Validation with the Dynamic Prediction Model of Protein and Amino Acid Requirements for Growth Performance and Health in Layer Chicks
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds, Diets, and Treatments
2.2. Sample Collection and Measurement
2.3. Serum Biochemistry Analyses
2.4. Statistical Analyses
3. Results
3.1. Growth Performance
3.2. Protein and Main Amino Acid Intake
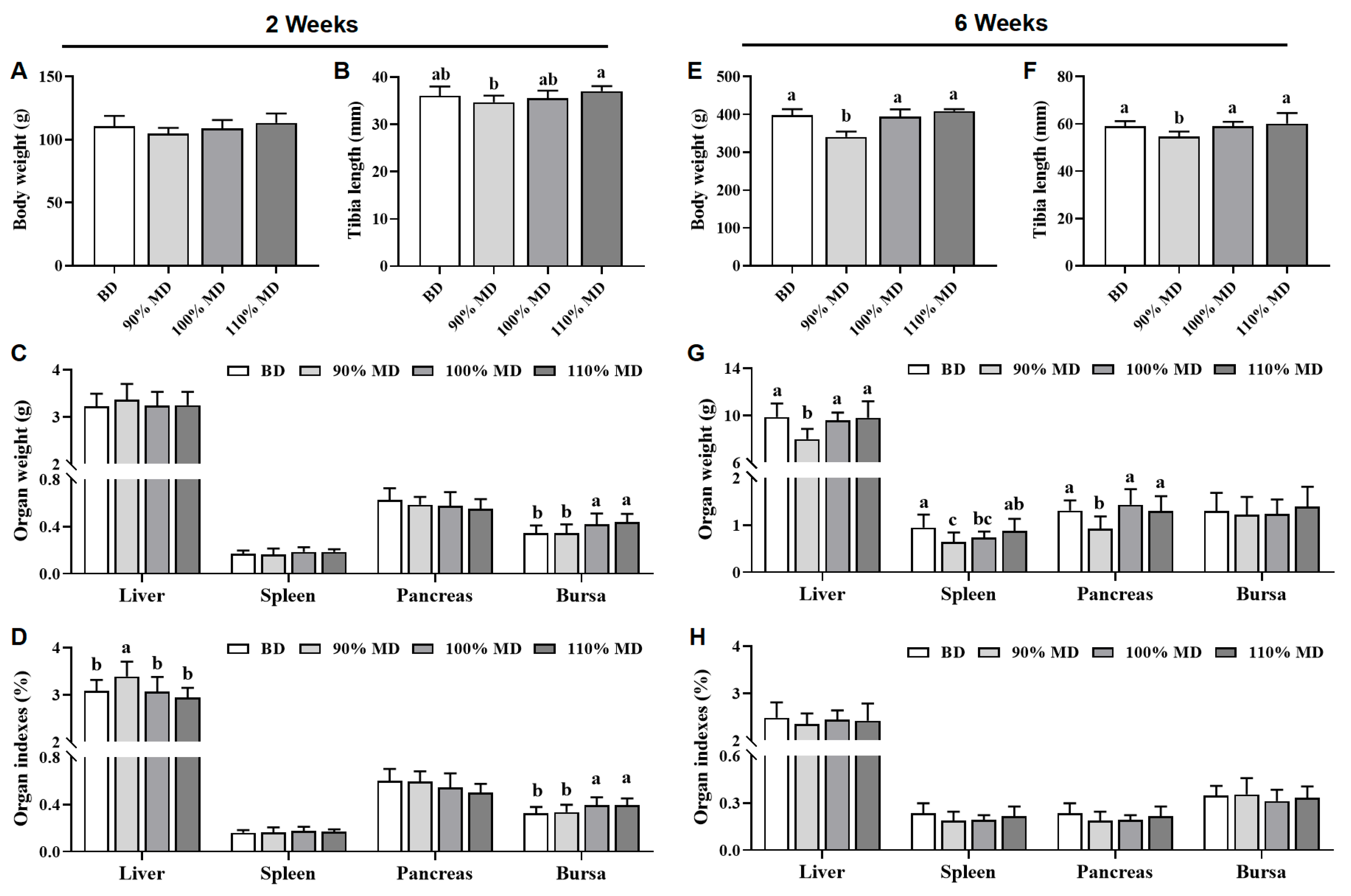
3.3. Organ Weight and Their Indexes
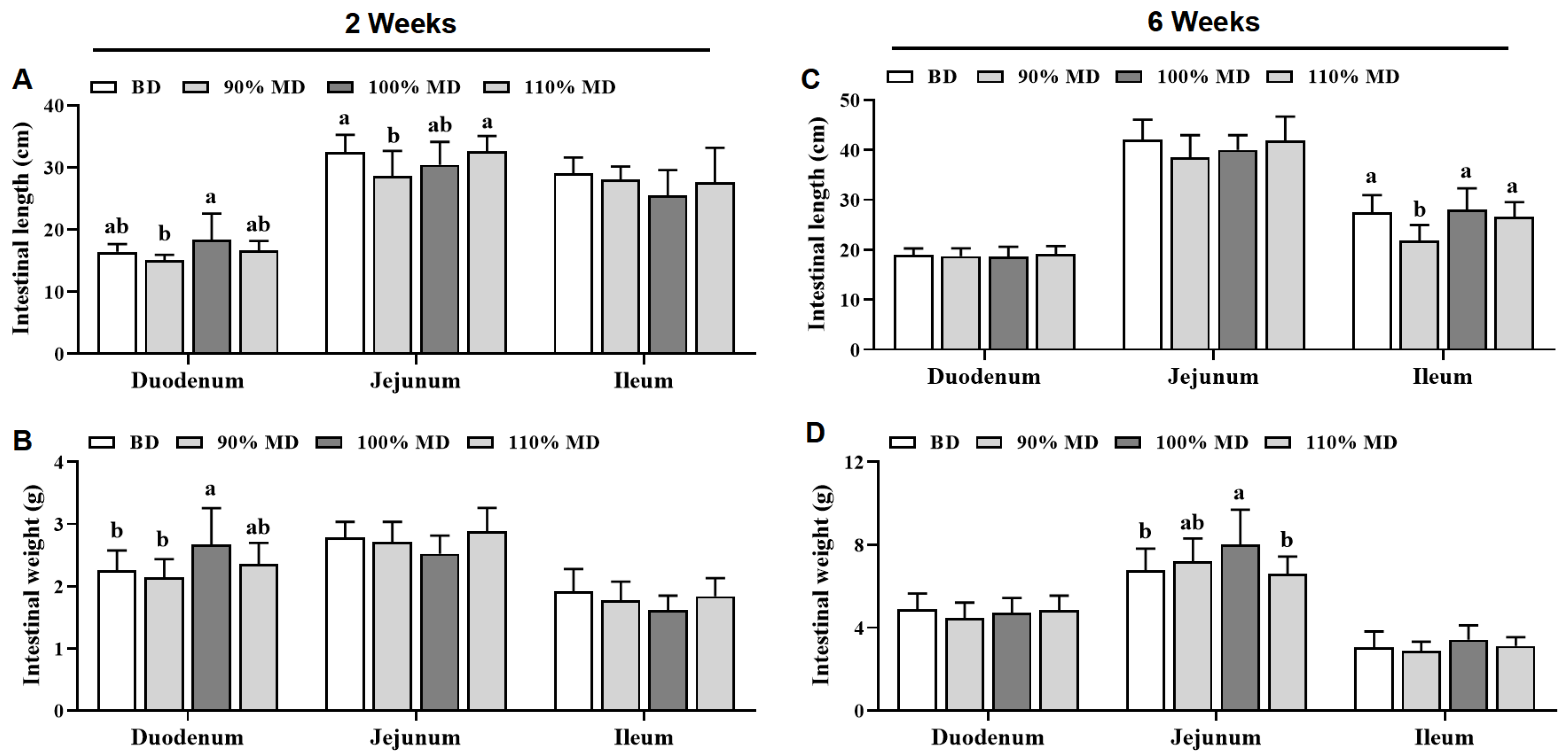
3.4. Small Intestine Length and Weight
3.5. Serum Biochemistry
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Caldas, J.V.; Boonsinchai, N.; Wang, J.R.; England, J.A.; Coon, C.N. The dynamics of body composition and body energy content in broilers. Poult. Sci. 2019, 98, 866–877. [Google Scholar] [CrossRef]
- Tian, Y.D. Dynamie Model Construction of Energy and Amino Acid Requirements for Broilers. Ph.D. Thesis, Chinese Academy of Agricultural Sciences, Beijing, China, 2005. [Google Scholar]
- Alfarisy, G.A.F.; Mahmudy, W.F.; Natsir, M.H. Good parameters for PSO in optimizing laying hen diet. Int. J. Electr. Comput. Eng. 2018, 8, 2419. [Google Scholar]
- Li, G.; Feng, Y.; Cui, J.; Hou, Q.; Li, T.; Jia, M.; Lv, Z.; Jiang, Q.; Wang, Y.; Zhang, M.; et al. The ionome and proteome landscape of aging in laying hens and relation to egg white quality. Sci. China Life Sci. 2023, 66, 2020–2040. [Google Scholar] [CrossRef]
- Pomar, C.; Hauschild, L.; Zhang, G.-H.; Pomar, J.; Lovatto, P.A. Applying precision feeding techniques in growing-finishing pig operations. Rev. Bras. Zootec. 2009, 38, 226–237. [Google Scholar] [CrossRef]
- Fan, L.; Xia, Y.; Wang, Y.; Han, D.; Liu, Y.; Li, J.; Fu, J.; Wang, L.; Gan, Z.; Liu, B.; et al. Gut microbiota bridges dietary nutrients and host immunity. Sci. China Life Sci. 2023, 66, 2466–2514. [Google Scholar] [CrossRef]
- Bamiro, O.M.; Shittu, A.M. Vertical integration and cost behavior in poultry industry in Ogun and Oyo States of Nigeria. Agribusiness 2009, 25, 1–15. [Google Scholar] [CrossRef]
- Cao, K.X.; Deng, Z.C.; Li, S.J.; Yi, D.; He, X.; Yang, X.J.; Guo, Y.M.; Sun, L.H. Poultry nutrition: Achievement, challenge, and strategy. J. Nutr. 2024, 154, 3554–3565. [Google Scholar] [CrossRef]
