Factors Affecting Hair Cortisol Concentration in Domestic Dogs: A Focus on Factors Related to Dogs and Their Guardians
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.1.1. Healthy Dogs (HD) Group
2.1.2. Chronic Gastroenteric Disease (CGD) Dogs Group
2.2. Questionnaire
2.3. Hair Samples Collection and Storage
2.4. Extraction of Cortisol from Hair Samples
- Perform a coarse cleaning of the hair using tweezers on a white sheet of paper to remove all impurities and foreign materials; if the hair is long, cut the part closest to the root and use that for the analyses. Weigh 150 mg of hair and perform 2 washes with 3 mL of isopropanol each, followed by drying overnight under a fume hood.
- Cut the hair sample with scissors for 1–2 min, and then, after including three zirconium beads for each sample, use the homogenizer performing 6 cycles at 4350 rpm for 30 s each. Weigh 50 mg of hair and proceed with the extraction (if extraction is not performed immediately, the samples can be stored in a dark environment).
- Add 1 mL of methanol, vortex for 20 s, and then place on a shaker for 24 h (200 rpm).
- After 24 h of shaking, centrifuge at 9000 rpm for 15 min and collect 0.6 mL of supernatant. Evaporate to dryness using nitrogen; if not proceeding immediately with the cortisol ELISA kit, freeze the samples.
- Before beginning with the analysis, allow the kit and the samples to reach room temperature by leaving them out of refrigeration for approximately 90 min. Each hair sample was reconstituted with 200 µL of buffer [21]. Cortisol was measured using the Salimetrics© (Carlsbad, CA, USA) enzyme immunoassay kit for high-sensitivity salivary cortisol. If cortisol levels exceed 3.0 μg/dl (82.77 nmol/L), the samples must be diluted with Assay Diluent and re-read to ensure accuracy; the final value is calculated by multiplying the measured result by the dilution factor. All samples were measured in duplicates, and the mean value was used for statistical analysis.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heimbürge, S.; Kanitz, E.; Otten, W. The Use of Hair Cortisol for the Assessment of Stress in Animals. Gen. Comp. Endocrinol. 2019, 270, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Mesarcova, L.; Kottferova, J.; Skurkova, L.; Leskova, L.; Kmecova, N. Analysis of Cortisol in Dog Hair—A Potential Biomarker of Chronic Stress: A Review. Vet. Med. 2017, 62, 363–376. [Google Scholar] [CrossRef]
