The Boss Is Back in Town: Insights into the Wolf Recolonization of a Highly Anthropized and Low-Ungulate-Density Environment
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Data Collection and Processing
2.3. Data Analyses
2.3.1. Wolf Distribution
2.3.2. Activity Pattern and Overlap
2.3.3. Diet Analysis
3. Results
3.1. Wolf Distribution
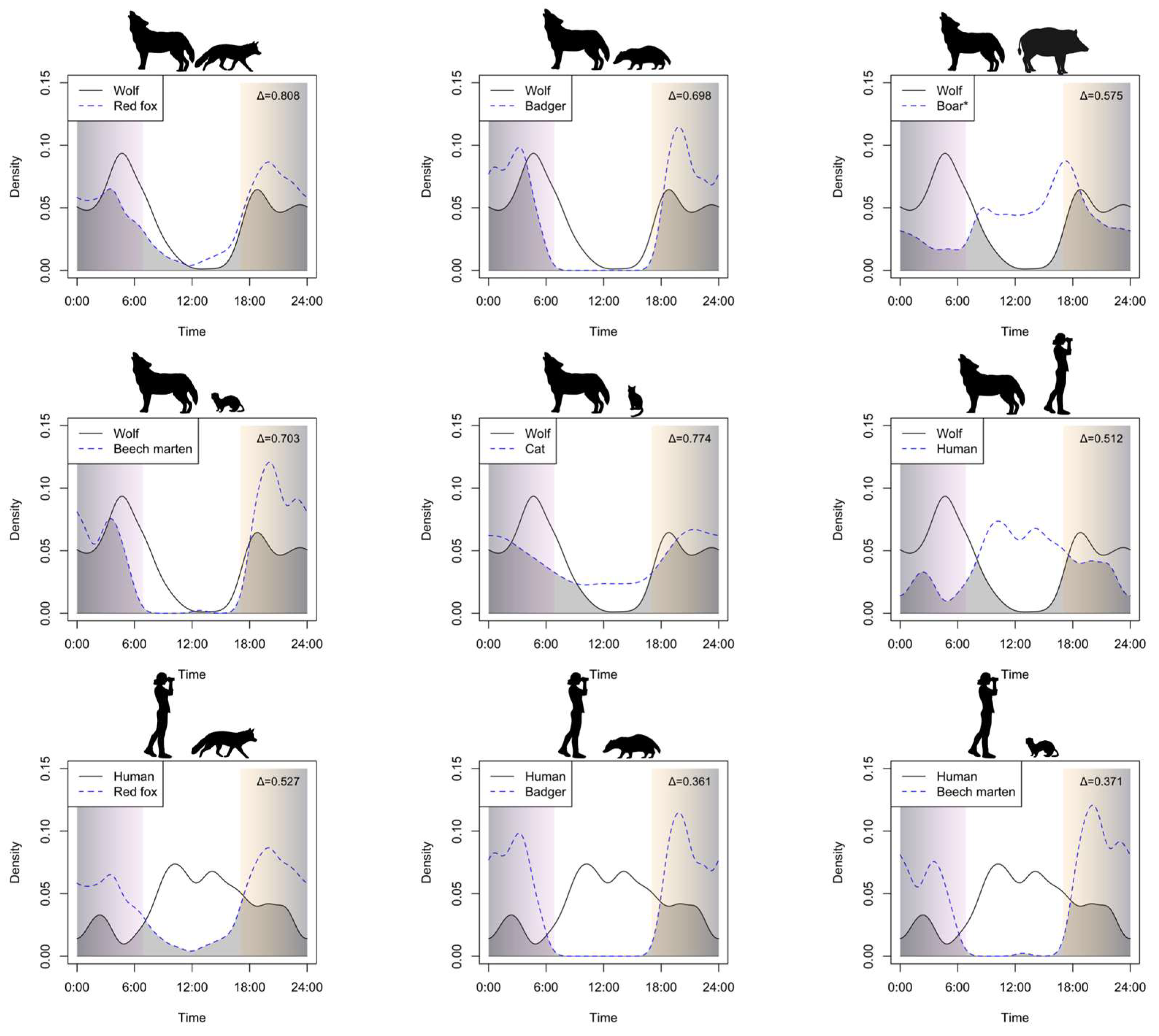
3.2. Activity Pattern and Overlap
3.3. Wolf Diet
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chapron, G.; Kaczensky, P.; Linnell, J.D.C.; Von Arx, M.; Huber, D.; Andrén, H.; López-Bao, J.V.; Adamec, M.; Álvares, F.; Anders, O.; et al. Recovery of Large Carnivores in Europe’s Modern Human-Dominated Landscapes. Science 2014, 346, 1517–1519. [Google Scholar] [CrossRef] [PubMed]
- Ripple, W.J.; Estes, J.A.; Beschta, R.L.; Wilmers, C.C.; Ritchie, E.G.; Hebblewhite, M.; Berger, J.; Elmhagen, B.; Letnic, M.; Nelson, M.P.; et al. Status and Ecological Effects of the World’s Largest Carnivores. Science 2014, 343, 1241484. [Google Scholar] [CrossRef] [PubMed]
- Elmhagen, B.; Ludwig, G.; Rushton, S.P.; Helle, P.; Lindén, H. Top Predators, Mesopredators and Their Prey: Interference Ecosystems along Bioclimatic Productivity Gradients. J. Anim. Ecol. 2010, 79, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Newsome, T.M.; Ripple, W.J. A Continental Scale Trophic Cascade from Wolves through Coyotes to Foxes. J. Anim. Ecol. 2015, 84, 49–59. [Google Scholar] [CrossRef]
- Ripple, W.J.; Beschta, R.L. Large Predators Limit Herbivore Densities in Northern Forest Ecosystems. Eur. J. Wildl. Res. 2012, 58, 733–742. [Google Scholar] [CrossRef]
- Laundré, J.W.; Hernández, L.; Altendorf, K.B. Wolves, Elk, and Bison: Reestablishing the “Landscape of Fear” in Yellowstone National Park, U.S.A. Can. J. Zool. 2001, 79, 1401–1409. [Google Scholar] [CrossRef]
- Haswell, P.M.; Kusak, J.; Jones, K.A.; Hayward, M.W. Fear of the Dark? A Mesopredator Mitigates Large Carnivore Risk through Nocturnality, but Humans Moderate the Interaction. Behav. Ecol. Sociobiol. 2020, 74, 62. [Google Scholar] [CrossRef]
- Hobbs, N.T.; Johnston, D.B.; Marshall, K.N.; Wolf, E.C.; Cooper, D.J. Does Restoring Apex Predators to Food Webs Restore Ecosystems? Large Carnivores in Yellowstone as a Model System. Ecol. Monogr. 2024, 94, e1598. [Google Scholar] [CrossRef]
- Groom, R.J.; Lannas, K.; Jackson, C.R. The Impact of Lions on the Demography and Ecology of Endangered African Wild Dogs. Anim. Conserv. 2017, 20, 382–390. [Google Scholar] [CrossRef]
- Wikenros, C.; Aronsson, M.; Liberg, O.; Jarnemo, A.; Hansson, J.; Wallgren, M.; Sand, H.; Bergström, R. Fear or Food—Abundance of Red Fox in Relation to Occurrence of Lynx and Wolf. Sci. Rep. 2017, 7, 9059. [Google Scholar] [CrossRef]
- Jiménez, J.; Nuñez-Arjona, J.C.; Mougeot, F.; Ferreras, P.; González, L.M.; García-Domínguez, F.; Muñoz-Igualada, J.; Palacios, M.J.; Pla, S.; Rueda, C.; et al. Restoring Apex Predators Can Reduce Mesopredator Abundances. Biol. Conserv. 2019, 238, 108234. [Google Scholar] [CrossRef]
- Bernardi, C.D.; Chapron, G.; Kaczensky, P.; Álvares, F.; Andrén, H.; Balys, V.; Blanco, J.C.; Chiriac, S.; Ćirović, D.; Drouet-Hoguet, N.; et al. Continuing Recovery of Wolves in Europe. PLoS Sustain. Transform. 2025, 4, e0000158. [Google Scholar] [CrossRef]
- Boitani, L. Wolf Conservation and Recovery. In Wolves: Behavior, Ecology and Conservation; Mech, L.D., Boitani, L., Eds.; University of Chicago: Chicago, IL, USA, 2003; pp. 317–340. [Google Scholar]
