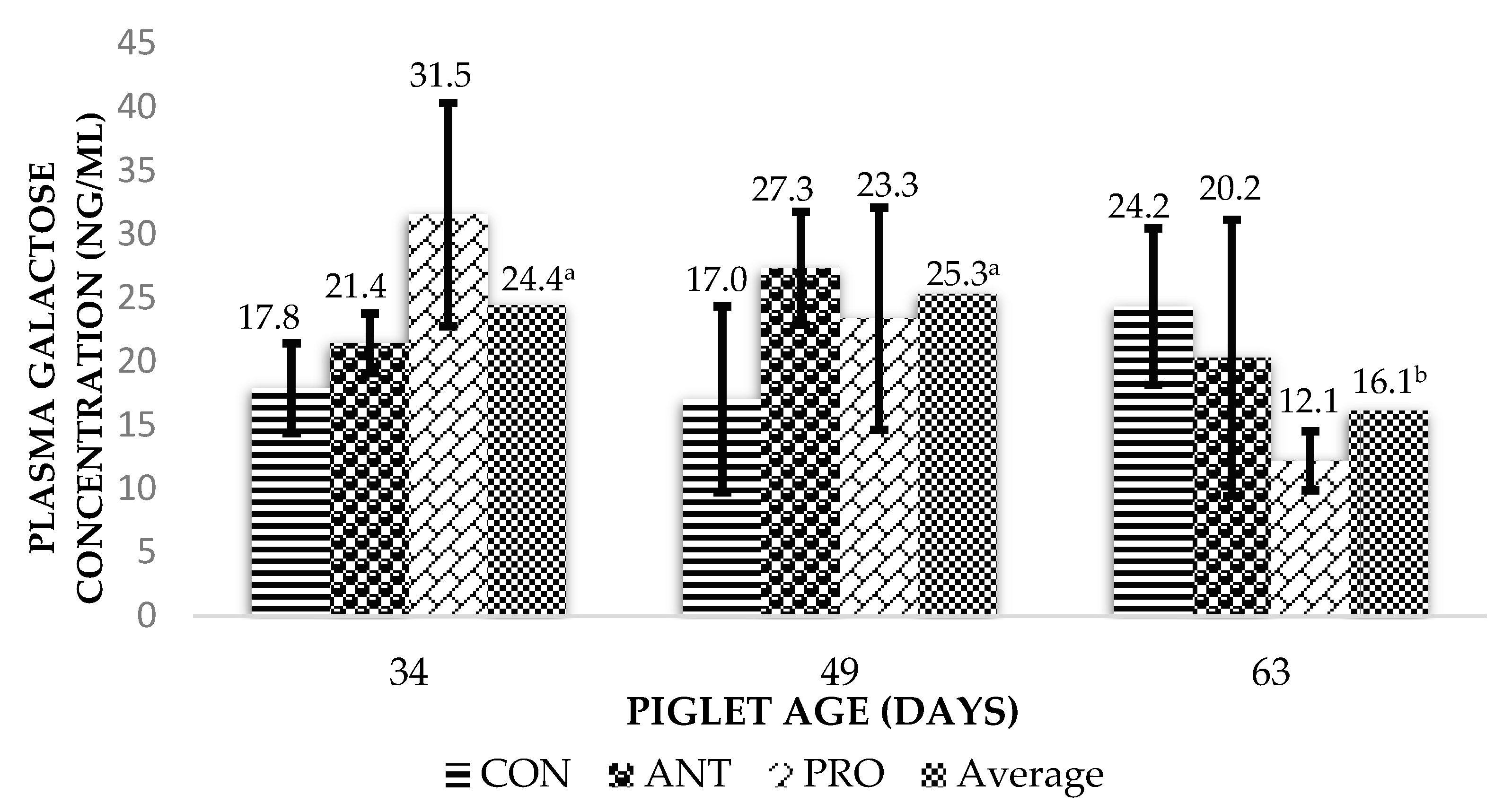
3.2. Intestinal Permeability
3.3. Concentration of Escherichia coli in Feces
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Santos, L.S.; Mascarenhas, A.G.; Oliveira, H.F. Digestive physiology and post-weaning nutrition in piglets. Nutr. Electron. J. 2016, 13, 4570–4584. [Google Scholar]
- Sutherland, M.A.; Backus, B.L.; McGlone, J.J. Effects of transport at weaning on the behavior physiology and performance of pigs. Animals 2014, 4, 657–669. [Google Scholar] [CrossRef]
- Nair, M.S.; Amalaradjou, M.A.; Venkitanarayanan, K. Antivirulence properties of probiotics in combating microbial pathogenesis. Adv. Appl. Microbiol. 2017, 98, 1–29. [Google Scholar] [CrossRef]
- Azizi, A.F.N.; Uemura, R.; Omori, M.; Sueyoshi, M.; Yasuda, M. Essects of probiotics on growth and immunity of piglets. Animals 2022, 12, 1786. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canina, R.B.; Flint, H.J.; Salminen, S.; et al. The international Scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Zhao, P.Y.; Kim, I.H. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim. Feed Sci. Technol. 2015, 200, 86–92. [Google Scholar] [CrossRef]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Review: Are we using probiotics correctly in post-weaning piglets? Animal 2018, 12, 2489–2498. [Google Scholar] [CrossRef] [PubMed]
- Khaneghah, A.M.; Abhari, K.; Es, I.; Soares, M.B.; Oliveira, R.B.A.; Hosseini, H.; Rezaei, M.; Balthazar, C.F.; Silva, R.; Crus, A.G.; et al. Interactions between probiotics and pathogenic microorganisms in hosts and foods: A review. Trends Food Sci. Technol. 2020, 95, 205–218. [Google Scholar] [CrossRef]
- Rostagno, H.S.; Albino, L.F.T.; Hannas, M.I.; Donzele, J.L.; Sakomura, N.S.; Perazzo, F.G.; Saraiva, A.; Teixeira, M.L.; Rodrigues, P.B.; Oliveira, R.F.; et al. Tabelas Brasileiras Para Aves e Suínos: Composição de Alimentos e Exigências Nutricionais, 4th ed.; Universidade Federal de Viçosa: Viçosa, Brazil, 2017. [Google Scholar]
- Thymann, T.; Burrin, D.G.; Tappenden, K.A.; Bjornvad, C.R.; Jensen, S.K.; Sangild, P.T. Formula-feeding reduces lactose digestive capacity in neonatal pigs. Br. J. Nutr. 2006, 95, 1075–1081. [Google Scholar] [CrossRef]