- Cook, N.J. Review: Minimally Invasive Sampling Media and the Measurement of Corticosteroids as Biomarkers of Stress in Animals. Can. J. Anim. Sci. 2012, 92, 227–259. [Google Scholar] [CrossRef]
- Accorsi, P.A.; Carloni, E.; Valsecchi, P.; Viggiani, R.; Gamberoni, M.; Tamanini, C.; Seren, E. Cortisol Determination in Hair and Faeces from Domestic Cats and Dogs. Gen. Comp. Endocrinol. 2008, 155, 398–402. [Google Scholar] [CrossRef]
- Meyer, J.S.; Novak, M.A. Minireview: Hair Cortisol: A Novel Biomarker of Hypothalamic-Pituitary-Adrenocortical Activity. Endocrinology 2012, 153, 4120–4127. [Google Scholar] [CrossRef]
- Bennett, A.; Hayssen, V. Measuring Cortisol in Hair and Saliva from Dogs: Coat Color and Pigment Differences. Domest. Anim. Endocrinol. 2010, 39, 171–180. [Google Scholar] [CrossRef]
- Russell, E.; Koren, G.; Rieder, M.; Van Uum, S. Hair Cortisol as a Biological Marker of Chronic Stress: Current Status, Future Directions and Unanswered Questions. Psychoneuroendocrinology 2012, 37, 589–601. [Google Scholar] [CrossRef]
- Wester, V.L.; Van Rossum, E.F.C. Clinical Applications of Cortisol Measurements in Hair. Eur. J. Endocrinol. 2015, 173, M1–M10. [Google Scholar] [CrossRef]
- González-de-la-Vara; Valdez, R.A.; Lemus-Ramirez, V.; Carlos Vázquez-Chagoyán, J.; Villa-Godoy, A.; Romano, M.C. Effects of Adrenocorticotropic Hormone Challenge and Age on Hair Cortisol Concentrations in Dairy Cattle. Can. J. Vet. Res. 2011, 75, 216–221. [Google Scholar]
- Wester, V.L.; van der Wulp, N.R.P.; Koper, J.W.; de Rijke, Y.B.; van Rossum, E.F.C. Hair Cortisol and Cortisone Are Decreased by Natural Sunlight. Psychoneuroendocrinology 2016, 72, 94–96. [Google Scholar] [CrossRef]
- Bryan, H.M.; Adams, A.G.; Invik, R.M.; Wynne-Edwards, K.E.; Smits, J.E. Hair as a Meaningful Measure of Baseline Cortisol Levels over Time in Dogs. J. Am. Assoc. Lab. Anim. Sci. 2013, 52, 189–196. [Google Scholar] [PubMed]
