Transcriptome Analyses Reveal the Molecular Response of Juvenile Greater Amberjack (Seriola dumerili) to Marine Heatwaves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
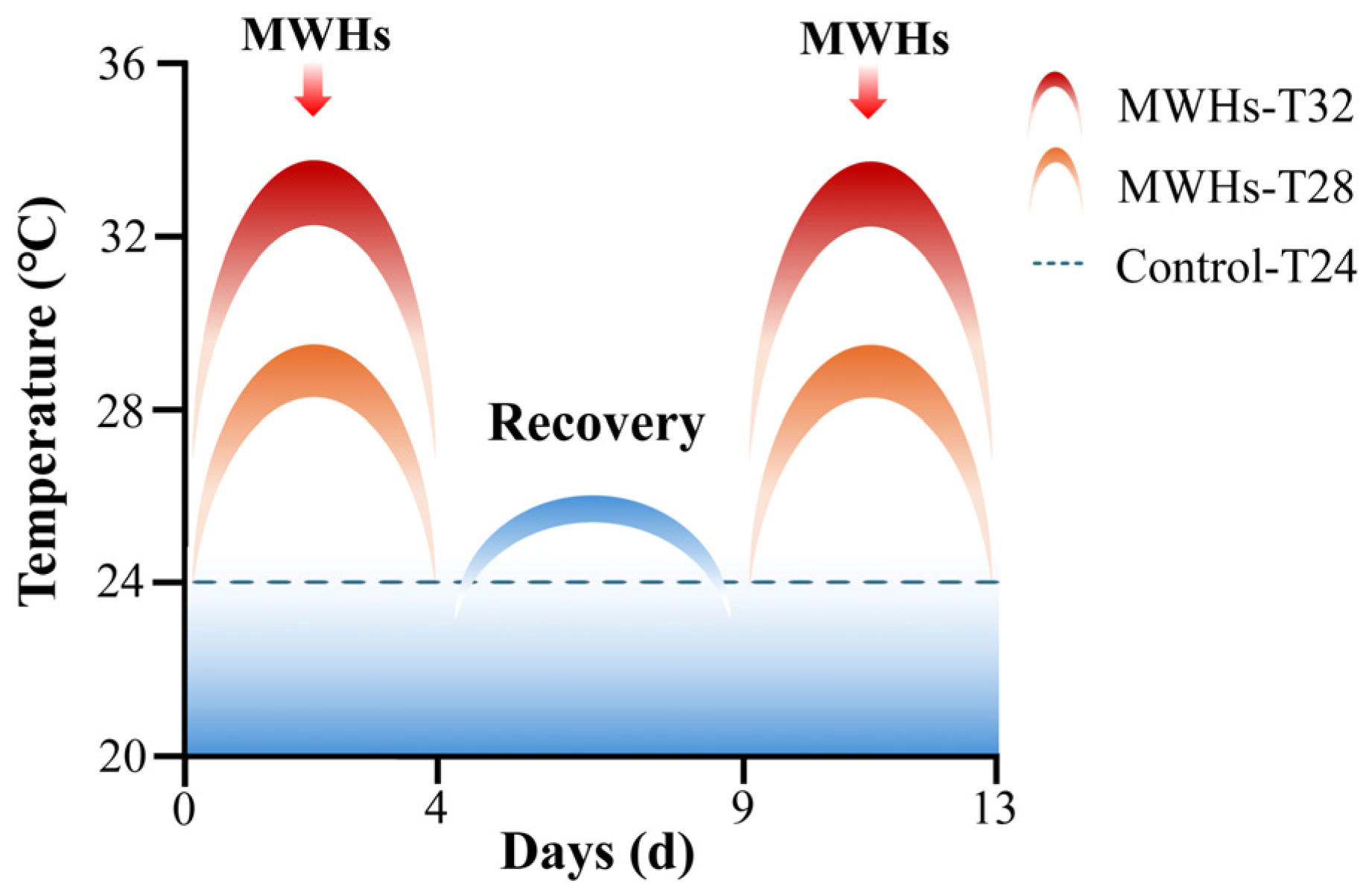
2.1. Experimental Design
2.2. Transcriptome Analysis
2.3. Quantitative Real-Time Reverse Transcription Polymerase Chain Reaction Validation
3. Results
3.1. Transcriptome Sequencing Quality
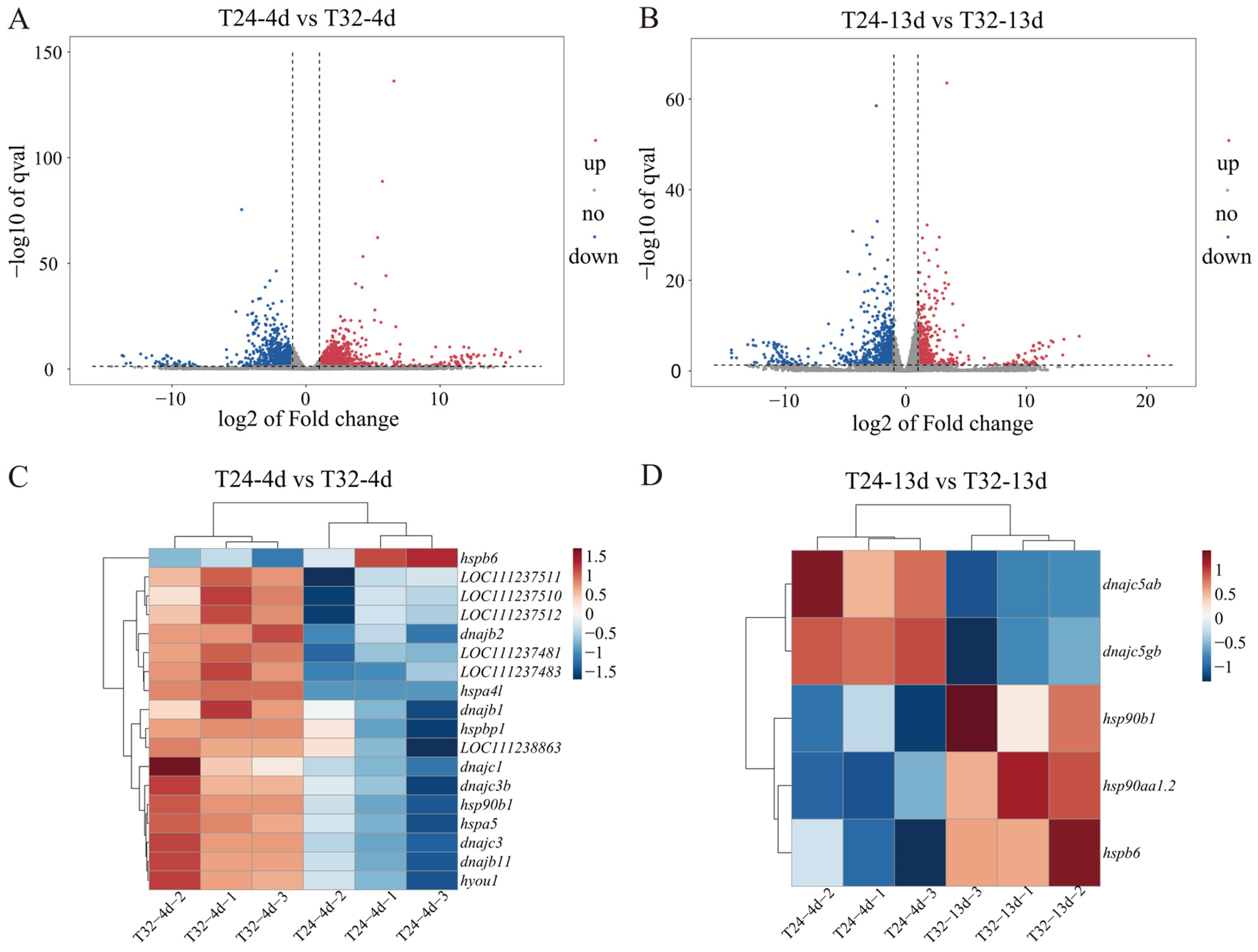
3.2. DEG Identification and Analysis
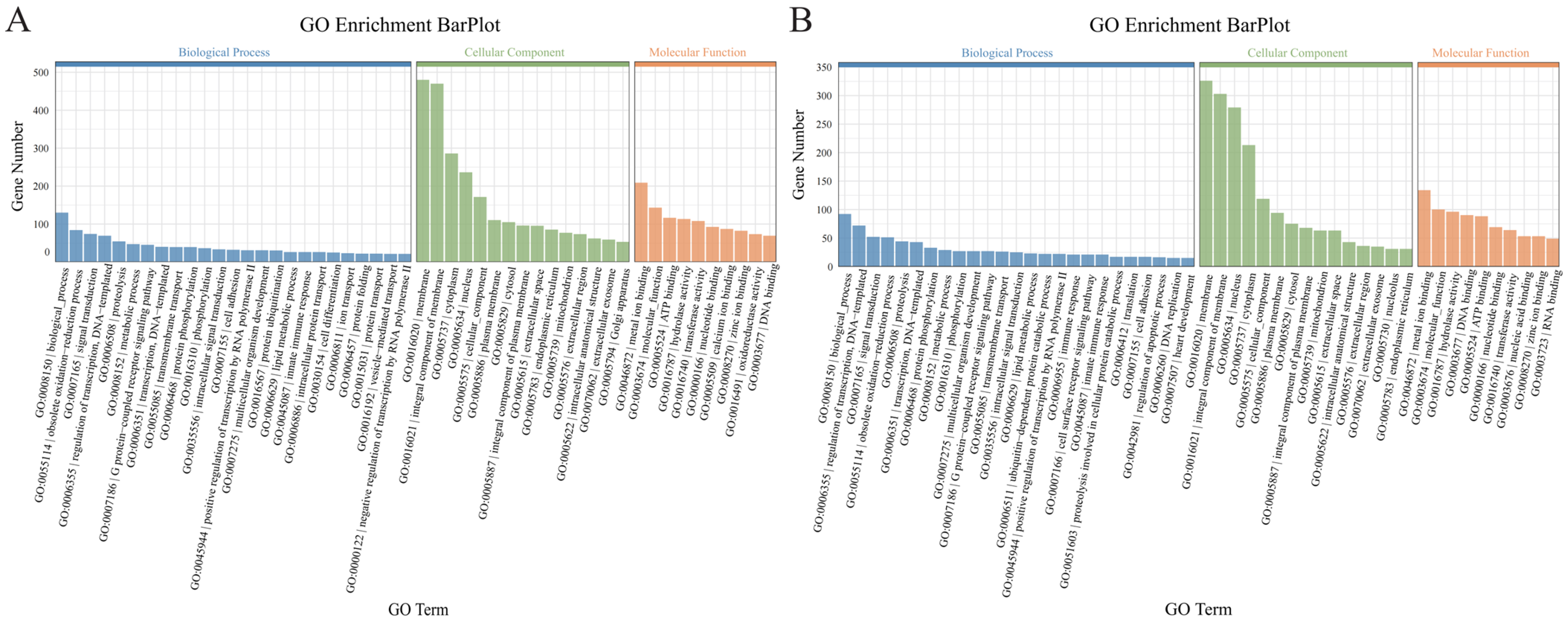
3.3. GO Enrichment Analysis
3.4. KEGG Pathway Enrichment Analysis
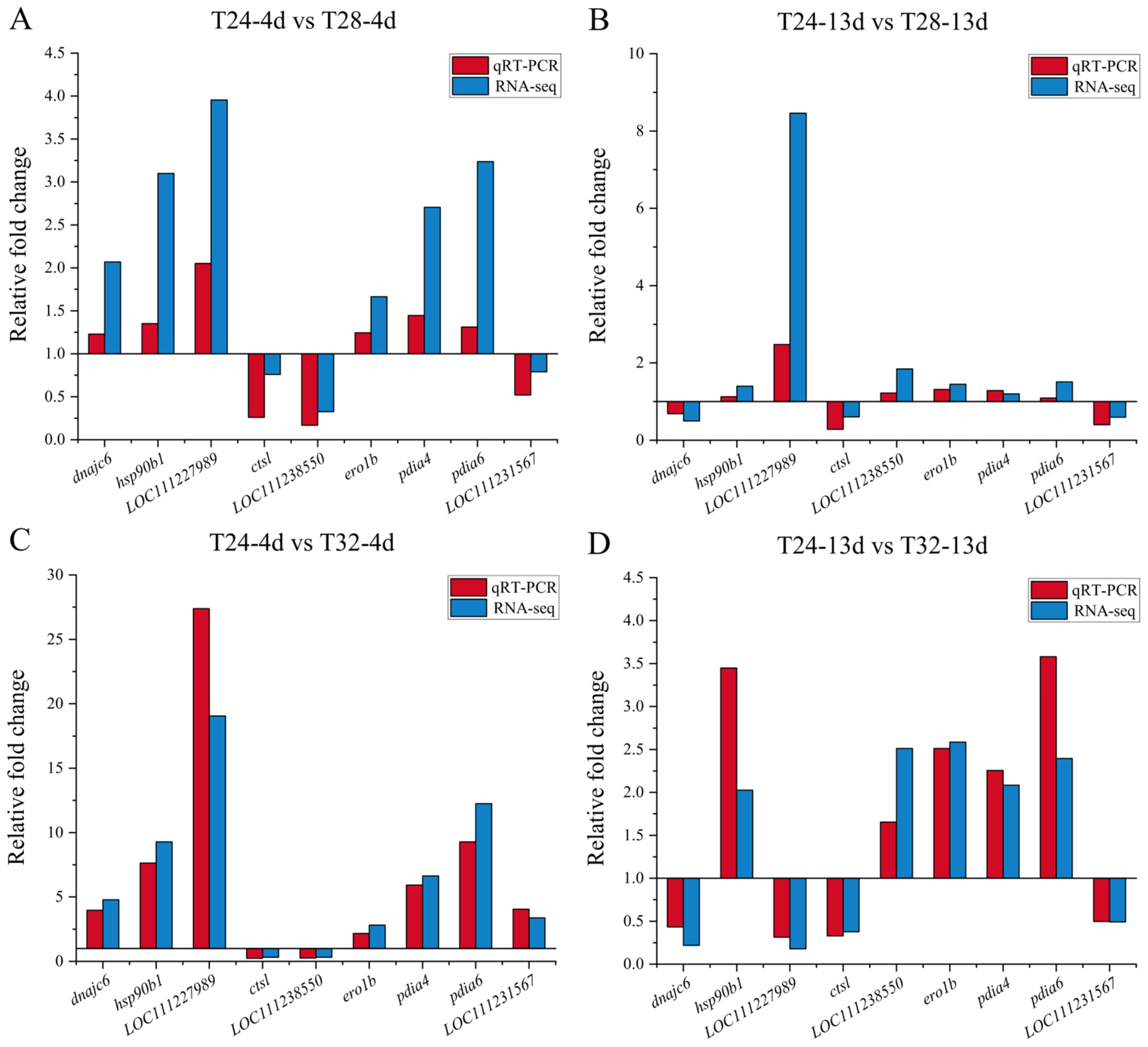
3.5. Validation of Transcriptome Data Using qRT-PCR
4. Discussion
4.1. Heat Shock Proteins’ Response to MHWs
4.2. Oxidative Stress Under MHWs
4.3. Immune Response to MWHs
4.4. Energy Metabolism Under MHWs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, W.F. Ocean warming and warning. Nat. Clim. Change 2024, 14, 118–119. [Google Scholar] [CrossRef]
- Pereira, L.A.L.; Amanajás, R.D.; De Oliveira, A.M.; Paula Da Silva, M.D.N.; Val, A.L. Health of the amazonian fish tambaqui (Colossoma macropomum): Effects of prolonged photoperiod and high temperature. Aquaculture 2021, 541, 736836. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Jiang, M.; Cai, X.F.; Zhang, J.; Lin, L.S.; Liu, X.D. iTRAQ-based quantitative proteome analysis in liver of large yellow croaker Larimichthys crocea under high temperature stress. Aquac. Rep. 2023, 28, 101444. [Google Scholar] [CrossRef]