- Silva, G.F.; Silva, B.A.N.; Sanglard, D.; Domingos, R.L.; Gonçalves, M.F.; Cardoso, H.M.C.; Cardoso, L.A.; Pereira, T.S.B.; Maia, B.C.A.; Brito, S.K.; et al. Performance and gut permeability of post-weaned piglets are influenced by different sources of lignocellulose fiber. Livest. Sci. 2023, 274, 105274. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, X.; Duan, Y.; Zhao, Y.; Zhang, W.; Azad, M.A.K.; Wang, Z.; Blachier, F.; Kong, X. Dietary supplementation with Bacillus subtilis promotes growth and gut health of weaned piglets. Front. Vet. Sci. 2021, 7, 600772. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Xu, H.; Mei, X.; Cao, X.; Gong, L.; Wu, Y.; Li, Y.; Yu, D.; Liu, S.; Wang, Y.; et al. Probiotic Bacillus enhance the intestinal epithelial cell barrier and immune function of piglets. Benef. Microbes 2018, 9, 743–754. [Google Scholar] [CrossRef]
- Xu, X.; Yang, C.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Gao, T.; Dang, X.; Lu, F. Dietary supplementation with compound probiotics and berberine alters piglet production performance and Fecal Microbiota. Animals 2020, 10, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Chen, X.; Fang, C.; Zhao, L.; Chen, F. The maturing development of gut microbiota in commercial piglets during the weaning transition. Front. Microbiol. 2017, 8, 1688. [Google Scholar] [CrossRef]
- Li, J. Current status and prospects for in-feed antibiotics in the different stages of pork production—A review. Asian-Australas. J. Anim. Sci. 2017, 30, 1667–1673. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Bahaddad, S.S.; Almalki, M.H.K.; Alghamdi, O.A.; Sohrad, S.S.; Yasir, M.; Azhar, E.I.; Chouayekh, H. Bacillus Species as direct-fed microbial antibiotic alternatives for monogastric production. Probiotics Antimicrob. Proteins 2023, 15, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Qian, Y.; Yu, B.; Zhang, T.; Gao, J.; He, J.; Huang, Z.; Zheng, X.M.; Luo, J.; Chen, D. Effects of Bacillus subtilis DSM32315 supplementation and dietary crude protein level on performance, barrier function and gut microbiota profile in weaned piglets. J. Anim. Sci. 2019, 97, 2125–2138. [Google Scholar] [CrossRef]
- Jiang, Z.; Yang, M.; Su, W.; Mei, L.; Li, Y.; Guo, Y.; Li, Y.; Liang, W.; Yang, B.; Huang, Z.; et al. Probiotics in piglet: From gut health to pathogen defense mechanisms. Front. Immunol. 2024, 15, 1468873. [Google Scholar] [CrossRef]