- Zuidhof, M.J.; Fedorak, M.V.; Ouellette, C.A.; Wenger, I.I. Precision feeding: Innovative management of broiler breeder feed intake and flock uniformity. Poult. Sci. 2017, 96, 2254–2263. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.; Lv, Z.; Liu, H.; Zhao, J.; Noblet, J.; Wang, F.; Lai, C.; Li, D. Net energy of corn, soybean meal and rapeseed meal in growing pigs. J. Anim. Sci. Biotechnol. 2017, 844, 1–10. [Google Scholar] [CrossRef]
- Pawlowska, J.; Sosnówka-Czajka, E. Factors affecting chick quality in Poland. Worlds Poult. Sci. J. 2019, 75, 621–631. [Google Scholar] [CrossRef]
- Gompertz, B. On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. Proc. R. Soc. Lond. 1815, 2, 252–253. [Google Scholar]
- Bridges, T.C.; Turner, L.W.; Stahly, T.S.; Usry, J.L.; Loewer, O.J. Modeling the physiological growth of swine part i: Model logic and growth concepts. Trans. ASAE 1992, 35, 1019–1028. [Google Scholar] [CrossRef]
- Liu, M.; Xia, Z.Y.; Li, H.L.; Huang, Y.X.; Refaie, A.; Deng, Z.C.; Sun, L.H. Estimation of protein and amino acid requirements in layer chicks depending on dynamic model. Animals 2024, 14, 764. [Google Scholar] [CrossRef]
- Hurwitz, S.; Plavnik, I.; Bartov, I.; Bornstein, S. The amino acid requirements of chicks: Experimental validation of model-calculated requirements. Poult. Sci. 1980, 59, 2470–2479. [Google Scholar] [CrossRef]
- Hurwitz, S.; Plavnik, I.; Bengal, I.; Talpaz, H.; Bartov, I. The amino acid requirements of growing turkeys. Experimental validation of model-calculated requirements for sulfur amino acids and lysine. Poult. Sci. 1983, 62, 2387–2393. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Liu, M.; Xu, Z.J.; Xu, Z.J.; Huang, Y.X.; Li, X.M.; Chen, C.J.; Zuo, G.; Yang, J.C.; Lei, X.G.; et al. Optimum doses and forms of selenium maintaining reproductive health via regulating homeostasis of gut microbiota and testicular redox, inflammation, cell proliferation, and apoptosis in roosters. J. Nutr. 2024, 154, 369–380. [Google Scholar] [CrossRef]
- Yang, J.C.; Huang, Y.X.; Sun, H.; Liu, M.; Zhao, L.; Sun, L.H. Selenium deficiency dysregulates one-carbon metabolism in nutritional muscular dystrophy of chicks. J. Nutr. 2023, 153, 47–55. [Google Scholar] [CrossRef]