- Zimen, E.; Boitani, L. Number and Distribution of Wolves in Italy. Zeitchrift Säugetierkunde 1975, 40, 102–112. [Google Scholar]
- La Morgia, V.; Marucco, F.; Aragno, P.; Salvatori, V.; Gervasi, V.; De Angelis, D.; Fabbri, E.; Caniglia, R.; Velli, E.; Avanzinelli, E.; et al. Stima Della Distribuzione e Consistenza del Lupo a Scala Nazionale 2020/2021; Technical Report ISPRA-Ministero Della Transizione Ecologica “Attività di Monitoraggio Nazionale Nell’ambito del Piano di Azione del Lupo”; Istituto Superiore per la Protezione e la Ricerca Ambientale: Roma, Italy, 2022. [Google Scholar]
- Zanni, M.; Brogi, R.; Merli, E.; Apollonio, M. The Wolf and the City: Insights on Wolves’ Conservation in the Anthropocene. Anim. Conserv. 2023, 26, 766–780. [Google Scholar] [CrossRef]
- Massolo, A.; Meriggi, A. Factors Affecting Habitat Occupancy by Wolves in Northern Apennines (Northern Italy): A Model of Habitat Suitability. Ecography 1998, 21, 97–107. [Google Scholar] [CrossRef]
- Karlsson, J.; Sjöström, M. Human attitudes towards wolves, a matter of distance. Biol. Conserv. 2007, 137, 610–616. [Google Scholar] [CrossRef]
- Coppola, F.; Baldanti, S.; Di Rosso, A.; Vecchio, G.; Casini, L.; Russo, C.; Lucchini, V.; Boni, C.B.; Malasoma, M.; Gabbani, C.; et al. Settlement of a stable wolf pack in a highly anthropic area of Pisan hills: Relationship with animal husbandry and hunting in a human–wolf coexistence perspective. Anim. Sci. J. 2022, 93, e13799. [Google Scholar] [CrossRef]
- Torretta, E.; Brangi, A.; Meriggi, A. Changes in Wolf Occupancy and Feeding Habits in the Northern Apennines: Results of Long-Term Predator–Prey Monitoring. Animals 2024, 14, 735. [Google Scholar] [CrossRef]
- Fardone, L.; Forlani, M.; Canova, L.; De Luca, M.; Meriggi, A. Can the Wolf (Canis lupus) Thrive in Highly Anthropised Lowlands? First Habitat Suitability Analysis of the Po Plain, Italy. Animals 2025, 15, 546. [Google Scholar] [CrossRef]
- Llaneza, L.; López-Bao, J.V.; Sazatornil, V. Insights into Wolf Presence in Human-dominated Landscapes: The Relative Role of Food Availability, Humans and Landscape Attributes. Divers. Distrib. 2012, 18, 459–469. [Google Scholar] [CrossRef]
- Bisi, J.; Kurki, S.; Svensberg, M.; Liukkonen, T. Human dimensions of wolf (Canis lupus) conflicts in Finland. Eur. Jour. Wildl. Res. 2007, 53, 304–314. [Google Scholar] [CrossRef]
- Salvatori, V.; Balian, E.; Blanco, J.C.; Carbonell, X.; Ciucci, P.; Demeter, L.; Marino, A.; Panzavolta, A.; Sólyom, A.; von Korff, Y.; et al. Are large carnivores the real issue? Solutions for improving conflict management through stakeholder participation. Sustainability 2021, 13, 4482. [Google Scholar] [CrossRef]
- Kaczensky, P.; Chapron, G.; von Arx, M.; Huber, D.; Andrén, H.; Linnell, J. Status, Management and Distribution of Large Carnivores—Bear, Lynx, Wolf & Wolverine—In Europe; Report to the EU Commission, Part 2; IUCN SSC Large Carnivore Initiative for Europe: Gland, Switzerland, 2013. [Google Scholar]
- Gervasi, V.; Aragno, P.; Salvatori, V.; Caniglia, R.; De Angelis, D.; Fabbri, E.; La Morgia, V.; Marucco, F.; Velli, E.; Genovesi, P. Estimating Distribution and Abundance of Wide-Ranging Species with Integrated Spatial Models: Opportunities Revealed by the First Wolf Assessment in South-Central Italy. Ecol. Evol. 2024, 14, e11285. [Google Scholar] [CrossRef]
- Gaudiano, L.; Sorino, R.; Corriero, G.; Frassanito, A.; Strizzi, C.; Notarnicola, G. Stato Delle Conoscenze del Lupo Canis lupus in Puglia. In Proceedings of the X Congresso Italiano di Teriologi, Acquapendente, Italy, 20–23 April 2016. [Google Scholar]