- Zhang, C.S.; Li, F.H.; Xiang, J.H. Effect of temperature on the standard metabolic rates of juvenile and adult Exopalaemon carinicauda. Chin. J. Ocean. Limnol. 2015, 33, 381–388. [Google Scholar] [CrossRef]
- Luo, L.; Zhao, Z.G.; Zhang, R.; Guo, K.; Wang, S.H.; Xu, W.; Wang, C.A. The effects of temperature changes on the isozyme and Hsp70 levels of the amur sturgeon, Acipenser schrenckii, at two acclimation temperatures. Aquaculture 2022, 551, 737743. [Google Scholar] [CrossRef]
- Zhao, H.; Ke, H.Y.; Zhang, L.; Zhao, Z.M.; Lai, J.S.; Zhou, J.; Huang, Z.P.; Li, H.D.; Du, J.; Li, Q. Integrated analysis about the effects of heat stress on physiological responses and energy metabolism in Gymnocypris chilianensis. Sci. Total Environ. 2022, 806, 151252. [Google Scholar] [CrossRef]
- Dettleff, P.; Toloza, C.; Fuentes, M.; Aedo, J.; Zuloaga, R.; Estrada, J.M.; Molina, A.; Valdés, J.A. Gills de novo assembly reveals oxidative stress, unfolded protein, and immune response on red cusk-eel (Genypterus chilensis) under thermal stress. Mar. Environ. Res. 2024, 196, 106440. [Google Scholar] [CrossRef]
- Somero, G.N. The cellular stress response and temperature: Function, regulation, and evolution. J. Exp. Zool. Part A 2020, 333, 379–397. [Google Scholar] [CrossRef]