- Huang, W.; Ma, T.; Liu, Y.; Kwok, L.Y.; Li, Y.; Jin, H.; Zhao, F.; Shen, X.; Shi, X.; Sun, Z.; et al. Spraying compound probiotics improves growth performance and immunity and modulates gut microbiota and blood metabolites of suckling piglets. Sci. China Life Sci. 2023, 66, 1092–1107. [Google Scholar] [CrossRef]
- Zhao, L.; Chu, X.H.; Liu, S.; Li, R.; Zhu, Y.F.; Li, F.N.; Jiang, J.; Zhou, J.C.; Lei, X.G.; Sun, L.H. Selenium-enriched cardamine violifolia increases selenium and decreases cholesterol concentrations in liver and pectoral muscle of broilers. J. Nutr. 2022, 152, 2072–2079. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, M.; Sun, H.; Yang, J.C.; Huang, Y.X.; Huang, J.Q.; Lei, X.; Sun, L.H. Selenium deficiency-induced multiple tissue damage with dysregulation of immune and redox homeostasis in broiler chicks under heat stress. Sci. China Life Sci. 2023, 66, 2056–2069. [Google Scholar] [CrossRef]
- Li, J.; Bai, G.; Gao, Y.; Gao, Q.; Zhong, R.; Chen, L.; Wang, Y.; Ma, T.; Zhang, H. Solid-state fermentation pro-enzymes supplementation benefits growth performance, health, and intestinal microbiota of broiler chickens fed wheat-based diet. Anim. Res. One Health 2024, 1–13. [Google Scholar] [CrossRef]
- Xu, K.L.; Gong, G.X.; Liu, M.; Yang, L.; Xu, Z.J.; Gao, S.; Xiao, M.Y.; Ren, T.; Zhao, B.J.; Khalil, M.M.; et al. Keratinase improves the growth performance, meat quality and redox status of broiler chickens fed a diet containing feather meal. Poult. Sci. 2022, 101, 101913. [Google Scholar] [CrossRef]
- Forester, S.M.; Jennings-Dobbs, E.M.; Sathar, S.A.; Layman, D.K. Perspective: Developing a nutrient-based framework for protein quality. J. Nutr. 2023, 153, 2137–2146. [Google Scholar] [CrossRef]
- Usigbe, M.J.; Uyeh, D.D.; Park, T.; Ha, Y.; Mallipeddi, R. Many objective optimization and decision support for dairy cattle feed formulation. Sci. Rep. 2025, 15, 13451. [Google Scholar] [CrossRef]
- Narinç, D.; Aydemir, E. Chick quality: An overview of measurement techniques and influencing factors. Worlds Poult. Sci. J. 2021, 77, 313–329. [Google Scholar] [CrossRef]