- Lavarra, P.; Angelini, P.; Augello, R.; Bianco, P.M.; Capogrossi, R.; Gennaio, R.; La Ghezza, V.; Marrese, M. Il Sistema Carta Della Natura Della Regione Puglia; Serie Rapporti; ISPRA: Roma, Italy, 2014; ISBN 978-88-448-0655-2. [Google Scholar]
- Marzano, G.; Crispino, F.; Rugge, M.; Gervasio, G. The Wolf, Canis Lupus Linnaeus, 1758 (Mammalia Canidae): Re-Colonization Is Still Ongoing in Southern Italy: A Breeding Pack Documented through Camera Traps in the Salento Peninsula. Biodivers. J. 2017, 8, 855–860. [Google Scholar]
- Costa, G. Fauna Salentina Ossia Enumerazione di Tutti Gli Animali Che Trovansi Nelle Diverse Contrade Della Provincia di Terra d’Otranto e Nelle Acque de’ Due Mari Che la Bagnano, Contenente la Descrizione de’ Nuovi o Poco Esattamente Conosciuti; Editrice Salentina: Galatina, Italy, 1871. [Google Scholar]
- Ghigi, A. Ricerche Faunstiche e Sistematiche Sui Mammiferi d’Italia Che Formano Oggetto Di Caccia. Natura 1911, 2, 289–337. [Google Scholar]
- Loy, A.; Aloise, G.; Ancillotto, L.; Angelici, F.M.; Bertolino, S.; Capizzi, D.; Castiglia, R.; Colangelo, P.; Contoli, L.; Cozzi, B.; et al. Mammals of Italy: An Annotated Checklist. Hystrix It. J. Mamm. 2019, 30, 87–106. [Google Scholar] [CrossRef]
- Tourani, M.; Moqanaki, E.M.; Boitani, L.; Ciucci, P. Anthropogenic Effects on the Feeding Habits of Wolves in an Altered Arid Landscape of Central Iran. Mammalia 2014, 78, 117–121. [Google Scholar] [CrossRef]
- Musto, C.; Cerri, J.; Capizzi, D.; Fontana, M.C.; Rubini, S.; Merialdi, G.; Berzi, D.; Ciuti, F.; Santi, A.; Rossi, A.; et al. First evidence of widespread positivity to anticoagulant rodenticides in grey wolves (Canis lupus). Sci. Tot. Env. 2024, 915, 169990. [Google Scholar] [CrossRef]
- Mele, C.; Medagli, P.; Accogli, R.; Beccarisi, L.; Marchiori, S.; Albano, A. Flora of Salento (Apulia, Southeastern Italy): An Annotated Checklist. Flora Mediterr. 2006, 16, 193–245. [Google Scholar]
- Marchiori, S.; Medagli, P.; Mele, C.; Scandura, S.; Albano, A. Caratteristiche Della Flora Vascolare Pugliese. In La Cooperazione Italo-Albanese per la Valorizzazione Della Biodiversità; CIHEAM-IAMB: Bari, Italy, 2000; pp. 67–75. [Google Scholar]
- Greenberg, S.; Godin, T.; Whittington, J. Design Patterns for Wildlife-Related Camera Trap Image Analysis. Ecol. Evol. 2019, 9, 13706–13730. [Google Scholar] [CrossRef]
- Ferretti, F.; Oliveira, R.; Rossa, M.; Belardi, I.; Pacini, G.; Mugnai, S.; Fattorini, N.; Lazzeri, L. Interactions between Carnivore Species: Limited Spatiotemporal Partitioning between Apex Predator and Smaller Carnivores in a Mediterranean Protected Area. Front. Zool. 2023, 20, 20. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.E.; Baker, R.J.; Ballard, W.B.; Kelly, B.T. Differentiating Mexican Gray Wolf and Coyote Scats Using DNA Analysis. Wildl. Soc. Bull. 2004, 32, 685–692. [Google Scholar] [CrossRef]
- Lovari, S.; Pokheral, C.P.; Jnawali, S.R.; Fusani, L.; Ferretti, F. Coexistence of the Tiger and the Common Leopard in a Prey-Rich Area: The Role of Prey Partitioning. J. Zool. 2015, 295, 122–131. [Google Scholar] [CrossRef]
- Teernik, B.J. Hair of West European Mammals. Atlas and Identification Key; Cambridge University Press: Cambridge, UK, 2004; ISBN 978-0521545778. [Google Scholar]
- Wilkens, B. Archeozoologia. Manuale per Lo Studio dei Resti Faunistici Dell’area Mediterranea; Edes: Sassari, Italy, 2012; ISBN 978-88-6025-241-8. [Google Scholar]
- Molinari-Jobin, A.; Kéry, M.; Marboutin, E.; Molinari, P.; Koren, I.; Fuxjäger, C.; Breitenmoser-Würsten, C.; Wölfl, S.; Fasel, M.; Kos, I.; et al. Monitoring in the Presence of Species Misidentification: The Case of the Eurasian Lynx in the Alps. Anim. Conserv. 2012, 15, 266–273. [Google Scholar] [CrossRef]