- Davenport, M.D.; Tiefenbacher, S.; Lutz, C.K.; Novak, M.A.; Meyer, J.S. Analysis of Endogenous Cortisol Concentrations in the Hair of Rhesus Macaques. Gen. Comp. Endocrinol. 2006, 147, 255–261. [Google Scholar] [CrossRef] [PubMed]
- Rothlin-Zachrisson, N.; Röcklinsberg, H.; Jettel, E.; Bergqvist, F.J.; Stadig, S.; Öhlund, M.; Mariti, C.; Holst, B.S. Hair Cortisol Concentrations in Clipped and Combed Hair and Associations with Characteristics, Health Status and Stress in Domestic Cats. Sci. Rep. 2024, 14, 21846. [Google Scholar] [CrossRef]
- Part, C.E.; Kiddie, J.L.; Hayes, W.A.; Mills, D.S.; Neville, R.F.; Morton, D.B.; Collins, L.M. Physiological, Physical and Behavioural Changes in Dogs (Canis Familiaris) When Kennelled: Testing the Validity of Stress Parameters. Physiol. Behav. 2014, 133, 260–271. [Google Scholar] [CrossRef]
- Sundman, A.S.; Van Poucke, E.; Svensson Holm, A.C.; Faresjö, Å.; Theodorsson, E.; Jensen, P.; Roth, L.S.V. Long-Term Stress Levels Are Synchronized in Dogs and Their Owners. Sci. Rep. 2019, 9, 7391. [Google Scholar] [CrossRef]
- Höglin, A.; Van Poucke, E.; Katajamaa, R.; Jensen, P.; Theodorsson, E.; Roth, L.S.V. Long-Term Stress in Dogs Is Related to the Human–Dog Relationship and Personality Traits. Sci. Rep. 2021, 11, 8612. [Google Scholar] [CrossRef]
- Dupouy-Manescau, N.; Méric, T.; Sénécat, O.; Drut, A.; Valentin, S.; Leal, R.O.; Hernandez, J. Updating the Classification of Chronic Inflammatory Enteropathies in Dogs. Animals 2024, 14, 681. [Google Scholar] [CrossRef]
- Marchetti, V.; Gori, E.; Mariotti, V.; Gazzano, A.; Mariti, C. The Impact of Chronic Inflammatory Enteropathy on Dogs’ Quality of Life and Dog-Owner Relationship. Vet. Sci. 2021, 8, 166. [Google Scholar] [CrossRef]
- Reid, J.; Wiseman-Orr, L.; Scott, M. Shortening of an Existing Generic Online Health-Related Quality of Life Instrument for Dogs. J. Small Anim. Pract. 2018, 59, 334–342. [Google Scholar] [CrossRef]
