Cervids as a Promising Pillar of an Integrated Surveillance System for Emerging Infectious Diseases in Hungary: A Pilot Study
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. ELISA
2.3. Virus Neutralization Assay
2.4. Detection of BTV by Real-Time RT-PCR
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BTV | Bluetongue virus |
| WNV | West Nile virus |
| EHDV | Epizootic hemorrhagic disease virus |
| TBEV | Tick-borne encephalitis virus |
| JEV | Japanese encephalitis virus |
| USUV | Usutu virus |
| ZIKAV | Zika virus |
| DENV | Dengue virus |
| ELISA | Enzyme-linked immunosorbent assay |
| pr-E | Envelope protein |
| VNT | Birus neutralization test |
| BSL-4 | Biosafety level 4 |
| IF | Immunofluorescence |
References
- Kreuder Johnson, C.; Hitchens, P.L.; Smiley Evans, T.; Goldstein, T.; Thomas, K.; Clements, A.; Joly, D.O.; Wolfe, N.D.; Daszak, P.; Karesh, W.B.; et al. Spillover and Pandemic Properties of Zoonotic Viruses with High Host Plasticity. Sci. Rep. 2015, 5, 14830. [Google Scholar] [CrossRef]
- Socha, W.; Kwasnik, M.; Larska, M.; Rola, J.; Rozek, W. Vector-Borne Viral Diseases as a Current Threat for Human and Animal Health—One Health Perspective. J. Clin. Med. 2022, 11, 3026. [Google Scholar] [CrossRef]
- Bouzid, M.; Colón-González, F.J.; Lung, T.; Lake, I.R.; Hunter, P.R. Climate Change and the Emergence of Vector-Borne Diseases in Europe: Case Study of Dengue Fever. BMC Public Health 2014, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Erazo, D.; Grant, L.; Ghisbain, G.; Marini, G.; Colón-González, F.J.; Wint, W.; Rizzoli, A.; Van Bortel, W.; Vogels, C.B.F.; Grubaugh, N.D.; et al. Contribution of Climate Change to the Spatial Expansion of West Nile Virus in Europe. Nat. Commun. 2024, 15, 1196. [Google Scholar] [CrossRef] [PubMed]
- Maclachlan, N.J.; Zientara, S.; Wilson, W.C.; Richt, J.A.; Savini, G. Bluetongue and Epizootic Hemorrhagic Disease Viruses: Recent Developments with These Globally Re-Emerging Arboviral Infections of Ruminants. Curr. Opin. Virol. 2019, 34, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Rana, E.A.; Prodhan, M.A.; Akter, S.H.; Gogoi-Tiwari, J.; Sarker, S.; Annandale, H.; Eagles, D.; Abraham, S.; Uddin, J.M. The Global Burden of Emerging and Re-Emerging Orbiviruses in Livestock: An Emphasis on Bluetongue Virus and Epizootic Hemorrhagic Disease Virus. Viruses 2024, 17, 20. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Paniagua, J.; Gutiérrez-Guzmán, A.V.; Lecollinet, S.; Boadella, M.; Arenas-Montes, A.; Cano-Terriza, D.; Lowenski, S.; Gortázar, C.; Höfle, U. Spatio-Temporal Trends and Risk Factors Affecting West Nile Virus and Related Flavivirus Exposure in Spanish Wild Ruminants. BMC Vet. Res. 2016, 12, 249. [Google Scholar] [CrossRef]
- Escribano-Romero, E.; Lupulović, D.; Merino-Ramos, T.; Blázquez, A.-B.; Lazić, G.; Lazić, S.; Saiz, J.-C.; Petrović, T. West Nile Virus Serosurveillance in Pigs, Wild Boars, and Roe Deer in Serbia. Vet. Microbiol. 2015, 176, 365–369. [Google Scholar] [CrossRef]