- Molinari-Jobin, A.; Wölfl, S.; Marboutin, E.; Molinari, P.; Wölfl, M.; Kos, I.; Fasel, M.; Koren, I.; Fuxjäger, C.; Breitenmoser, C.; et al. Monitoring the Lynx in the Alps. Hystrix It. J. Mamm. 2012, 23, 49–53. [Google Scholar] [CrossRef]
- Marucco, F.; La Morgia, V.; Aragno, P.; Salvatori, V.; Caniglia, R.; Fabbri, E.; Mucci, N.; Genovesi, P. Linee Guida e Protocolli per Il Monitoraggio Nazionale del Lupo in Italia; Realizzate Nell’ambito Della Convenzione ISPRA-Ministero Dell’Ambiente e Della Tutela Del Territorio e Del Mare per “Attività Di Monitoraggio Nazionale Nell’ambito Del Piano Di Azione Del Lupo”; Istituto Superiore per la Protezione e la Ricerca Ambientale: Roma, Italy, 2020. [Google Scholar]
- Marucco, F.; Avanzinelli, E.; Boiani, M.; Menzano, A.; Perrone, S.; Dupont, P.; Bischof, R.; Milleret, C.; von Hardenberg, A.; Pilgrim, K.; et al. La Popolazione di Lupo Nelle Regioni Alpine Italiane 2020–2021; Technical Report on National Scale Monitoring Following the Wolf Action Plan, Supported by ISPRA-MITE Convention and for LIFE WolfAlps EU18 NAT/IT/000972 WOLFALPS EU.; Istituto Superiore per la Protezione e la Ricerca Ambientale: Roma, Italy, 2022. [Google Scholar]
- Wolf Alpine Group. The Wolf Alpine Population in 2020–2022 over 7 Countries; Technical Report for LIFE WolfAlps EU Project LIFE18 NAT/IT/000972, Action C4; LIFE WolfAlps EU: Roma, Italy, 2023. [Google Scholar]
- Ranc, N.; Acosta-Pankov, I.; Balys, V.; Bučko, J.; Cirovic, D.; Fabijanić, N.; Filacorda, S.; Giannatos, G.; Guimaraes, N.; Hatlauf, J.; et al. Distribution of Large Carnivores in Europe 2012–2016: Distribution Map for Golden Jackal (Canis aureus); Zenodo: Geneva, Switzerland, 2022. [Google Scholar] [CrossRef]
- Bassi, E.; Willis, S.G.; Passilongo, D.; Mattioli, L.; Apollonio, M. Predicting the Spatial Distribution of Wolf (Canis lupus) Breeding Areas in a Mountainous Region of Central Italy. PLoS ONE 2015, 10, e0124698. [Google Scholar] [CrossRef]
- Frangini, L.; Sterrer, U.; Franchini, M.; Pesaro, S.; Rüdisser, J.; Filacorda, S. Stay Home, Stay Safe? High Habitat Suitability and Environmental Connectivity Increases Road Mortality in a Colonizing Mesocarnivore. Landsc. Ecol. 2022, 37, 2343–2361. [Google Scholar] [CrossRef]
- Octenjak, D.; Pađen, L.; Šilić, V.; Reljić, S.; Vukičević, T.T.; Kusak, J. Wolf Diet and Prey Selection in Croatia. Mamm. Res. 2020, 65, 647–654. [Google Scholar] [CrossRef]
- Mohammadi, A.; Kaboli, M.; Sazatornil, V.; López-Bao, J.V. Anthropogenic Food Resources Sustain Wolves in Conflict Scenarios of Western Iran. PLoS ONE 2019, 14, e0218345. [Google Scholar] [CrossRef]
- Hartig, F. DHARMa: Residual Diagnostics for Hierarchical (Multi-Level/Mixed) Regression Models 2016, version 0.4.7.; R Group: New York, NY, USA, 2016.
- Akaike, H. A New Look at the Statistical Model Identification. In Selected papers of Hirotugu Akaike Springer Series in Statistics (Perspectives in Statistics); Parzen, E., Tanabe, K., Kitagawa, G., Eds.; Springer: New York, NY, USA, 1974; pp. 215–216. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach; Springer-Verlag: New York, NY, USA, 2002. [Google Scholar] [CrossRef]
- Burnham, K.P.; Anderson, D.R. Multi Model Inference: Understanding AIC and BIC in Model Selection. Sociol. Methods Res. 2004, 33, 261–304. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference 2010, version 1.48.11.; R Group: New York, NY, USA, 2010.