- Mridul, M.M.I.; Zeehad, M.S.K.; Aziz, D.; Salin, K.R.; Hurwood, D.A.; Rahi, M.L. Temperature induced biological alterations in the major carp, rohu (Labeo rohita): Assessing potential effects of climate change on aquaculture production. Aquac. Rep. 2024, 35, 101954. [Google Scholar] [CrossRef]
- Islam, M.J.; Kunzmann, A.; Slater, M.J. Responses of aquaculture fish to climate change-induced extreme temperatures: A review. J. World Aquac. Soc. 2022, 53, 314–366. [Google Scholar] [CrossRef]
- Steiner, K.; Laroche, O.; Walker, S.P.; Symonds, J.E. Effects of water temperature on the gut microbiome and physiology of chinook salmon (Oncorhynchus tshawytscha) reared in a freshwater recirculating system. Aquaculture 2022, 560, 738529. [Google Scholar] [CrossRef]
- Hobday, A.J.; Alexander, L.V.; Perkins, S.E.; Smale, D.A.; Straub, S.C.; Oliver, E.C.J.; Benthuysen, J.A.; Burrows, M.T.; Donat, M.G.; Feng, M.; et al. A hierarchical approach to defining marine heatwaves. Prog. Oceanogr. 2016, 141, 227–238. [Google Scholar] [CrossRef]
- Laufkötter, C.; Zscheischler, J.; Frölicher, T.L. High-impact marine heatwaves attributable to human-induced global warming. Science 2020, 369, 1621–1625. [Google Scholar] [CrossRef] [PubMed]