- Noli, C.; Colombo, S.; Cornegliani, L.; Ghibaudo, G.; Persico, P.; Vercelli, A.; Galzerano, M. Quality of Life of Dogs with Skin Disease and of Their Owners. Part 2: Administration of a Questionnaire in Various Skin Diseases and Correlation to Efficacy of Therapy. Vet. Dermatol. 2011, 22, 344–351. [Google Scholar] [CrossRef]
- Mariti, C.; Diverio, S.; Gutierrez, J.; Baragli, P.; Gazzano, A. Partial Analytic Validation of Determination of Cortisol in Dog Hair Using a Commercial EIA Kit. Dog Behav. 2020, 6, 1–15. [Google Scholar] [CrossRef]
- Alberghina, D.; Statelli, A.; Monteverde, V.; Vazzana, I.; Cascone, G.; Panzera, M. Serum Cortisol and Its Correlation with Leucocyte Profile and Circulating Lipids in Donkeys (Equus Asinus). Animals 2022, 12, 841. [Google Scholar] [CrossRef] [PubMed]
- de Kruijff, I.; Noppe, G.; Kieviet, N.; Choenni, V.; Lambregtse-van den Berg, M.P.; Begijn, D.G.A.; Tromp, E.; Dorst, K.; van Rossum, E.F.C.; de Rijke, Y.B.; et al. LC-MS/MS-Based Reference Intervals for Hair Cortisol in Healthy Children. Psychoneuroendocrinology 2020, 112, 104539. [Google Scholar] [CrossRef]
- Nicholson, S.L.; Meredith, J.E. Should Stress Management Be Part of the Clinical Care Provided to Chronically Ill Dogs? J. Vet. Behav. 2015, 10, 489–495. [Google Scholar] [CrossRef]
- Bowland, G.B.; Bernstein, R.M.; Koster, J.; Fiorello, C.; Brenn-White, M.; Liu, J.; Schwartz, L.; Campbell, A.; von Stade, D.; Beagley, J.; et al. Fur Color and Nutritional Status Predict Hair Cortisol Concentrations of Dogs in Nicaragua. Front. Vet. Sci. 2020, 7, 565346. [Google Scholar] [CrossRef]
- Packer, R.M.A.; Davies, A.M.; Volk, H.A.; Puckett, H.L.; Hobbs, S.L.; Fowkes, R.C. What Can We Learn from the Hair of the Dog? Complex Effects of Endogenous and Exogenous Stressors on Canine Hair Cortisol. PLoS ONE 2019, 14, e0216000. [Google Scholar] [CrossRef]
- Park, S.H.; Kim, S.A.; Shin, N.S.; Hwang, C.Y. Elevated Cortisol Content in Dog Hair with Atopic Dermatitis. Jpn. J. Vet. Res. 2016, 64, 123–129. [Google Scholar] [CrossRef]
- Roth, L.S.V.; Faresjö, Å.; Theodorsson, E.; Jensen, P. Hair Cortisol Varies with Season and Lifestyle and Relates to Human Interactions in German Shepherd Dogs. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef]