- Ridout, M.S.; Linkie, M. Estimating Overlap of Daily Activity Patterns from Camera Trap Data. J. Agric. Biol. Environ. Stat. 2009, 14, 322–337. [Google Scholar] [CrossRef]
- Monterroso, P.; Alves, P.C.; Ferreras, P. Plasticity in Circadian Activity Patterns of Mesocarnivores in Southwestern Europe: Implications for Species Coexistence. Behav. Ecol. Sociobiol. 2014, 68, 1403–1417. [Google Scholar] [CrossRef]
- Meredith, M.; Ridout, M.; Campbell, L.A.D. Overlap: Estimates of Coefficient of Overlapping for Animal Activity Patterns 2013, version 0.3.9.; R Group: New York, NY, USA, 2013.
- Brillouin, L. Science and Information Theory; Academic Press: New York, NY, USA, 1956; p. 320. [Google Scholar]
- Capitani, C.; Chynoweth, M.; Kusak, J.; Çoban, E.; Şekercioğlu, Ç.H. Wolf diet in an agricultural landscape of north-eastern Turkey. Mammalia 2015, 80, 329–334. [Google Scholar] [CrossRef]
- Frerebeau, N. Analysis and Visualization of Archaeological Count Data 2025, version 3.3.1; Zenodo: Geneva, Switzerland, 2025.
- Valière, N.; Fumagalli, L.; Gielly, L.; Miquel, C.; Lequette, B.; Poulle, M.-L.; Weber, J.-M.; Arlettaz, R.; Taberlet, P. Long-Distance Wolf Recolonization of France and Switzerland Inferred from Non-Invasive Genetic Sampling over a Period of 10 Years. Anim. Conserv. 2003, 6, 83–92. [Google Scholar] [CrossRef]
- Fabbri, E.; Miquel, C.; Lucchini, V.; Santini, A.; Caniglia, R.; Duchamp, C.; Weber, J.-M.; Lequette, B.; Marucco, F.; Boitani, L.; et al. From the Apennines to the Alps: Colonization Genetics of the Naturally Expanding Italian Wolf (Canis lupus) Population. Mol. Ecol. 2007, 16, 1661–1671. [Google Scholar] [CrossRef]
- Blanco, J.C.; Cortés, Y. Dispersal Patterns, Social Structure and Mortality of Wolves Living in Agricultural Habitats in Spain. J. Zool. 2007, 273, 114–124. [Google Scholar] [CrossRef]
- Morales-González, A.; Fernández-Gil, A.; Quevedo, M.; Revilla, E. Patterns and Determinants of Dispersal in Grey Wolves (Canis lupus). Biol. Rev. 2022, 97, 466–480. [Google Scholar] [CrossRef]
- Marucco, F.; Pilgrim, K.L.; Avanzinelli, E.; Schwartz, M.K.; Rossi, L. Wolf Dispersal Patterns in the Italian Alps and Implications for Wildlife Diseases Spreading. Animals 2022, 12, 1260. [Google Scholar] [CrossRef]
- Barry, T.; Gurarie, E.; Cheraghi, F.; Kojola, I.; Fagan, W.F. Does Dispersal Make the Heart Grow Bolder? Avoidance of Anthropogenic Habitat Elements across Wolf Life History. Anim. Behav. 2020, 166, 219–231. [Google Scholar] [CrossRef]
- Torretta, E.; Corradini, A.; Pedrotti, L.; Bani, L.; Bisi, F.; Dondina, O. Hide-and-Seek in a Highly Human-Dominated Landscape: Insights into Movement Patterns and Selection of Resting Sites of Rehabilitated Wolves (Canis lupus) in Northern Italy. Animals 2023, 13, 46. [Google Scholar] [CrossRef]