- Ruiz-Fons, F.; Sánchez-Matamoros, A.; Gortázar, C.; Sánchez-Vizcaíno, J.M. The Role of Wildlife in Bluetongue Virus Maintenance in Europe: Lessons Learned after the Natural Infection in Spain. Virus Res. 2014, 182, 50–58. [Google Scholar] [CrossRef]
- Angelini, P.; Tamba, M.; Finarelli, A.C.; Bellini, R.; Albieri, A.; Bonilauri, P.; Cavrini, F.; Dottori, M.; Gaibani, P.; Martini, E.; et al. West Nile Virus Circulation in Emilia-Romagna, Italy: The Integrated Surveillance System 2009. Eurosurveillance 2010, 15, 19547. [Google Scholar] [CrossRef]
- Hofmann, M.; Griot, C.; Chaignat, V.; Perler, L.; Thür, B. Blauzungenkrankheit Erreicht Die Schweiz. Schweiz. Arch. Für Tierheilkd. 2008, 150, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Guzmán, A.-V.; Vicente, J.; Sobrino, R.; Perez-Ramírez, E.; Llorente, F.; Höfle, U. Antibodies to West Nile Virus and Related Flaviviruses in Wild Boar, Red Foxes and Other Mesomammals from Spain. Vet. Microbiol. 2012, 159, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Milićević, V.; Sapundžić, Z.Z.; Glišić, D.; Kureljušić, B.; Vasković, N.; Đorđević, M.; Mirčeta, J. Cross-Sectional Serosurvey of Selected Infectious Diseases in Wild Ruminants in Serbia. Res. Vet. Sci. 2024, 170, 105183. [Google Scholar] [CrossRef]
- Hubálek, Z.; Juricová, Z.; Straková, P.; Blazejová, H.; Betásová, L.; Rudolf, I. Serological Survey for West Nile Virus in Wild Artiodactyls, Southern Moravia (Czech Republic). Vector-Borne Zoonotic Dis. 2017, 17, 654–657. [Google Scholar] [CrossRef]
- Bournez, L.; Umhang, G.; Faure, E.; Boucher, J.-M.; Boué, F.; Jourdain, E.; Sarasa, M.; Llorente, F.; Jiménez-Clavero, M.A.; Moutailler, S.; et al. Exposure of Wild Ungulates to the Usutu and Tick-Borne Encephalitis Viruses in France in 2009–2014: Evidence of Undetected Flavivirus Circulation a Decade Ago. Viruses 2019, 12, 10. [Google Scholar] [CrossRef] [PubMed]
- Grassi, L.; Drigo, M.; Zelená, H.; Pasotto, D.; Cassini, R.; Mondin, A.; Franzo, G.; Tucciarone, C.M.; Ossola, M.; Vidorin, E.; et al. Wild Ungulates as Sentinels of Flaviviruses and Tick-Borne Zoonotic Pathogen Circulation: An Italian Perspective. BMC Vet. Res. 2023, 19, 155. [Google Scholar] [CrossRef]
- Zana, B.; Erdélyi, K.; Nagy, A.; Mezei, E.; Nagy, O.; Takács, M.; Bakonyi, T.; Forgách, P.; Korbacska-Kutasi, O.; Fehér, O.; et al. Multi-Approach Investigation Regarding the West Nile Virus Situation in Hungary, 2018. Viruses 2020, 12, 123. [Google Scholar] [CrossRef]
- Rossi, S.; Pioz, M.; Beard, E.; Durand, B.; Gibert, P.; Gauthier, D.; Klein, F.; Maillard, D.; Saint-Andrieux, C.; Saubusse, T.; et al. Bluetongue Dynamics in French Wildlife: Exploring the Driving Forces. Transbound. Emerg. Dis. 2014, 61, e12–e24. [Google Scholar] [CrossRef]