- Gous, R.M. Nutritional limitations on growth and development in poultry. Livest. Sci. 2010, 130, 25–32. [Google Scholar] [CrossRef]
- Andrews, T.L.; Harms, R.H.; Wilson, H.R. Protein requirement of the bobwhite chick. Poult. Sci. 1973, 52, 2199–2201. [Google Scholar] [CrossRef]
- Macelline, S.P.; Toghyani, M.; Chrystal, P.V.; Selle, P.H.; Liu, S.Y. Amino acid requirements for laying hens: A comprehensive review. Poult. Sci. 2021, 100, 101036. [Google Scholar] [CrossRef]
- Macelline, S.P.; Chrystal, P.V.; Liu, S.Y.; Selle, P.H. The dynamic conversion of dietary protein and amino acids into chicken-meat protein. Animals 2021, 11, 2288. [Google Scholar] [CrossRef]
- Wang, J.; Yue, H.; Wu, S.; Zhang, H.; Qi, G. Nutritional modulation of health, egg quality and environmental pollution of the layers. Anim. Nutr. 2017, 3, 91–96. [Google Scholar] [CrossRef]
- Lesuisse, J.; Li, C.; Schallier, S.; Leblois, J.; Everaert, N.; Buyse, J. Feeding broiler breeders a reduced balanced protein diet during the rearing and laying period impairs reproductive performance but enhances broiler offspring performance. Poult. Sci. 2017, 96, 3949–3959. [Google Scholar] [CrossRef]
- Castro, F.L.S.; Kim, H.Y.; Hong, Y.G.; Kim, W.K. The effect of total sulfur amino acid levels on growth performance, egg quality, and bone metabolism in laying hens subjected to high environmental temperature. Poult. Sci. 2019, 98, 4982–4993. [Google Scholar] [CrossRef]
- Dao, H.T.; Moss, A.F.; Bradbury, E.J.; Swick, R.A. Effects of L-arginine, guanidinoacetic acid and L-citrulline supplementation in reduced-protein diets on bone morphology and mineralization of laying hens. Anim. Nutr. 2023, 14, 225–234. [Google Scholar] [CrossRef]
- Fu, G.; Zhang, M.; Huang, Y.; Han, R.; Qi, K.; Yin, L.; Zhao, D.; Huang, Y.; Ma, T.; Wang, L. Effects of different addition levels of CHM-JM113 on growth performance, antioxidant capacity, organ index, and intestinal health of AA broilers. Front. Vet. Sci. 2024, 11, 1388173. [Google Scholar] [CrossRef]
- Madej, J.P.; Stefaniak, T.; Bednarczyk, M. Effect of in ovo-delivered prebiotics and synbiotics on lymphoid-organs’ morphology in chickens. Poult. Sci. 2015, 94, 1209–1219. [Google Scholar] [CrossRef]
