Effect of Pre-IVM Duration with cAMP Modulators on the Production of Cloned Equine Embryos and Foals
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Mares
2.2. Chemicals and Media
2.3. Collection of Immature Oocytes
2.4. Oocyte In Vitro Maturation (IVM)
2.5. Somatic Cell Nuclear Transfer (SCNT)
2.6. Blastocyst Vitrification and Thawing
2.7. Embryo Transfer (ET)
2.8. Statistical Analysis
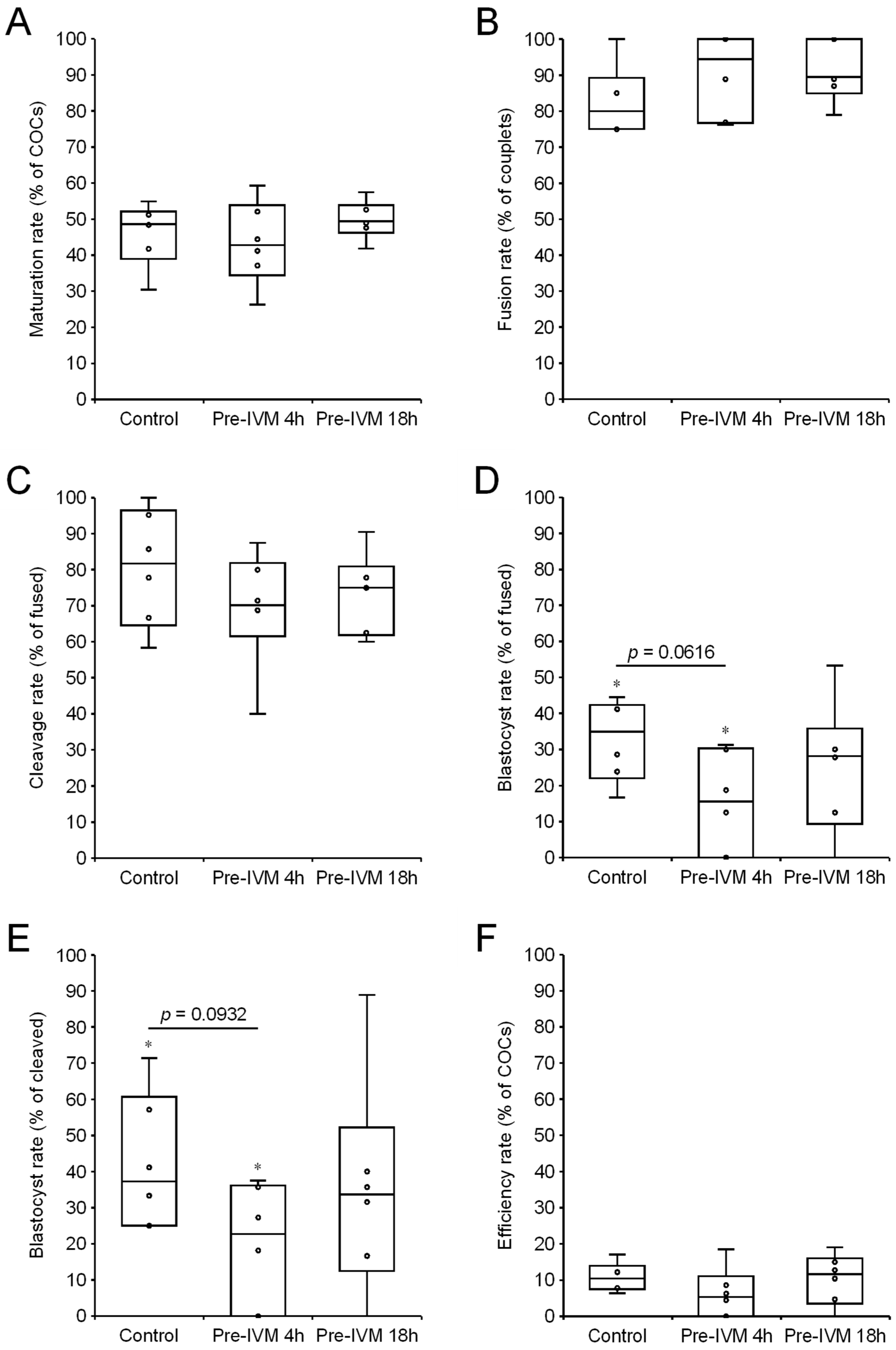
3. Results
3.1. In Vitro Development of SCNT Embryos
3.1.1. Effect of cAMP-Modulating Pre-IVM Treatments
3.1.2. Effect of Donor Cell Lines
3.2. Pregnancy Outcomes After ET
3.2.1. Effect of cAMP-Modulating Pre-IVM Treatments
3.2.2. Effect of Donor Cell Lines
3.2.3. Effect of Embryo Grade
3.2.4. Effect of Recipient Mare’s Day Post-Ovulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Squires, E. Current reproductive technologies impacting equine embryo production. J. Equine Vet. Sci. 2020, 89, 102981. [Google Scholar] [CrossRef]
- Galli, C.; Colleoni, S.; Duchi, R.; Lagutina, I.; Lazzari, G. Developmental competence of equine oocytes and embryos obtained by in vitro procedures ranging from in vitro maturation and ICSI to embryo culture, cryopreservation and somatic cell nuclear transfer. Anim. Reprod. Sci. 2007, 98, 39–55. [Google Scholar] [CrossRef] [PubMed]
- Gilchrist, R.B.; Thompson, J.G. Oocyte maturation: Emerging concepts and technologies to improve developmental potential in vitro. Theriogenology 2007, 67, 6–15. [Google Scholar] [CrossRef]
- Hinrichs, K. Assisted reproductive techniques in mares. Reprod. Domest. Anim. 2018, 53 (Suppl. S2), 4–13. [Google Scholar] [CrossRef]
- Gilchrist, R.B. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod. Fertil. Dev. 2011, 23, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Appeltant, R.; Somfai, T.; Maes, D.; Van Soom, A.; Kikuchi, K. Porcine oocyte maturation in vitro: Role of cAMP and oocyte-secreted factors—a practical approach. J. Reprod. Dev. 2016, 62, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Rose, R.D.; Gilchrist, R.B.; Kelly, J.M.; Thompson, J.G.; Sutton-McDowall, M.L. Regulation of sheep oocyte maturation using cAMP modulators. Theriogenology 2013, 79, 142–148. [Google Scholar] [CrossRef]
- Downs, S.M. Regulation of the G2/M transition in rodent oocytes. Mol. Reprod. Dev. 2010, 77, 566–585. [Google Scholar] [CrossRef]