- García, I.; Napp, S.; Casal, J.; Perea, A.; Allepuz, A.; Alba, A.; Carbonero, A.; Arenas, A. Bluetongue Epidemiology in Wild Ruminants from Southern Spain. Eur. J. Wildl. Res. 2009, 55, 173–178. [Google Scholar] [CrossRef]
- Domán, M.; Marton, S.; Malik, P.; Bányai, K.; Hornyák, Á. Country-Wide Distribution of Bluetongue Virus with Expanding Host Spectrum and Evidence of Vector Competence in Hungary. Acta Virol. 2019, 63, 229–234. [Google Scholar] [CrossRef]
- Rodríguez-Sánchez, B.; Gortázar, C.; Ruiz-Fons, F.; Sánchez-Vizcaíno, J.M. Bluetongue Virus Serotypes 1 and 4 in Red Deer, Spain. Emerg. Infect. Dis. 2010, 16, 518–520. [Google Scholar] [CrossRef] [PubMed]
- Barroso, P.; Risalde, M.A.; García-Bocanegra, I.; Acevedo, P.; Barasona, J.Á.; Palencia, P.; Carro, F.; Jiménez-Ruiz, S.; Pujols, J.; Montoro, V.; et al. Long-Term Determinants of the Seroprevalence of the Bluetongue Virus in Deer Species in Southern Spain. Res. Vet. Sci. 2021, 139, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Lorca-Oró, C.; López-Olvera, J.R.; Ruiz-Fons, F.; Acevedo, P.; García-Bocanegra, I.; Oleaga, Á.; Gortázar, C.; Pujols, J. Long-Term Dynamics of Bluetongue Virus in Wild Ruminants: Relationship with Outbreaks in Livestock in Spain, 2006-2011. PLoS ONE 2014, 9, e100027. [Google Scholar] [CrossRef] [PubMed]
- Rivera, N.A.; Varga, C.; Ruder, M.G.; Dorak, S.J.; Roca, A.L.; Novakofski, J.E.; Mateus-Pinilla, N.E. Bluetongue and Epizootic Hemorrhagic Disease in the United States of America at the Wildlife–Livestock Interface. Pathogens 2021, 10, 915. [Google Scholar] [CrossRef]
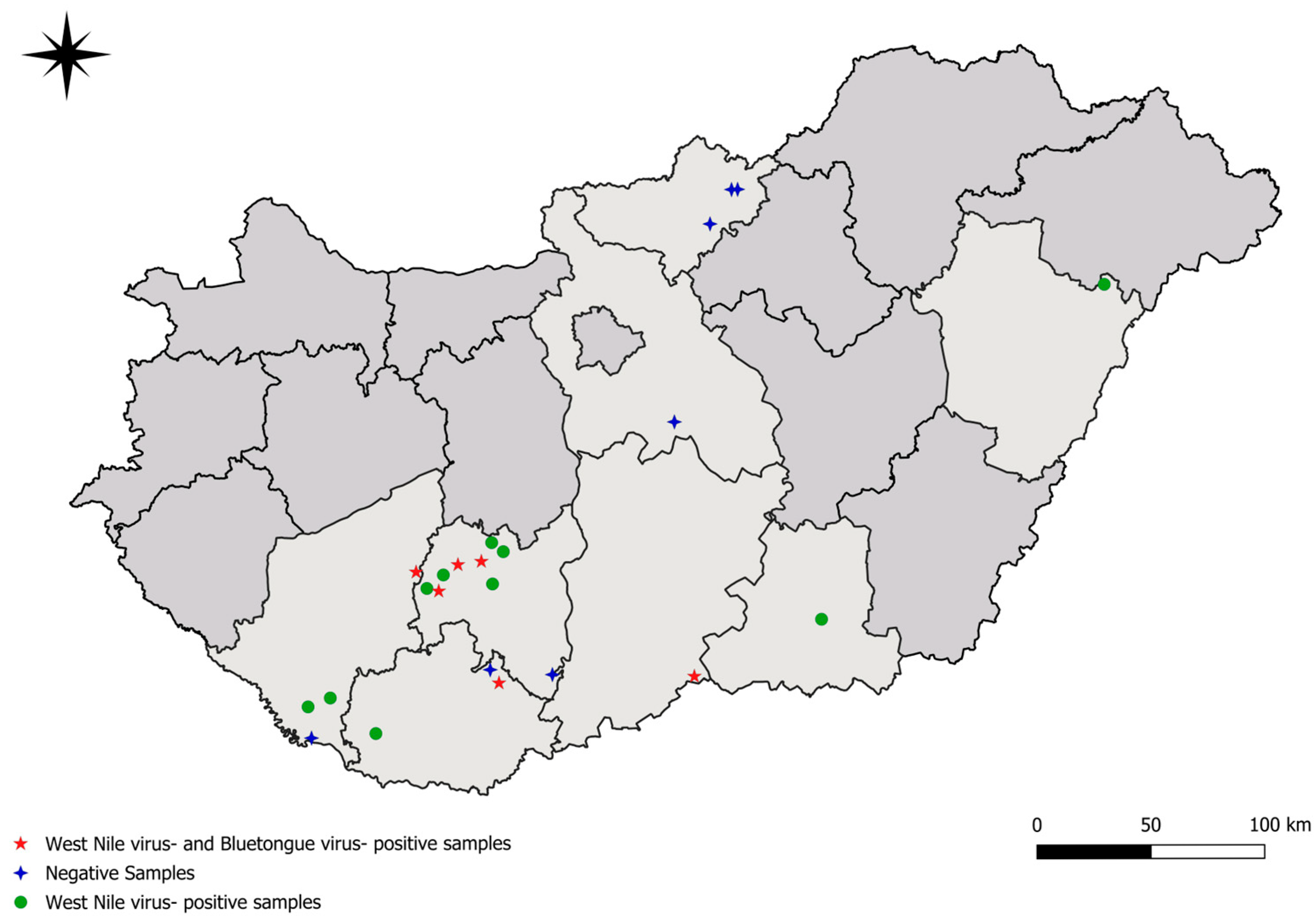
| European Fallow Deer (Dama dama) | Red Deer (Cervus elaphus) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| County | Locality | N.o. Animals Tested | EHDV ELISA | WNV ELISA | WNV neutr. | BTV ELISA | Seroprevalence WNV % 2 | Seroprevalence BTV % | N.o. Animals Tested | EHDV ELISA | WNV ELISA | WNV neutr. | BTV ELISA | Seroprevalence WNV % 2 | Seroprevalence BTV % |
| Bács-Kiskun | Kelebia | 32 | 0 | 5 | 5 | 1 | 15.6 | 3.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Baranya | Mecseknádasd | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mészkemence | 15 | 0 | 1 | 1 | 2 | 6.7 | 13.3 | 5 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Szentegát | 1 | 0 | 1 | 1 | 0 | 100 | 0 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Csongrád-Csanád | Hódmezővásárhely | 1 | 0 | 1 | 1 | 0 | 100 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Hajdú-Bihar | Guth | 45 | 0 | 4 | 1 | 0 | 2.2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Nógrád | Kazár | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Pásztó | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Vizslás | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Pest | Pusztavacs | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Somogy | Barcs | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Csokonyavisonta | 28 | 0 | 24 | 18 | 0 | 64.3 | 0 | 11 | 0 | 8 | 6 | 0 | 54.5 | 0 | |