- Tsuji, T.; Miyoshi, M. A Scanning and transmission electron microscopic study of the lymphoreticular framework in the chicken fabricius’ bursa. Med. Bull. Fukuoka Univ. 2001, 28, 63–75. [Google Scholar]
- Cheng, J.; Lei, H.; Xie, C.; Chen, J.; Yi, X.; Zhao, F.; Yuan, Y.; Chen, P.; He, J.; Luo, C.; et al. Lymphocyte development in the bursa of fabricius of young broilers is influenced by the gut microbiota. Microbiol. Spectr. 2023, 11, e0479922. [Google Scholar] [CrossRef]
- Chukwudi, P.; Umeugokwe, P.I.; Ikeh, N.E.; Amaefule, B.C. The effects of organic acids on broiler chicken nutrition: A review. Anim. Res. One Health 2025, 3, 43–53. [Google Scholar] [CrossRef]
- Deng, Z.C.; Wang, J.; Wang, J.; Yan, Y.Q.; Huang, Y.X.; Chen, C.Q.; Sun, L.H.; Liu, M. Tannic acid extracted from gallnut improves intestinal health with regulation of redox homeostasis and gut microbiota of weaned piglets. Anim. Res. One Health 2024, 2, 16–27. [Google Scholar] [CrossRef]
- Ravindran, V.; Abdollahi, M.R. Nutrition and digestive physiology of the broiler chick: State of the art and outlook. Animals 2021, 11, 2795. [Google Scholar] [CrossRef]
- Beski, S.S.M.; Swick, R.A.; Iji, P.A. Specialized protein products in broiler chicken nutrition: A review. Anim. Nutr. 2015, 1, 47–53. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Liu, C.; Ma, N.; Feng, Y.; Zhou, M.; Li, H.; Zhang, X.; Ma, X. From probiotics to postbiotics: Concepts and applications. Anim. Res. One Health 2023, 1, 92–114. [Google Scholar] [CrossRef]
- Marín-García, P.J.; Llobat, L.; López-Lujan, M.C.; Cambra-López, M.; Blas, E.; Pascual, J.J. Urea nitrogen metabolite can contribute to implementing the ideal protein concept in monogastric animals. Animals 2022, 12, 2344. [Google Scholar] [CrossRef]
- Jegatheesan, P.; Beutheu, S.; Ventura, G.; Sarfati, G.; Nubret, E.; Kapel, N.; Waligora-Dupriet, A.J.; Bergheim, I.; Cynober, L.; De-Bandt, J.P. Effect of specific amino acids on hepatic lipid metabolism in fructose-induced non-alcoholic fatty liver disease. Clin. Nutr. 2016, 35, 175–182. [Google Scholar] [CrossRef]
- Deng, J.; Peng, Z.; Xia, Z.Y.; Mo, Y.X.; Guo, L.J.; Wei, J.T.; Sun, L.H.; Liu, M. Five glutathione S-transferase isozymes played crucial role in the detoxification of aflatoxin B1 in chicken liver. J. Anim. Sci. Biotechnol. 2025, 16, 54. [Google Scholar] [CrossRef]