- Mech, L.D.; Boitani, L. Wolves: Behavior, Ecology, and Conservation; University of Chicago: Chicago, IL, USA, 2003. [Google Scholar]
- Ferreiro-Arias, I.; García, E.J.; Palacios, V.; Sazatornil, V.; Rodríguez, A.; López-Bao, J.V.; Llaneza, L. Drivers of Wolf Activity in a Human-Dominated Landscape and Its Individual Variability Toward Anthropogenic Disturbance. Ecol. Evol. 2024, 14, e70397. [Google Scholar] [CrossRef] [PubMed]
- Dennehy, E.; Llaneza, L.; López-Bao, J.V. Contrasting Wolf Responses to Different Paved Roads and Traffic Volume Levels. Biodivers. Conserv. 2021, 30, 3133–3150. [Google Scholar] [CrossRef]
- Kukielka, E.; Barasona, J.A.; Cowie, C.E.; Drewe, J.A.; Gortazar, C.; Cotarelo, I.; Vicente, J. Spatial and Temporal Interactions between Livestock and Wildlife in South Central Spain Assessed by Camera Traps. Prev. Vet. Med. 2013, 112, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsov, S.S.; Zheltukhin, A.S.; Kotlov, I.P. Daily Activity Patterns of the Large and Medium-Sized Mammals Based on Camera Traps Datain the Central Forest Nature Reserve, Valdai Upland, Russia. Nat. Conserv. Res. Заповедная наука 2018, 3, 68–88. [Google Scholar]
- Wevers, J.; Fattebert, J.; Casaer, J.; Artois, T.; Beenaerts, N. Trading Fear for Food in the Anthropocene: How Ungulates Cope with Human Disturbance in a Multi-Use, Suburban Ecosystem. Sci. Total Environ. 2020, 741, 140369. [Google Scholar] [CrossRef]
- Brivio, F.; Grignolio, S.; Brogi, R.; Benazzi, M.; Bertolucci, C.; Apollonio, M. An Analysis of Intrinsic and Extrinsic Factors Affecting the Activity of a Nocturnal Species: The Wild Boar. Mamm. Biol. 2017, 84, 73–81. [Google Scholar] [CrossRef]
- Cruz, P.; Iezzi, M.E.; Angelo, C.D.; Varela, D.; Bitetti, M.S.D.; Paviolo, A. Effects of Human Impacts on Habitat Use, Activity Patterns and Ecological Relationships among Medium and Small Felids of the Atlantic Forest. PLoS ONE 2018, 13, e0200806. [Google Scholar] [CrossRef]
- Oberosler, V.; Groff, C.; Iemma, A.; Pedrini, P.; Rovero, F. The Influence of Human Disturbance on Occupancy and Activity Patterns of Mammals in the Italian Alps from Systematic Camera Trapping. Mamm. Biol. 2017, 87, 50–61. [Google Scholar] [CrossRef]
- Vivas, I.; Zafra, A.; Barja, I. Hunting Activity Modulates Wolves’ Activity Patterns During Pup Caring; Research Square: Durham, NC, USA, 2024. [Google Scholar] [CrossRef]
- Vorel, A.; Kadlec, I.; Toulec, T.; Selimovic, A.; Horníček, J.; Vojtěch, O.; Mokrý, J.; Pavlačík, L.; Arnold, W.; Cornils, J.; et al. Home Range and Habitat Selection of Wolves Recolonising Central European Human-Dominated Landscapes. Wildl. Biol. 2024, 2024, e01245. [Google Scholar] [CrossRef]