- Tang, X.; Zeng, Y.; Xiong, K.; Zhong, J. Bacillus spp. as potential probiotics: Promoting piglet growth by improving intestinal health. Front. Vet. Sci. 2024, 11, 1429233. [Google Scholar] [CrossRef]
- Turpin, D.L.; Langendijk, P.L.; Pluske, R. Mannitol and galactose as markers of gastrointestinal tract morphology in pigs after gradual or converntional weaning. Anim. Prod. Sci. 2017, 57, 2408. [Google Scholar] [CrossRef]
- Dmytriv, T.; Storey, K.; Lushchak, V.I. Intestinal barrier permeability: The influence of gutmicrobiota, nutrition, and exercise. Front. Physiol. 2024, 15, 1380713. [Google Scholar] [CrossRef]
- Sciascia, Q.L.; Metges, C.C. Review: Methods and biomarkers to investigate intestinal function and health in pigs. Animal 2023, 17, 100860. [Google Scholar] [CrossRef] [PubMed]
- Araujo, W.A.G.; Ferreira, A.S.; Renaudeau, D.; Brustolini, P.C.; Silva, B.A.N. Effects of diet protein source on the behavior of piglets after weaning. Livest. Sci. 2010, 132, 35–40. [Google Scholar] [CrossRef]
- Júnior, V.D.T.; Rodrigues, G.A.; Soares, M.H.; Rocha, G.C.; Campos, P.H.R.F.; Saraiva, A. Crude protein and lactose effects on performance, intestinal and immune function of piglets fed diets without antimicrobials growth promoters. Livest. Sci. 2021, 250, 104566. [Google Scholar] [CrossRef]
- Wang, H.; Kim, K.P.; Kim, I.H. Influence of Bacillus subtilis GCB-13-001 on growth performance, nutrient digestibility, blood characteristics, faecal microbiota and faecal score in weanling pigs. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1919–1925. [Google Scholar] [CrossRef]
- Fairbrother, J.M.; Nadeau, É.; Gyles, C.L. Escherichia coli in post-weaning diarrhea in pigs: An update on bacterial types, pathogenesis, and prevention strategies. Anim. Health Res. Rev. 2005, 6, 17–39. [Google Scholar] [CrossRef]
- Dong, X.; Zhang, N.; Zhou, M.; Tu, Y.; Deng, K.; Diao, Q. Effects of dietary probiotics on growth performance, fecal microbiota and serum profiles in weaned piglets. Anim. Prod. Sci. 2014, 54, 616–621. [Google Scholar] [CrossRef]
- Giang, H.H.; Viet, T.Q.; Ogle, B.; Lindberg, J.E. Effects of supplementation of probiotics on the performance, nutrient digestibility and faecal microflora in growing finishing pigs. Asian Australas. J. Anim. Sci. 2011, 24, 655–661. [Google Scholar] [CrossRef]
| Phases * | ||||
|---|---|---|---|---|
| Ingredients | 1 | 2 | 3 | 4 |
| Corn | 31.490 | 37.660 | 48.130 | 59.004 |