- Sun, X.; Ma, J.; Wang, C.; Ren, Z.; Yang, X.; Yang, X.; Liu, Y. Functional roles of folic acid in alleviating dexamethasone-induced fatty liver syndrome in laying hens. Anim. Res. One Health 2025, 3, 114–128. [Google Scholar] [CrossRef]

| Component, % | Basal Diet (BD) | Model Diet (MD) | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1–2 Week | 3–6 Week | 1 Week | 2 Week | 3 Week | 4 Week | 5 Week | 6 Week | |||||||||||||
| 90% | 100% | 110% | 90% | 100% | 110% | 90% | 100% | 110% | 90% | 100% | 110% | 90% | 100% | 110% | 90% | 100% | 110% | |||
| Corn | 63.80 | 66.64 | 65.77 | 59.04 | 53.22 | 67.93 | 61.25 | 54.25 | 73.89 | 68.30 | 62.52 | 72.64 | 66.84 | 60.60 | 75.12 | 69.93 | 64.37 | 78.35 | 73.73 | 68.74 |
| Soybean meal | 31.37 | 28.60 | 28.85 | 34.88 | 40.72 | 27.21 | 33.09 | 39.04 | 21.47 | 26.63 | 31.82 | 22.73 | 28.03 | 33.44 | 20.29 | 25.26 | 30.32 | 17.09 | 21.67 | 26.32 |
| CaCo3 | 1.16 | 1.43 | 1.75 | 1.70 | 1.10 | 1.63 | 1.60 | 1.66 | 1.85 | 1.77 | 1.66 | 1.85 | 1.735 | 1.72 | 1.85 | 1.81 | 1.71 | 1.85 | 1.84 | 1.76 |
| Calcium hydrogen phosphate | 1.90 | 1.86 | 1.90 | 1.90 | 1.86 | 1.92 | 1.90 | 1.90 | 1.87 | 1.86 | 1.87 | 1.89 | 1.86 | 1.87 | 1.89 | 1.86 | 1.88 | 1.91 | 1.92 | 1.90 |
| Soybean oil | 0.80 | 0.50 | 0.80 | 1.50 | 2.08 | 0.40 | 1.20 | 2.15 | - | 0.45 | 1.10 | - | 0.60 | 1.40 | - | 0.25 | 0.80 | - | - | 0.40 |
| Salt | 0.42 | 0.43 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.42 | 0.43 | 0.42 | 0.42 | 0.43 | 0.42 | 0.43 | 0.43 | 0.42 | 0.43 | 0.43 | 0.42 |
| Premix 1 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 | 0.07 |
| 50% choline chloride | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 | 0.10 |
| L-lysine | 0.04 | 0.015 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| DL Methionine | 0.21 | 0.21 | - | - | - | - | - | - | - | - | - | - | - | - | - | 0.01 | 0.01 | 0.04 | 0.045 | 0.055 |
| L-valine | - | - | 0.27 | 0.32 | 0.37 | 0.25 | 0.30 | 0.35 | 0.23 | 0.28 | 0.33 | 0.20 | 0.245 | 0.29 | 0.15 | 0.19 | 0.23 | 0.07 | 0.10 | 0.14 |