- Reinhardt, I.; Kluth, G.; Nowak, C.; Szentiks, C.A.; Krone, O.; Ansorge, H.; Mueller, T. Military Training Areas Facilitate the Recolonization of Wolves in Germany. Conserv. Lett. 2019, 12, e12635. [Google Scholar] [CrossRef]
- Lewis, J.S.; Bailey, L.L.; VandeWoude, S.; Crooks, K.R. Interspecific Interactions between Wild Felids Vary across Scales and Levels of Urbanization. Ecol. Evol. 2015, 5, 5946–5961. [Google Scholar] [CrossRef] [PubMed]
- Ståhlberg, S.; Bassi, E.; Viviani, V.; Apollonio, M. Quantifying Prey Selection of Northern and Southern European Wolves (Canis lupus). Mamm. Biol. 2017, 83, 34–43. [Google Scholar] [CrossRef]
- Meriggi, A.; Brangi, A.; Schenone, L.; Signorelli, D.; Milanesi, P. Changes of Wolf (Canis lupus) Diet in Italy in Relation to the Increase of Wild Ungulate Abundance. Ethol. Ecol. Evol. 2011, 23, 195–210. [Google Scholar] [CrossRef]
- Di Rosso, A.; Boni, C.B.; Baldanti, S.; Casini, L.; Coppola, F.; Felicioli, A. Pup feeding habits of a mixed wolf-hybrid pack living in a human-modified landscape in Central Italy. Hystrix 2023, 34, 144–146. [Google Scholar] [CrossRef]
- Leccisi, F. Farms Development in the Salento Area. In Regional Architecture in the Mediterranean Area; Bucci, A., Mollo, L., Eds.; Alinea Editrice: Firenze, Italy, 2010; pp. 349–356. ISBN 978-88-6055-293-8. [Google Scholar]
- Mariella, L.; Palma, M.; Pellegrino, D. A WebGIS for Stray Dogs: Methodological and Practical Aspects. Geoinformatics Geostat. An. Overv. 2019, S2. [Google Scholar]
- Prato, L.; Cerfolli, F. Programma di Intervento Sul Territorio Dell’ATC Provincia di Lecce Annata 2018–2019. Final Report. In Ampliamento Aree di Censimento Della Specie Volpe Vulpes Vulpes; ATC: Lecce, Italy, 2019. [Google Scholar]

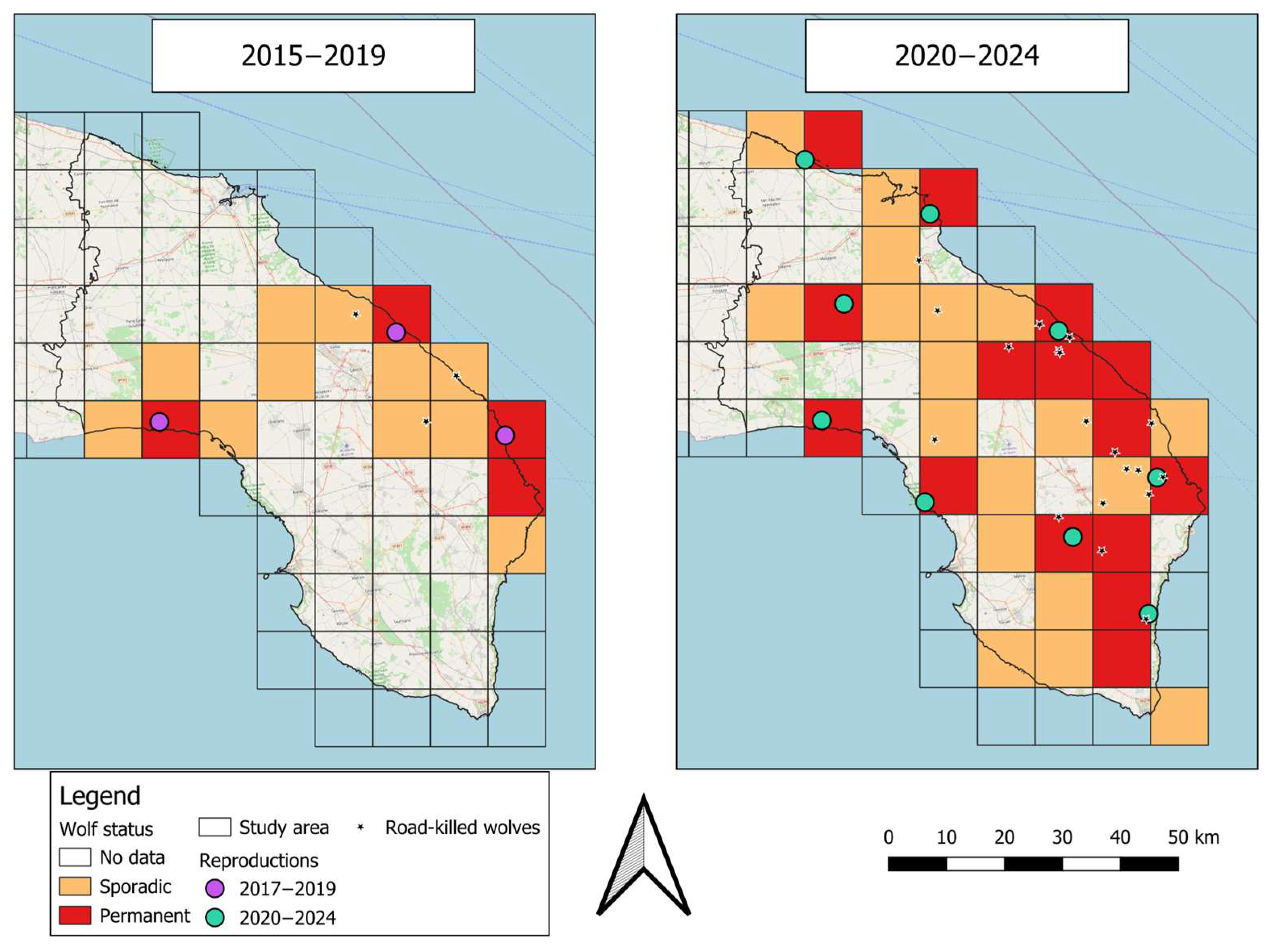

| 2015–2019 | 2020–2024 | ||
|---|---|---|---|
| Occurrence data with SCALP code | C1 | 43 | 377 |
| C2 | 4 | 30 | |
| C3 | / | / | |
| Tot | 47 | 407 | |
| Number of square grids | Sporadic | 11 | 18 |
| Permanent | 4 | 15 | |
| Tot | 15 | 33 | |
| Number of unique reproductions | 3 | 8 |
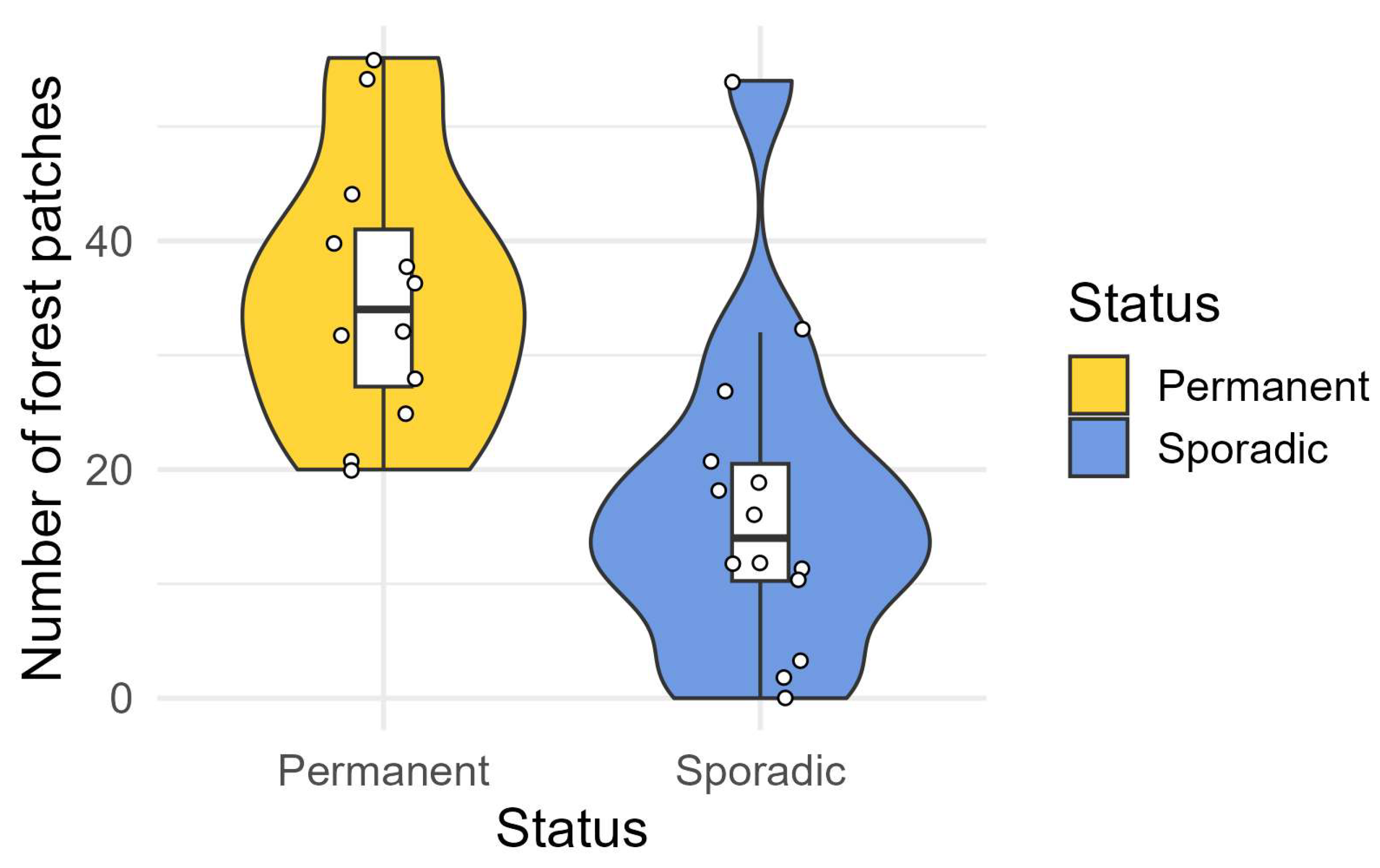
| Year of Reproduction | % Forest | Number of Forested Patches | Total Forested Area (km2) | Number of Ovicaprine Farms | % Protected Areas | Road Density (km/km2) |
|---|---|---|---|---|---|---|
| 2017 | 36.73 | 28 | 11.61 | 6 | 0.72 | 4.55 |
| 2018 | 27.40 | 10 | 5.84 | 3 | 0 | 4.31 |
| 2019 | 27.37 | 32 | 7.98 | 2 | 0 | 3.17 |
| 2020 | 4.311 | 25 | 4.16 | 14 | 5.37 | 4.58 |
| 2020 | 4.09 | 40 | 2.92 | 12 | 8.30 | 4.14 |
| 2022 | 2.11 | 32 | 2.11 | 10 | 0 | 3.27 |
| 2023 | 2.2 | 22 | 0.96 | / | 5.89 | 4.55 |
| 2024 | 0.99 | 20 | 0.99 | 36 | 0.29 | 5.88 |