- Richani, D.; Gilchrist, R.B. Approaches to oocyte meiotic arrest in vitro and impact on oocyte developmental competence. Biol. Reprod. 2022, 106, 243–252. [Google Scholar] [CrossRef]
- Albuz, F.K.; Sasseville, M.; Lane, M.; Armstrong, D.T.; Thompson, J.G.; Gilchrist, R.B. Simulated physiological oocyte maturation (SPOM): A novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Hum. Reprod. 2010, 25, 2999–3011. [Google Scholar] [CrossRef]
- Bernal-Ulloa, S.M.; Heinzmann, J.; Herrmann, D.; Hadeler, K.G.; Aldag, P.; Winkler, S.; Pache, D.; Baulain, U.; Lucas-Hahn, A.; Niemann, H. Cyclic AMP affects oocyte maturation and embryo development in prepubertal and adult cattle. PLoS ONE 2016, 11, e0150264. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Sutton-McDowall, M.L.; Wang, X.; Sugimura, S.; Thompson, J.G.; Gilchrist, R.B. Extending prematuration with cAMP modulators enhances the cumulus contribution to oocyte antioxidant defence and oocyte quality via gap junctions. Hum. Reprod. 2016, 31, 810–821. [Google Scholar] [CrossRef] [PubMed]
- Suresh, A.; Shukla, M.K.; Kumar, D.; Shrivastava, O.P.; Verma, N. Simulated physiological oocyte maturation (SPOM) improves developmental competence of in vitro produced goat embryos. Theriogenology 2021, 172, 193–199. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Masterson, K.R.; Battaglia, D.; Thompson, J.G.; Foss, R.; Beck, R.; Cook, N.L.; O’Leary, T. Conditions to optimise the developmental competence of immature equine oocytes. Reprod. Fertil. Dev. 2020, 32, 1012–1021. [Google Scholar] [CrossRef]
- Zeng, H.T.; Richani, D.; Sutton-McDowall, M.L.; Ren, Z.; Smitz, J.E.; Stokes, Y.; Gilchrist, R.B.; Thompson, J.G. Prematuration with cyclic adenosine monophosphate modulators alters cumulus cell and oocyte metabolism and enhances developmental competence of in vitro-matured mouse oocytes. Biol. Reprod. 2014, 91, 47. [Google Scholar] [CrossRef]
- Leal, G.R.; Monteiro, C.A.S.; Carvalheira, L.R.; Souza-Fabjan, J.M.G. The Simulated Physiological Oocyte Maturation (SPOM) system in domestic animals: A systematic review. Theriogenology 2022, 188, 90–99. [Google Scholar] [CrossRef]
- NHMRC. Australian Code for the Care and Use of Animals for Scientific Purposes, 8th ed.; National Health and Medical Research Council: Canberra, Australia, 2013.