| L-threonine | 0.13 | 0.145 | 0.07 | 0.07 | 0.06 | 0.07 | 0.07 | 0.06 | 0.10 | 0.11 | 0.11 | 0.10 | 0.09 | 0.09 | 0.10 | 0.09 | 0.09 | 0.09 | 0.095 | 0.095 |
| Total | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Nutritional level 2 | ||||||||||||||||||||
| Metabolic energy (Mcal/kg) | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 | 2.92 |
| Crude protein | 20.00 | 19.00 | 19.03 | 21.15 | 23.26 | 18.49 | 20.54 | 22.60 | 16.43 | 18.26 | 20.09 | 16.89 | 18.77 | 20.64 | 16.01 | 17.79 | 19.57 | 14.86 | 16.51 | 18.16 |
| Calcium | 0.98 | 1.06 | 1.18 | 1.18 | 0.98 | 1.14 | 1.14 | 1.18 | 1.20 | 1.18 | 1.15 | 1.20 | 1.17 | 1.18 | 1.20 | 1.19 | 1.17 | 1.19 | 1.20 | 1.18 |
| Total phosphorus | 0.46 | 0.45 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Effective phosphorus | 0.46 | 0.45 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.46 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 | 0.45 |
| Methionine | 0.46 | 0.45 | 0.25 | 0.27 | 0.29 | 0.24 | 0.26 | 0.29 | 0.22 | 0.24 | 0.26 | 0.22 | 0.24 | 0.27 | 0.21 | 0.24 | 0.26 | 0.24 | 0.26 | 0.29 |
| Met + Cys | 0.92 | 0.89 | 0.69 | 0.75 | 0.82 | 0.67 | 0.73 | 0.80 | 0.60 | 0.66 | 0.72 | 0.62 | 0.68 | 0.74 | 0.59 | 0.66 | 0.71 | 0.59 | 0.65 | 0.71 |
| Lysine | 1.10 | 1.00 | 0.99 | 1.16 | 1.32 | 0.95 | 1.11 | 1.27 | 0.79 | 0.93 | 1.08 | 0.83 | 0.97 | 1.12 | 0.76 | 0.90 | 1.04 | 0.67 | 0.80 | 0.93 |
| Threonine | 0.75 | 0.73 | 0.66 | 0.74 | 0.81 | 0.64 | 0.72 | 0.79 | 0.60 | 0.67 | 0.74 | 0.61 | 0.67 | 0.74 | 0.58 | 0.64 | 0.70 | 0.53 | 0.59 | 0.65 |
| Isoleucine | 0.98 | 0.91 | 0.92 | 1.06 | 1.20 | 0.88 | 1.02 | 1.16 | 0.74 | 0.87 | 0.99 | 0.77 | 0.90 | 1.03 | 0.71 | 0.83 | 0.95 | 0.64 | 0.75 | 0.86 |
| Valine | 0.76 | 0.73 | 0.99 | 1.10 | 1.21 | 0.96 | 1.06 | 1.17 | 0.89 | 0.98 | 1.08 | 0.87 | 0.96 | 1.06 | 0.79 | 0.88 | 0.97 | 0.69 | 0.76 | 0.84 |
| BD | 90% MD | 100% MD | 110% MD | p-Value | |
|---|---|---|---|---|---|
| 0–2 Weeks | |||||
| FI (g/chick) | 143.46 ± 3.28 b | 143.45 ± 3.89 b | 144.13 ± 6.16 b | 156.47 ± 4.65 a | 0.0001 |
| BWG (g/chick) | 77.26 ± 8.12 | 71.46 ± 4.40 | 75.54 ± 6.46 | 79.81 ± 7.59 | 0.2212 |
| FCR | 1.87 ± 0.18 | 2.01 ± 0.11 | 1.91 ± 0.10 | 1.97 ± 0.17 | 0.3797 |
| 3–6 Weeks | |||||