- van der Laan, J.E.; Vinke, C.M.; Arndt, S.S. Evaluation of Hair Cortisol as an Indicator of Long-Term Stress Responses in Dogs in an Animal Shelter and after Subsequent Adoption. Sci. Rep. 2022, 12, 19631. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Goy-Thollot, I.; Decosne-Junot, C.; Bonnet, J.M. Influence of Aging on Adrenal Responsiveness in a Population of Eleven Healthy Beagles. Res. Vet. Sci. 2007, 82, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Palazzolo, D.L.; Quadri, S.K. The Effects of Aging on the Circadian Rhythm of Serum Cortisol in the Dog. Exp. Gerontol. 1987, 22, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Svendsen, K.; Sondergaard, A. Hair and Saliva as Biomarkers for Stress Evaluation in Labrador Retrievers in Relation to HD Scores. Ph.D. Thesis, University of Copenhagen, Copenhagen, Denmark, 2014. [Google Scholar]
- Fourie, N.H.; Jolly, C.J.; Phillips-Conroy, J.E.; Brown, J.L.; Bernstein, R.M. Variation of Hair Cortisol Concentrations among Wild Populations of Two Baboon Species (Papio Anubis, P. Hamadryas) and a Population of Their Natural Hybrids. Primates 2015, 56, 259–272. [Google Scholar] [CrossRef]
- Chauntry, A.J.; Bishop, N.C.; Hamer, M.; Kingsnorth, A.P.; Chen, Y.-L.; Paine, N.J. Sedentary Behaviour Is Associated with Heightened Cardiovascular, Inflammatory and Cortisol Reactivity to Acute Psychological Stress. Psychoneuroendocrinology 2022, 141, 105756. [Google Scholar] [CrossRef]
- Nicolson, N.A.; Van Diest, R. Salivary Cortisol Patterns in Vital Exhaustion. J. Psychosom. Res. 2000, 49, 335–342. [Google Scholar] [CrossRef]
- Santos, N.S.; Domingues, T.D.; Tardo, A.M.; Dinis, M.; Mateus, L.; Fracassi, F.; Leal, R.O. Can We Predict Hypoadrenocorticism in Dogs with Resting Hypocortisolemia? A Predictive Model Based on Clinical, Haematological, and Biochemical Variables. Front. Vet. Sci. 2024, 11, 1523170. [Google Scholar] [CrossRef]
- Gallego, A.F.; Gow, A.G.; Boag, A.M. Evaluation of Resting Cortisol Concentration Testing in Dogs with Chronic Gastrointestinal Signs. J. Vet. Intern. Med. 2022, 36, 525–531. [Google Scholar] [CrossRef]