- Thompson, J.G.; Simpson, A.C.; Pugh, P.A.; Donnelly, P.E.; Tervit, H.R. Effect of oxygen concentration on in-vitro development of preimplantation sheep and cattle embryos. J. Reprod. Fertil. 1990, 89, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Cortez, J.V.; Hardwicke, K.; Cuervo-Arango, J.; Grupen, C.G. Cloning horses by somatic cell nuclear transfer: Effects of oocyte source on development to foaling. Theriogenology 2023, 203, 99–108. [Google Scholar] [CrossRef]
- Metcalf, E.S.; Masterson, K.R.; Battaglia, D.; Beck, R.; Cook, N.L. The effect of a simulated physiological oocyte maturation system on the in vitro production of equine embryos. J. Equine Vet. Sci. 2016, 41, 65. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Metcalf, E.S. Morphology, developmental stages and quality parameters of in vitro-produced equine embryos. Reprod. Fertil. Dev. 2019, 31, 1758–1770. [Google Scholar] [CrossRef]
- Conti, M.; Andersen, C.B.; Richard, F.; Mehats, C.; Chun, S.Y.; Horner, K.; Jin, C.; Tsafriri, A. Role of cyclic nucleotide signaling in oocyte maturation. Mol. Cell. Endocrinol. 2002, 187, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E.; Armstrong, D.T.; Gilchrist, R.B. Bovine cumulus cell-oocyte gap junctional communication during in vitro maturation in response to manipulation of cell-specific cyclic adenosine 3′,5′-monophosophate levels. Biol. Reprod. 2004, 70, 548–556. [Google Scholar] [CrossRef]
- Shu, Y.M.; Zeng, H.T.; Ren, Z.; Zhuang, G.L.; Liang, X.Y.; Shen, H.W.; Yao, S.Z.; Ke, P.Q.; Wang, N.N. Effects of cilostamide and forskolin on the meiotic resumption and embryonic development of immature human oocytes. Hum. Reprod. 2008, 23, 504–513. [Google Scholar] [CrossRef]
- Hinrichs, K. The equine oocyte: Factors affecting meiotic and developmental competence. Mol. Reprod. Dev. 2010, 77, 651–661. [Google Scholar] [CrossRef]
- Morris, L.H.A. The development of in vitro embryo production in the horse. Equine Vet. J. 2018, 50, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Love, L.B.; Varner, D.D.; Hinrichs, K. Blastocyst development in equine oocytes with low meiotic competence after suppression of meiosis with roscovitine prior to in vitro maturation. Zygote 2006, 14, 1–8. [Google Scholar] [CrossRef]
- Ezoe, K.; Yabuuchi, A.; Tani, T.; Mori, C.; Miki, T.; Takayama, Y.; Beyhan, Z.; Kato, Y.; Okuno, T.; Kobayashi, T.; et al. Developmental competence of vitrified-warmed bovine oocytes at the germinal-vesicle stage is improved by cyclic adenosine monophosphate modulators during in vitro maturation. PLoS ONE 2015, 10, e0126801. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, A.L.; Pereira, S.A.; Leme, L.O.; Dode, M.A. Evaluation of the simulated physiological oocyte maturation system for improving bovine in vitro embryo production. Theriogenology 2015, 83, 52–57. [Google Scholar] [CrossRef]
- Leal, G.R.; Monteiro, C.A.D.S.; Saraiva, H.F.R.D.A.; Camargo, A.J.D.R.; Rodrigues, A.L.R.; Oliveira, C.S.; Vasconcelos, C.O.P.; Nogueira, L.A.G.; Serapião, R.V. Evaluation of the simulated physiological oocyte maturation (SPOM) system on F1 Gyr × Holstein oocytes and embryos. Anim. Prod. Sci. 2018, 59, 634–640. [Google Scholar] [CrossRef]
- Razza, E.M.; Pedersen, H.S.; Stroebech, L.; Fontes, P.K.; Kadarmideen, H.N.; Callesen, H.; Pihl, M.; Nogueira, M.F.G.; Hyttel, P. Simulated physiological oocyte maturation has side effects on bovine oocytes and embryos. J. Assist. Reprod. Genet. 2019, 36, 413–424. [Google Scholar] [CrossRef]