| FI (g/chick) | 696.45 ± 22.75 b | 652.71 ± 22.63 c | 689.26 ± 16.47 b | 758.27 ± 24.20 a | 0.0001 |
| BWG (g/chick) | 287.72 ± 22.05 a | 235.74 ± 9.89 b | 285.09 ± 21.13 a | 295.54 ± 6.93 a | 0.0001 |
| FCR | 2.43 ± 0.22 b | 2.77 ± 0.14 a | 2.43 ± 0.16 b | 2.57 ± 0.10 ab | 0.0047 |
| 0–6 Weeks | |||||
| FI (g/chick) | 839.90 ± 24.89 b | 796.15 ± 20.39 c | 833.39 ± 11.53 b | 914.75 ± 23.52 a | 0.0001 |
| BWG (g/chick) | 364.98 ± 15.50 a | 307.20 ± 13.93 b | 360.63 ± 19.28 a | 375.35 ± 5.19 a | 0.0001 |
| FCR | 2.31 ± 0.13 b | 2.60 ± 0.12 a | 2.32 ± 0.12 b | 2.44 ± 0.05 b | 0.0005 |
| BD | 90% MD | 100% MD | 110% MD | p-Value | |
|---|---|---|---|---|---|
| Crude protein intake (g/chick) | 161.02 ± 4.75 b | 130.56 ± 3.23 d | 151.68 ± 1.99 c | 183.18 ± 4.70 a | 0.0001 |
| Methionine intake (g/chick) | 3.79 ± 0.11 a | 1.81 ± 0.05 d | 2.08 ± 0.03 c | 2.51 ± 0.06 b | 0.0001 |
| Lysine intake (g/chick) | 8.54 ± 0.25 b | 6.28 ± 0.15 d | 7.74 ± 0.10 c | 9.82 ± 0.25 a | 0.0001 |
| Threonine intake (g/chick) | 6.16 ± 0.18 b | 4.67 ± 0.12 d | 5.43 ± 0.07 c | 6.56 ± 0.17 a | 0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Z.-Y.; Refaie, A.; Liu, M.; Wei, Y.-Y.; Sun, L.-H.; Deng, Z.-C. Validation with the Dynamic Prediction Model of Protein and Amino Acid Requirements for Growth Performance and Health in Layer Chicks. Animals 2025, 15, 1968. https://doi.org/10.3390/ani15131968
Xia Z-Y, Refaie A, Liu M, Wei Y-Y, Sun L-H, Deng Z-C. Validation with the Dynamic Prediction Model of Protein and Amino Acid Requirements for Growth Performance and Health in Layer Chicks. Animals. 2025; 15(13):1968. https://doi.org/10.3390/ani15131968
Chicago/Turabian StyleXia, Zhi-Yuan, Alainaa Refaie, Miao Liu, You-Yang Wei, Lv-Hui Sun, and Zhang-Chao Deng. 2025. "Validation with the Dynamic Prediction Model of Protein and Amino Acid Requirements for Growth Performance and Health in Layer Chicks" Animals 15, no. 13: 1968. https://doi.org/10.3390/ani15131968
APA StyleXia, Z.-Y., Refaie, A., Liu, M., Wei, Y.-Y., Sun, L.-H., & Deng, Z.-C. (2025). Validation with the Dynamic Prediction Model of Protein and Amino Acid Requirements for Growth Performance and Health in Layer Chicks. Animals, 15(13), 1968. https://doi.org/10.3390/ani15131968