- Oliver, E.C.J.; Donat, M.G.; Burrows, M.T.; Moore, P.J.; Smale, D.A.; Alexander, L.V.; Benthuysen, J.A.; Feng, M.; Sen Gupta, A.; Hobday, A.J.; et al. Longer and more frequent marine heatwaves over the past century. Nat. Commun. 2018, 9, 1324. [Google Scholar] [CrossRef]
- Smale, D.A.; Wernberg, T.; Oliver, E.C.J.; Thomsen, M.; Harvey, B.P.; Straub, S.C.; Burrows, M.T.; Alexander, L.V.; Benthuysen, J.A.; Donat, M.G.; et al. Marine heatwaves threaten global biodiversity and the provision of ecosystem services. Nat. Clim. Change 2019, 9, 306–312. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.G.; Gambi, M.C.; Kersting, D.K.; et al. Mass mortality in Northwestern Mediterranean rocky benthic communities: Effects of the 2003 heat wave. Glob. Change Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Donovan, M.K.; Burkepile, D.E.; Kratochwill, C.; Shlesinger, T.; Sully, S.; Oliver, T.A.; Hodgson, G.; Freiwald, J.; Van Woesik, R. Local conditions magnify coral loss after marine heatwaves. Science 2021, 372, 977–980. [Google Scholar] [CrossRef]
- Sen Gupta, A.; Thomsen, M.; Benthuysen, J.A.; Hobday, A.J.; Oliver, E.; Alexander, L.V.; Burrows, M.T.; Donat, M.G.; Feng, M.; Holbrook, N.J.; et al. Drivers and impacts of the most extreme marine heatwave events. Sci. Rep. 2020, 10, 19359. [Google Scholar] [CrossRef]