| Homokszentgyörgy | 6 | 0 | 5 | 4 | 0 | 66.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Törökkoppány | 31 | 0 | 13 | 9 | 2 | 29.0 | 6.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tolna | Belecska | 23 | 0 | 2 | 2 | 1 | 8.7 | 4.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Gemenc | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Gyönk | 21 | 0 | 6 | 6 | 0 | 28.6 | 0 | 2 | 0 | 1 | 1 | 0 | 50.0 | 0 | |
| Kisszékely | 7 | 0 | 3 | 2 | 0 | 28.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Kocsola | 33 | 0 | 6 | 5 | 1 | 15.2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Nagykónyi | 9 | 0 | 6 | 3 | 0 | 33.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Szakcs | 7 | 0 | 4 | 3 | 0 | 42.9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tamási | 42 | 0 | 7 | 7 | 1 | 16.7 | 2.4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Tolnanémedi | 8 | 0 | 5 | 3 | 0 | 37.5 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Sum | 318 | 0 | 94 | 71 | 8 | 22.3 | 2.5 | 22 | 0 | 9 | 7 | 0 | 31.8 | 0 | |
| European Fallow Deer | Red Deer | Roe Deer | ||||
|---|---|---|---|---|---|---|
| Sampling Period | N.o. Individuals | N.o. Positives | N.o. Individuals | N.o. Positives | N.o. Individuals | N.o. Positives |
| 2020/2021 | 125 | 20 (WNV) | 0 | 0 | 0 | 0 |
| 4 (BTV) | ||||||
| 2021/2022 | 74 | 17 (WNV) | 19 | 4 (WNV) | 1 | 0 |
| 1 (BTV) | ||||||
| 2022/2023 | 120 | 25 (WNV) | 3 | 2 (WNV) | 0 | 0 |
| 3 (BTV) | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lakatos, I.; Malik, P.; Bodó, K.; Szőke, Z.; Sükösd, F.; Lanszki, Z.; Szemethy, L.; Kurucz, K.; Bányai, K.; Kemenesi, G.; et al. Cervids as a Promising Pillar of an Integrated Surveillance System for Emerging Infectious Diseases in Hungary: A Pilot Study. Animals 2025, 15, 1948. https://doi.org/10.3390/ani15131948
Lakatos I, Malik P, Bodó K, Szőke Z, Sükösd F, Lanszki Z, Szemethy L, Kurucz K, Bányai K, Kemenesi G, et al. Cervids as a Promising Pillar of an Integrated Surveillance System for Emerging Infectious Diseases in Hungary: A Pilot Study. Animals. 2025; 15(13):1948. https://doi.org/10.3390/ani15131948
Chicago/Turabian StyleLakatos, István, Péter Malik, Kornélia Bodó, Zsuzsanna Szőke, Farkas Sükösd, Zsófia Lanszki, László Szemethy, Kornélia Kurucz, Krisztián Bányai, Gábor Kemenesi, and et al. 2025. "Cervids as a Promising Pillar of an Integrated Surveillance System for Emerging Infectious Diseases in Hungary: A Pilot Study" Animals 15, no. 13: 1948. https://doi.org/10.3390/ani15131948
APA StyleLakatos, I., Malik, P., Bodó, K., Szőke, Z., Sükösd, F., Lanszki, Z., Szemethy, L., Kurucz, K., Bányai, K., Kemenesi, G., & Zana, B. (2025). Cervids as a Promising Pillar of an Integrated Surveillance System for Emerging Infectious Diseases in Hungary: A Pilot Study. Animals, 15(13), 1948. https://doi.org/10.3390/ani15131948