- Reagan, K.L.; McLarty, E.; Marks, S.L.; Sebastian, J.; McGill, J.; Gilor, C. Characterization of Clinicopathologic and Abdominal Ultrasound Findings in Dogs with Glucocorticoid Deficient Hypoadrenocorticism. J. Vet. Intern. Med. 2022, 36, 1947–1957. [Google Scholar] [CrossRef]
- Corradini, S.; Accorsi, P.A.; Boari, A.; Beghelli, V.; Mattioli, M.; Famigli-Bergamini, P.; Fracassi, F. Evaluation of Hair Cortisol in the Diagnosis of Hypercortisolism in Dogs. J. Vet. Intern. Med. 2013, 27, 1268–1272. [Google Scholar] [CrossRef]
- Van Uum, S.H.M.; Sauvé, B.; Fraser, L.A.; Morley-Forster, P.; Paul, T.L.; Koren, G. Elevated Content of Cortisol in Hair of Patients with Severe Chronic Pain: A Novel Biomarker for Stress. Stress 2008, 11, 483–488. [Google Scholar] [CrossRef]
- Wojtaś, J.; Garbiec, A.; Karpiński, M.; Skowronek, P.; Strachecka, A. Are Hair Cortisol Levels of Humans, Cats, and Dogs from the Same Household Correlated? Animals 2022, 12, 1472. [Google Scholar] [CrossRef] [PubMed]
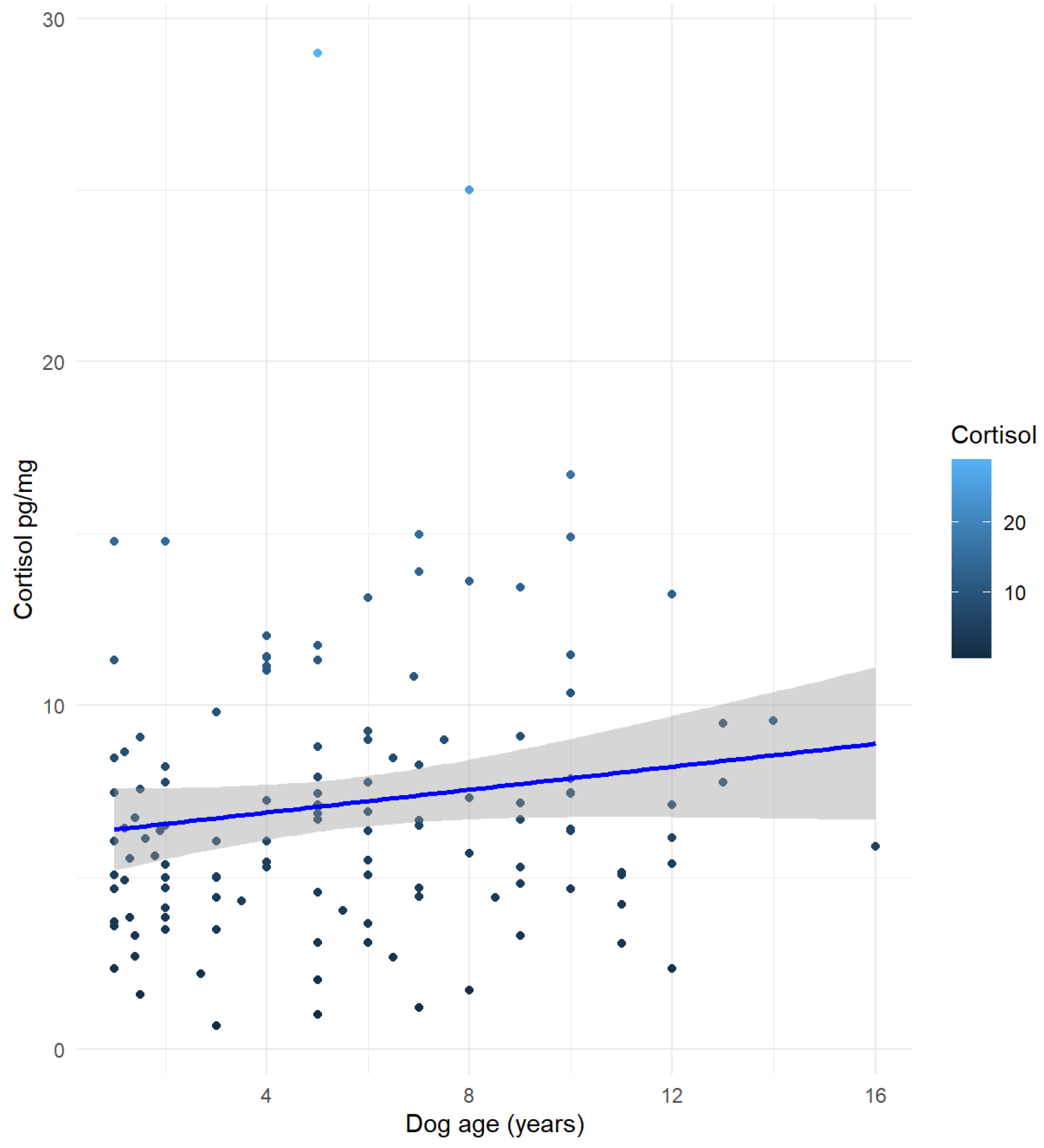
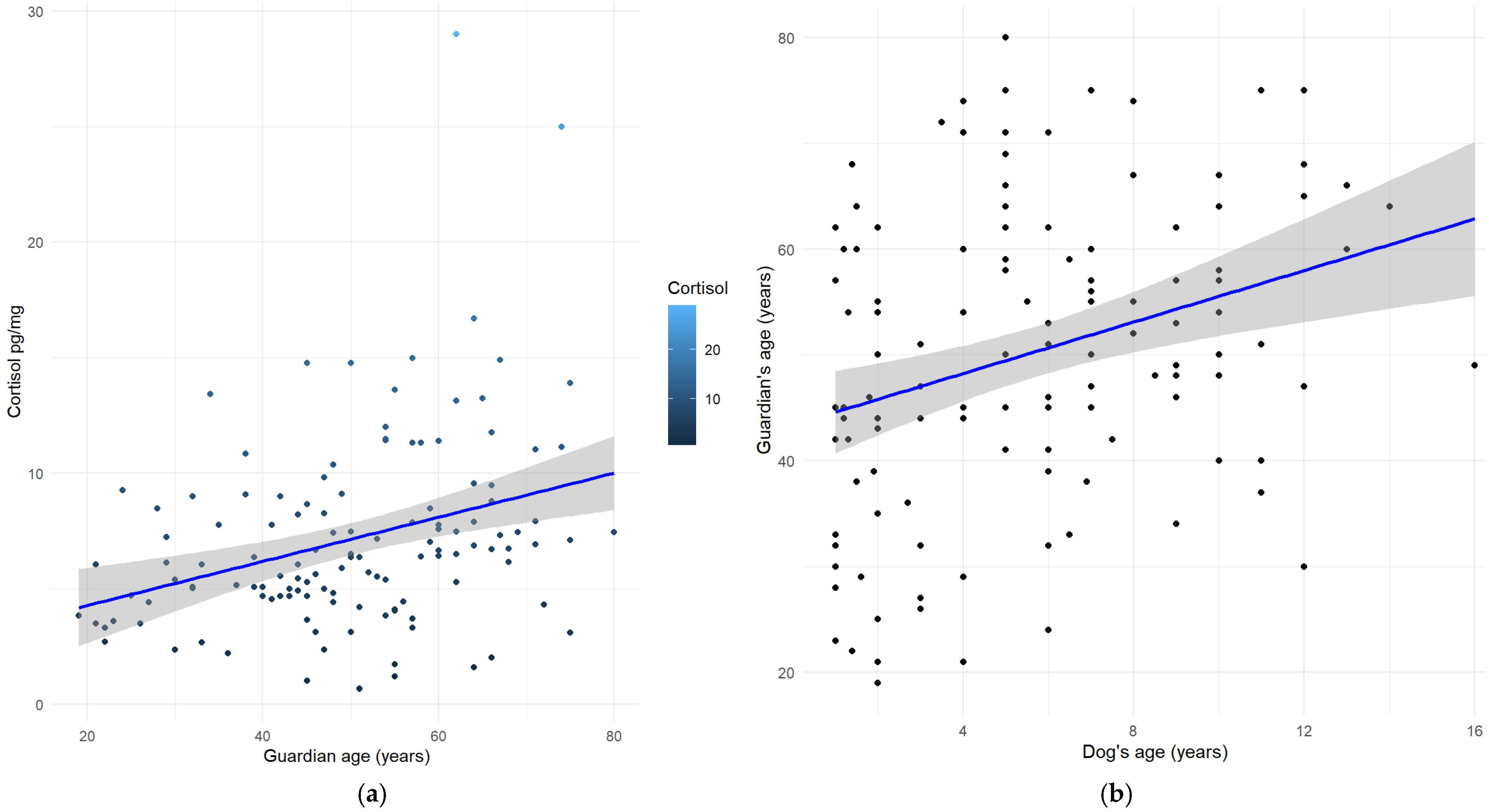
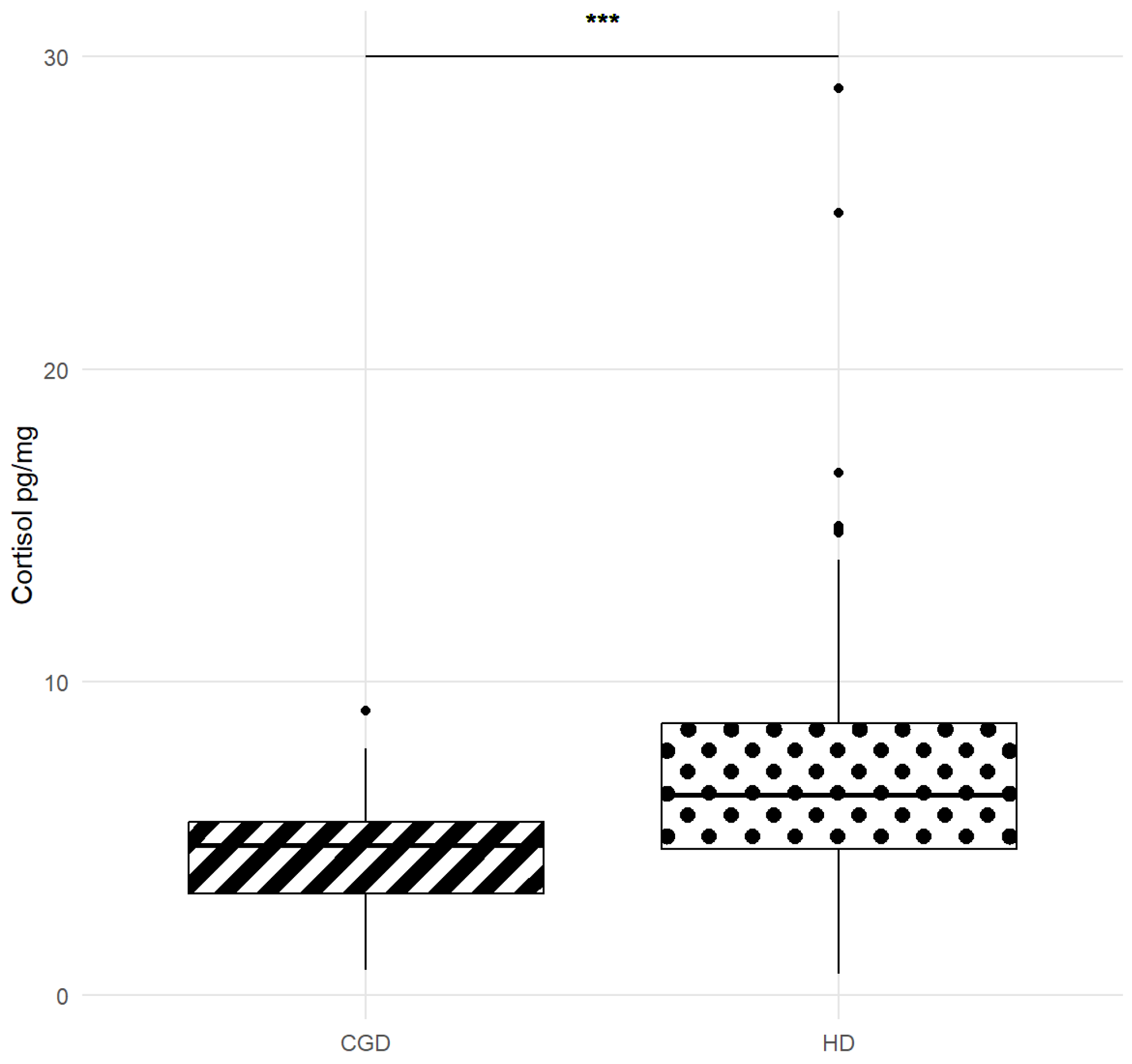
| Breed | Number of Dogs for Each Breed | Mean (±SD) |
|---|---|---|
| Mixed-Breed | 51 | 6.34 (±3.51) |
| Dachshund | 7 | 10.39 (±8.52) |
| Labrador Retriever Jack Russell Terrier Cocker Spaniel | 6 | 6.03 (±2.89) 8.46 (±2.04) 4.96 (±2.96) |
| Golden Retriever Poodle | 5 | 5.08 (±0.46) 8.72 (±4.78) |
| Australian Shepherd English Setter | 4 | |
| Épagneul Breton Maremmano Sheepdog German Shepherd Beagle | 3 | |
| Italian Spinone Bolognese Dog Basset Hound Belgian Shepherd Lagotto Romagnolo | 2 | |
| Great Dane Cavalier King Charles Spaniel German Wirehaired Pointer Dogo Argentino German Hunting Terrier Miniature Pinscher Shiba Inu Boxer Siberian Husky Italian Volpino Springer Spaniel Border Collie | 1 |
| HD Group | Guardian’s Age (Years) | Dog’s Age (Years) | Canine HCC (pg/mg) |
|---|---|---|---|
| Mean | 50.2 | 5.6 | 7.16 |
| Standard Deviation (SD) | 14.3 | 3.6 | 4.18 |
| Median | 50.0 | 5.0 | 6.41 |
| IQR1 | 41.8 | 2.0 | 4.66 |