- Richani, D.; Wang, X.; Zeng, H.T.; Smitz, J.; Thompson, J.G.; Gilchrist, R.B. Pre-maturation with cAMP modulators in conjunction with EGF-like peptides during in vitro maturation enhances mouse oocyte developmental competence. Mol. Reprod. Dev. 2014, 81, 422–435. [Google Scholar] [CrossRef] [PubMed]
- Buell, M.; Chitwood, J.L.; Ross, P.J. cAMP modulation during sheep in vitro oocyte maturation delays progression of meiosis without affecting oocyte parthenogenetic developmental competence. Anim. Reprod. Sci. 2015, 154, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Herrick, J.R. Reversible meiotic arrest in feline oocytes. Reprod. Fertil. Dev. 2014, 26, 258–267. [Google Scholar] [CrossRef] [PubMed]
- Dall’Acqua, P.C.; Leao, B.; Rocha-Frigoni, N.A.S.; Gottardi, F.P.; Mingoti, G.Z. Delaying meiotic resumption during transportation of bovine cumulus-oocyte complexes: Effects on development, apoptosis and caspases activity of in vitro-produced embryos. Zygote 2017, 25, 740–750. [Google Scholar] [CrossRef]
- Leal, G.R.; Graciosa, M.A.G.; Monteiro, C.A.S.; Pasolini, R.; Dos Reis Camargo, A.J.; Oliveira, C.S.; de Paula Vasconcelos, C.O.; Garcia Nogueira, L.A.; Reis Ferreira, A.M.; Serapião, R.V. The SPOM-adapted IVM system improves in vitro production of bovine embryos. Theriogenology 2020, 158, 277–282. [Google Scholar] [CrossRef]
- Galli, C.; Colleoni, S.; Turini, P.; Crotti, G.; Dieci, C.; Lodde, V.; Luciano, A.M.; Lazzari, G. Holding equine oocytes at room temperature for 18 hours prior to in vitro maturation maintains their developmental competence. J. Equine Vet. Sci. 2014, 34, 174–175. [Google Scholar] [CrossRef]
- Hinrichs, K. Advances in holding and cryopreservation of equine oocytes and embryos. J. Equine Vet. Sci. 2020, 89, 102990. [Google Scholar] [CrossRef]
- Hinrichs, K. Equine cloning. Vet. Clin. N. Am. Equine Pract. 2006, 22, 857–866. [Google Scholar] [CrossRef]
- Vanderwall, D.K.; Woods, G.L.; Roser, J.F.; Schlafer, D.H.; Sellon, D.C.; Tester, D.F.; White, K.L. Equine cloning: Applications and outcomes. Reprod. Fertil. Dev. 2006, 18, 91–98. [Google Scholar] [CrossRef]
- Fulka, H.; Loi, P.; Czernik, M.; Surani, A.; Fulka, J. Omne vivum ex ovo: The oocyte reprogramming and remodeling activities. Reproduction 2023, 165, R75–R89. [Google Scholar] [CrossRef]
- Niemann, H. Epigenetic reprogramming in mammalian species after SCNT-based cloning. Theriogenology 2016, 86, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Olivera, R.; Moro, L.N.; Jordan, R.; Luzzani, C.; Miriuka, S.; Radrizzani, M.; Donadeu, F.X.; Vichera, G. In vitro and in vivo development of horse cloned embryos generated with iPSCs, mesenchymal stromal cells and fetal or adult fibroblasts as nuclear donors. PLoS ONE 2016, 11, e0164049. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Claes, A.N.; Stout, T.A.E. In vitro-produced horse embryos exhibit a very narrow window of acceptable recipient mare uterine synchrony compared with in vivo-derived embryos. Reprod. Fertil. Dev. 2019, 31, 1904–1911. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, G.; Colleoni, S.; Crotti, G.; Turini, P.; Fiorini, G.; Barandalla, M.; Landriscina, L.; Dolci, G.; Benedetti, M.; Duchi, R.; et al. Laboratory production of equine embryos. J. Equine Vet. Sci. 2020, 89, 103097. [Google Scholar] [CrossRef]
- Lewis, N.; Canesin, H.; Choi, Y.H.; Foss, R.; Felix, M.; Rader, K.; Hinrichs, K. Equine in vitro produced blastocysts: Relationship of embryo morphology, stage and speed of development to foaling rate. Reprod. Fertil. Dev. 2023, 35, 338–351. [Google Scholar] [CrossRef]