| 2024 | 0.70 | 7 | 7 | / | 0 | 1.36 |
| Model | df | logLik | AICc | Δ AIC | Weight |
|---|---|---|---|---|---|
| Forest_count * | 2 | −12.5049 | 29.5316 | 0 | 0.2020 |
| Forest_count * + PA_perc | 3 | −11.7519 | 30.5948 | 1.0632 | 0.1187 |
| Forest_count * + Forest_perc | 3 | −11.7657 | 30.6224 | 1.0908 | 0.1171 |
| Forest_count * + N_farms + Forest_perc | 4 | −10.3976 | 30.7000 | 1.1684 | 0.1126 |
| Forest_count * + N_farms | 3 | −12.1603 | 31.4114 | 1.8798 | 0.0789 |
| Monitoring Area | Number of Camera Traps | Camera-Trap Nights | Species | Number of Independent Detections | RAI |
|---|---|---|---|---|---|
| Site 1 | 11 | 732 | Wolf | 363 | 49.59 |
| Red fox | 1548 | 211.48 | |||
| Badger | 300 | 40.98 | |||
| Beech marten | 215 | 29.37 | |||
| Wild boar | 303 | 41.39 | |||
| Domestic cat | 68 | 9.29 | |||
| Human | 554 | 75.68 | |||
| Site 2 | 4 | 480 | Wolf | 19 | 3.96 |
| Red fox | 163 | 33.96 | |||
| Badger | 28 | 5.83 | |||
| Beech marten | 7 | 1.46 | |||
| Wild boar | / | / | |||
| Domestic cat | 8 | 1.67 | |||
| Human | 221 | 46.04 | |||
| Site 3 | 2 | 190 | Wolf | 26 | 13.68 |
| Red fox | 134 | 70.53 | |||
| Badger | 11 | 5.79 | |||
| Beech marten | / | / | |||
| Wild boar | / | / | |||
| Domestic cat | 3 | 1.58 | |||
| Human | 92 | 48.42 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frangini, L.; Marzano, G.; Comuzzi, A.; De Giovanni, A.; Gallizia, A.; Franchini, M.; Rugge, M.; De Luca, M.; De Matteis, G.; Filacorda, S. The Boss Is Back in Town: Insights into the Wolf Recolonization of a Highly Anthropized and Low-Ungulate-Density Environment. Animals 2025, 15, 1958. https://doi.org/10.3390/ani15131958
Frangini L, Marzano G, Comuzzi A, De Giovanni A, Gallizia A, Franchini M, Rugge M, De Luca M, De Matteis G, Filacorda S. The Boss Is Back in Town: Insights into the Wolf Recolonization of a Highly Anthropized and Low-Ungulate-Density Environment. Animals. 2025; 15(13):1958. https://doi.org/10.3390/ani15131958
Chicago/Turabian StyleFrangini, Lorenzo, Giacomo Marzano, Alice Comuzzi, Andrea De Giovanni, Andrea Gallizia, Marcello Franchini, Michela Rugge, Marco De Luca, Giuseppe De Matteis, and Stefano Filacorda. 2025. "The Boss Is Back in Town: Insights into the Wolf Recolonization of a Highly Anthropized and Low-Ungulate-Density Environment" Animals 15, no. 13: 1958. https://doi.org/10.3390/ani15131958
APA StyleFrangini, L., Marzano, G., Comuzzi, A., De Giovanni, A., Gallizia, A., Franchini, M., Rugge, M., De Luca, M., De Matteis, G., & Filacorda, S. (2025). The Boss Is Back in Town: Insights into the Wolf Recolonization of a Highly Anthropized and Low-Ungulate-Density Environment. Animals, 15(13), 1958. https://doi.org/10.3390/ani15131958