| IQR3 | 60.5 | 8.1 | 8.69 |
| VAR | 204.2 | 13.0 | 17.44 |
| Min | 19.0 | 1.0 | 0.68 |
| Max | 80.0 | 16.0 | 28.98 |
| Range | 61.0 | 15.0 | 28.31 |
| Coefficient of Variation (CV) | 0.3 | 0.6 | 0.58 |
| Dogs (N = 128) | Canine HCC (pg/mg) | ||||
| N | % | Median (IQR1; IQR3) | Min | Max | |
| Sex | |||||
| Female (entire + neutered) Female entire Female neutered | 67 | 52.3 | 7.10 (4.67; 9.09) | 0.68 | 28.98 |
| 29 | 22.7 | 6.04 (4.66; 9.55) | 2.19 | 28.98 | |
| 38 | 29.7 | 7.10 (4.68; 9.06) | 0.68 | 25.00 | |
| Male (entire + neutered) Male entire Male neutered | 61 | 47.7 | 6.35 (4.55; 7.77) | 1.02 | 14.98 |
| 51 | 39.8 | 6.42 (5.03; 7.99) | 1.02 | 14.98 | |
| 10 | 7.8 | 5.26 (4.13; 6.48) | 1.72 | 11.48 | |
| Neutered (male + female) Entire (male + female) | 48 80 | 37.5 62.5 | 6.42 (4.61; 9.02) 6.41 (4.68; 8.31) | 0.68 1.02 | 25.00 28.98 |
| Guardian (N = 128) | Canine HCC (pg/mg) | ||||
| N | % | Median (IQR1; IQR3) | Min | Max | |
| Gender | |||||
| Female Male | 78 | 60.9 | 6.46 (4.58; 7.68) | 1.60 | 25.00 |
| 50 | 39.1 | 6.38(4.67; 9.06) | 0.68 | 28.98 | |
| Education level | |||||
| Elementary or Middle School High School Diploma Bachelor’s or higher Degree | 20 | 15.6 | 5.58 (3.83; 7.28) | 1.21 | 28.98 |
| 68 | 53.1 | 6.35 (4.68; 9.02) | 0.68 | 25.00 | |
| 40 | 31.3 | 7.13 (4.94; 9.28) | 2.19 | 16.70 | |
| Occupation | |||||
| Employed Self-employed professionals Retired Manual labourers Worked directly with animals Students Other | 30 | 23.4% | 7.81 (5.08; 9.10) | 2.36 | 14.76 |
| 23 | 18.0% | 6.67 (4.54; 8.21) | 1.72 | 25.00 | |
| 21 | 16.4% | 7.10 (6.14; 11.02) | 1.60 | 16.70 | |
| 19 | 14.8% | 6.42 (3.99; 7.84) | 0.68 | 14.76 | |
| 8 | 6.3% | 4.61 (3.48; 5.39) | 1.02 | 10.36 | |
| 5 | 3.9% | 3.59 (3.31; 3.83) | 2.69 | 6.04 | |
| 22 | 17.2% | 5.90 (4.67; 7.67) | 2.19 | 28.98 | |
| Variable | Estimate (β) | SE (Standard Error) | t-Value | Pr (>|t|) |
|---|---|---|---|---|
| Intercept | 1.619530 | 0.265339 | 6.104 | 1.27 × 10−8 *** |
| Dog’s age | 0.010134 | 0.015061 | 0.673 | 0.50228 |
| Dog’s sex | −0.053461 | 0.057238 | −0.934 | 0.35214 |
| Guardian’s age | 0.011936 | 0.003724 | 3.205 | 0.00172 ** |