- Kajtar, J.B.; Holbrook, N.J.; Lyth, A.; Hobday, A.J.; Mundy, C.N.; Ugalde, S.C. A stakeholder-guided marine heatwave hazard index for fisheries and aquaculture. Clim. Change 2024, 177, 26. [Google Scholar] [CrossRef]
- Galli, G.; Solidoro, C.; Lovato, T. Marine heat waves hazard 3D maps and the risk for low motility organisms in a warming Mediterranean Sea. Front. Mar. Sci. 2017, 4, 136. [Google Scholar] [CrossRef]
- Holbrook, N.J.; Sen Gupta, A.; Oliver, E.C.J.; Hobday, A.J.; Benthuysen, J.A.; Scannell, H.A.; Smale, D.A.; Wernberg, T. Keeping pace with marine heatwaves. Nat. Rev. Earth Environ. 2020, 1, 482–493. [Google Scholar] [CrossRef]
- Tian, Y.L.; Li, H.; Zhang, D.Y.; Wang, C.; Hao, R.J.; Ru, X.Y.; Hu, Q.; Huang, Y.; Zhu, C.H. Effect of marine heatwaves on juvenile greater amberjack (Seriola dumerili). Mar. Environ. Res. 2024, 193, 106302. [Google Scholar] [CrossRef] [PubMed]
- Corriero, A.; Wylie, M.J.; Nyuji, M.; Zupa, R.; Mylonas, C.C. Reproduction of greater amberjack (Seriola dumerili) and other members of the family carangidae. Rev. Aquacult. 2021, 13, 1781–1815. [Google Scholar] [CrossRef]
- Fernández-Montero, A.; Caballero, M.J.; Torrecillas, S.; Tuset, V.M.; Lombarte, A.; Ginés, R.R.; Izquierdo, M.; Robaina, L.; Montero, D. Effect of temperature on growth performance of greater amberjack (Seriola dumerili Risso 1810) juveniles. Aquac. Res. 2018, 49, 908–918. [Google Scholar] [CrossRef]