- Awadalla, M.; Vestal, N.; McGinnis, L.; Ahmady, A. Effect of age and morphology on live birth rate after cleavage stage embryo transfer. Reprod. Sci. 2021, 28, 43–51. [Google Scholar] [CrossRef]
- Cuervo-Arango, J.; Claes, A.N.; Stout, T.A. The recipient’s day after ovulation and the number of corpora lutea influence the likelihood of pregnancy in mares following transfer of ICSI frozen embryos. Theriogenology 2019, 135, 181–188. [Google Scholar] [CrossRef]
- Carnevale, E.M.; Ramirez, R.J.; Squires, E.L.; Alvarenga, M.A.; Vanderwall, D.K.; McCue, P.M. Factors affecting pregnancy rates and early embryonic death after equine embryo transfer. Theriogenology 2000, 54, 965–979. [Google Scholar] [CrossRef]
- Donato, G.G.; Necchi, D.; Vandaele, H.; Vita, M.E.; Bertero, A.; Vincenti, L.; Nervo, T. Influence of intrauterine fluid detection, number of transfers and age of the recipient on pregnancy rate and early embryonic loss in a commercial embryo transfer program. Animals 2023, 13, 1799. [Google Scholar] [CrossRef]
- Cortez, J.V.; Hardwicke, K.; Grupen, C.G. Pre-maturation of oocytes for equine cloning using cAMP modulators. J. Equine Vet. Sci. 2023, 125, 104641. [Google Scholar] [CrossRef]

| Group | Recipient Mares Total | Pregnant at Day 14 1 | Pregnant at Day 45 1 | Pregnant at Day 90 | Mares Foaling |
|---|---|---|---|---|---|
| Control | 4 | 1 (25.0%) | 1 (25.0%) | 1 | 1 |
| Pre-IVM 4 h | 2 | 1 (50.0%) | 1 (50.0%) | 1 | 1 |
| Pre-IVM 18 h | 17 | 8 (47.1%) | 2 (11.8%) | 2 | 2 |
| Total | 23 | 10 (43.5%) | 4 (17.4%) | 4 | 4 |
| Donor Cell Line | Recipient Mares Total | Pregnant at Day 14 1 | Pregnant at Day 45 1 | Pregnant at Day 90 | Mares Foaling |
|---|---|---|---|---|---|
| #01 | 10 | 3 (30.0%) | 1 (10.0%) | 1 | 1 |
| #02 | 5 | 1 (20.0%) | 1 (20.0%) | 1 | 1 |
| #03 | 4 | 3 (75.0%) | 1 (25.0%) | 1 | 1 |
| #05 | 4 | 3 (75.0%) | 1 (25.0%) | 1 | 1 |
| Embryo Grade | Recipient Mares Total | Pregnant at Day 14 1 | Pregnant at Day 45 1 | Pregnant at Day 90 | Mares Foaling |
|---|---|---|---|---|---|
| Grade 1 | 16 | 8 (50.0%) | 4 (25.0%) | 4 | 4 |
| Grade 2 | 7 | 2 (28.6%) | 0 (0.0%) | 0 | 0 |
| Day Post- Ovulation | Recipient Mares Total | Pregnant at Day 14 1 | Pregnant at Day 45 1 | Pregnant at Day 90 | Mares Foaling |
|---|---|---|---|---|---|
| Day 4 | 7 | 3 (42.9%) | 2 (28.6%) | 2 | 2 |
| Day 5 | 16 | 7 (43.8%) | 2 (12.5%) | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cortez, J.V.; Hardwicke, K.; Méndez-Calderón, C.E.; Grupen, C.G. Effect of Pre-IVM Duration with cAMP Modulators on the Production of Cloned Equine Embryos and Foals. Animals 2025, 15, 1961. https://doi.org/10.3390/ani15131961
Cortez JV, Hardwicke K, Méndez-Calderón CE, Grupen CG. Effect of Pre-IVM Duration with cAMP Modulators on the Production of Cloned Equine Embryos and Foals. Animals. 2025; 15(13):1961. https://doi.org/10.3390/ani15131961
Chicago/Turabian StyleCortez, Jenin V., Kylie Hardwicke, Carlos E. Méndez-Calderón, and Christopher G. Grupen. 2025. "Effect of Pre-IVM Duration with cAMP Modulators on the Production of Cloned Equine Embryos and Foals" Animals 15, no. 13: 1961. https://doi.org/10.3390/ani15131961
APA StyleCortez, J. V., Hardwicke, K., Méndez-Calderón, C. E., & Grupen, C. G. (2025). Effect of Pre-IVM Duration with cAMP Modulators on the Production of Cloned Equine Embryos and Foals. Animals, 15(13), 1961. https://doi.org/10.3390/ani15131961