| Occupation | −0.033424 | 0.024930 | −1.341 | 0.18250 |
| Education | −0.119572 | 0.079009 | −1.513 | 0.13276 |
| Variable | Estimate (β) | SE (Standard Error) | t-Value | Pr (>|t|) |
|---|---|---|---|---|
| Intercept | 1.514083 | 0.220353 | 6.871 | 2.74 × 10−10 *** |
| Guardian’s age | 0.012826 | 0.003492 | 3.672 | 0.000356 *** |
| Occupation | −0.038260 | 0.024364 | −1.570 | 0.118888 |
| Education | −0.113831 | 0.076526 | −1.487 | 0.139426 |
| Outcome | β (Cortisol) | SE | z | p-Value |
|---|---|---|---|---|
| Energetic/lively | 0.0486 | 0.0442 | 1.099 | 0.272 |
| Happy/satisfied | 0.0362 | 0.0431 | 0.840 | 0.401 |
| Active/serene | 0.0302 | 0.0421 | 0.718 | 0.473 |
| Calm/relaxed | −0.0350 | 0.0374 | −0.936 | 0.349 |
| Quality of life | 0.02603 | 0.0414 | 0.629 | 0.529 |
| Stress level | −0.03899 | 0.0393 | −0.991 | 0.322 |
| Anxiety level | −0.06730 | 0.0393 | −1.711 | 0.087(.) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariti, C.; Russo, G.; Mazzoni, C.; Borrelli, C.; Gori, E.; Habermaass, V.; Marchetti, V. Factors Affecting Hair Cortisol Concentration in Domestic Dogs: A Focus on Factors Related to Dogs and Their Guardians. Animals 2025, 15, 1901. https://doi.org/10.3390/ani15131901
Mariti C, Russo G, Mazzoni C, Borrelli C, Gori E, Habermaass V, Marchetti V. Factors Affecting Hair Cortisol Concentration in Domestic Dogs: A Focus on Factors Related to Dogs and Their Guardians. Animals. 2025; 15(13):1901. https://doi.org/10.3390/ani15131901
Chicago/Turabian StyleMariti, Chiara, Giulia Russo, Chiara Mazzoni, Carmen Borrelli, Eleonora Gori, Verena Habermaass, and Veronica Marchetti. 2025. "Factors Affecting Hair Cortisol Concentration in Domestic Dogs: A Focus on Factors Related to Dogs and Their Guardians" Animals 15, no. 13: 1901. https://doi.org/10.3390/ani15131901
APA StyleMariti, C., Russo, G., Mazzoni, C., Borrelli, C., Gori, E., Habermaass, V., & Marchetti, V. (2025). Factors Affecting Hair Cortisol Concentration in Domestic Dogs: A Focus on Factors Related to Dogs and Their Guardians. Animals, 15(13), 1901. https://doi.org/10.3390/ani15131901