| Soybean meal | 20.000 | 22.000 | 26.000 | 28.000 |
| Pre-cooked corn | 10.000 | 10.000 | 7.000 | 0 |
| Spray-dried blood plasma | 5.000 | 3.000 | 0 | 0 |
| Bakery by-product meal | 5.000 | 5.000 | 5.000 | 5.000 |
| Soybean oil | 3.550 | 3.350 | 3.400 | 3.800 |
| Milk whey | 21.000 | 15.000 | 6.000 | 0 |
| Calcium phosphate | 1.694 | 1.901 | 2.090 | 2.025 |
| Calcium carbonate | 0 | 0 | 0.106 | 0.265 |
| Sodium chloride | 0.400 | 0.400 | 0.400 | 0.400 |
| Flavoring additive 1 | 0.030 | 0.030 | 0.030 | 0.030 |
| Organic acid blend 2 | 0.200 | 0.000 | 0.000 | 0.000 |
| L-Lysine | 0.415 | 0.475 | 0.587 | 0.511 |
| DL-Methionine | 0.240 | 0.237 | 0.270 | 0.200 |
| L-Threonine | 0.159 | 0.188 | 0.281 | 0.239 |
| L-Tryptophan | 0.092 | 0.096 | 0.090 | 0.067 |
| L-Valine | 0.100 | 0.120 | 0.166 | 0.110 |
| Mineral supplement 3 | 0.100 | 0.100 | 0.100 | 0.100 |
| Vitamin premix 4 | 0.050 | 0.050 | 0.050 | 0.050 |
| Micotoxin deactivator 5 | 0.100 | 0.100 | 0.100 | 0.100 |
| Zinc oxide 80% | 0.280 | 0.240 | 0.200 | 0.100 |
| Nutritional Specifications—Nutritional Composition | ||||
| Metabolizable energy (kcal/kg) | 3.435 | 3.412 | 3.377 | 3.367 |
| CP (%) | 19.48 | 18.76 | 18.04 | 18.27 |
| Calcium (%) | 0.683 | 0.710 | 0.715 | 0.720 |
| Digestible phosphorus (%) | 0.439 | 0.430 | 0.400 | 0.360 |
| SID lysine | 1.35 | 1.30 | 1.26 | 1.20 |
| SID methionine + cystine | 0.81 | 0.78 | 0.78 | 0.72 |
| SID threonine | 0.88 | 0.84 | 0.84 | 0.80 |
| SID valine | 0.95 | 0.91 | 0.88 | 0.84 |
| SID tryptophan | 0.30 | 0.29 | 0.25 | 0.23 |
| Treatments | |||||
|---|---|---|---|---|---|
| Parameters | CON | ANT | PRO | RSD | p-Value |
| Phase 1 (27 to 34 days) | |||||
| Initial BW, kg | 9.29 | 9.29 | 9.30 | 0.61 | 0.7468 |
| Final BW, kg | 10.44 | 11.05 | 10.96 | 1.21 | 0.5427 |
| ADWG, g/d | 164 | 251 | 236 | 10 | 0.0789 |
| ADFI, g/d | 277 | 308 | 310 | 4 | 0.4863 |
| FC | 1.68 | 1.23 | 1.31 | 1.12 | 0.0605 |
| Phase 2 (35 to 42 days) | |||||
| Final BW, kg | 13.59 | 14.92 | 14.47 | 4.04 | 0.1450 |
| ADWG, g/d | 450 c | 553 a | 505 b | 10 | 0.0296 |
| ADFI, g/d | 539 b | 611 a | 636 a | 10 | 0.0224 |
| FC | 1.20 | 1.11 | 1.26 | 0.13 | 0.2358 |
| Phase 3 (43 to 56 days) | |||||
| Final BW, kg | 20.39 c | 22.71 a | 21.39 b | 8.22 | 0.0291 |
| ADWG, g/d | 523 b | 599 a | 532 b | 10 | 0.0207 |
| ADFI, g/d | 784 b | 845 a | 837 a | 5 | 0.0258 |
| FC | 1.49 | 1.41 | 1.57 | 0.42 | 0.1388 |
| Phase 4 (57 to 73 days) | |||||
| Final BW, kg | 30.46 | 31.74 | 32.02 | 19.91 | 0.5554 |
| ADWG, g/d | 630 | 565 | 664 | 14 | 0.4110 |
| ADFI, g/d | 1.125 b | 1.089 b | 1.169 a | 49 | 0.0097 |
| FC | 1.78 | 1.93 | 1.76 | 1.27 | 0.6273 |
| Total period | |||||
| TWG, kg | 21.17 b | 22.45 a | 22.47 a | 1.41 | 0.0503 |
| ADWG, g/d | 492 | 522 | 506 | 4 | 0.4415 |
| ADFI, g/d | 778 b | 810 b | 842 a | 74 | 0.0460 |
| FC | 1.58 | 1.55 | 1.66 | 0.04 | 0.7862 |
| Period | CON | ANT | PRO | p-Value |
|---|---|---|---|---|
| 27 to 34 days | 4.12 × 105 | 3.33 × 106 | 9.48 × 105 | 0.208 |
| 35 to 42 days | 0 | 0 | 0 | 0.406 |
| 43 to 56 days | 2.60 × 104 | 1.74 × 105 | 3.30 × 105 | 0.900 |
| 57 to 73 days | 0 | 9.75 × 103 | 3.03 × 103 | 0.700 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carvalho, I.C.S.; Moraes, E.A.; Carvalho, D.C.d.O.; Luna, F.d.S.; Sanglard, D.A.; Miranda, A.L.; Correa, I.S.; Martins, L.T.S.; Brito, S.K.; Nery, G.R.R.; et al. Effect of Bacillus subtilis DSM 32315 in Diets on Performance and Gut Integrity of Post-Weaning Piglets. Animals 2025, 15, 1977. https://doi.org/10.3390/ani15131977
Carvalho ICS, Moraes EA, Carvalho DCdO, Luna FdS, Sanglard DA, Miranda AL, Correa IS, Martins LTS, Brito SK, Nery GRR, et al. Effect of Bacillus subtilis DSM 32315 in Diets on Performance and Gut Integrity of Post-Weaning Piglets. Animals. 2025; 15(13):1977. https://doi.org/10.3390/ani15131977
Chicago/Turabian StyleCarvalho, Illa Carla Santos, Elenice Andrade Moraes, Débora Cristiane de Oliveira Carvalho, Fabrina de Sousa Luna, Demerson Arruda Sanglard, Afonso Luna Miranda, Isabela Santos Correa, Larissa Tayna Silva Martins, Sara Kauane Brito, Gustavo Roberto Ribeiro Nery, and et al. 2025. "Effect of Bacillus subtilis DSM 32315 in Diets on Performance and Gut Integrity of Post-Weaning Piglets" Animals 15, no. 13: 1977. https://doi.org/10.3390/ani15131977
APA StyleCarvalho, I. C. S., Moraes, E. A., Carvalho, D. C. d. O., Luna, F. d. S., Sanglard, D. A., Miranda, A. L., Correa, I. S., Martins, L. T. S., Brito, S. K., Nery, G. R. R., Brand, H. G., Moreira, G. R., & Silva, B. A. N. (2025). Effect of Bacillus subtilis DSM 32315 in Diets on Performance and Gut Integrity of Post-Weaning Piglets. Animals, 15(13), 1977. https://doi.org/10.3390/ani15131977