- Araki, K.; Aokic, J.; Kawase, J.; Hamada, K.; Ozaki, A.; Fujimoto, H.; Yamamoto, I.; Usuki, H. Whole genome sequencing of greater amberjack (Seriola dumerili) for SNP identification on aligned scaffolds and genome structural variation analysis using parallel resequencing. Int. J. Genom. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Lyu, F.; Han, F.R.; Ge, C.L.; Mao, W.K.; Chen, L.; Hu, H.P.; Chen, G.G.; Lang, Q.L.; Fang, C. OmicStudio: A composable bioinformatics cloud platform with real-time feedback that can generate high-quality graphs for publication. iMeta 2023, 2, e85. [Google Scholar] [CrossRef]
- Fuentes, J.; Fonseca, F.; Gregorio, S.F.; Kussaba, L.; Perera, E.; Alarcón-López, F.J.; Martos-Sitcha, J.A. High plant protein diet impairs growth performance and intestinal integrity in greater amberjack (Seriola dumerili): Molecular and physiological insights. Aquaculture 2025, 597, 741925. [Google Scholar] [CrossRef]
- Andersen, C.L.; Jensen, J.L.; Ørntoft, T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Lindquist, S. The heat-shock response. Annu. Rev. Biochem. 1986, 55, 1151–1191. [Google Scholar] [CrossRef]
- Schneider, C.; Sepp-Lorenzino, L.; Nimmesgern, E.; Ouerfelli, O.; Danishefsky, S.; Rosen, N.; Hartl, F.U. Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90. Proc. Natl. Acad. Sci. USA 1996, 93, 14536–14541. [Google Scholar] [CrossRef]
- Lei, L.; Yu, J.M.; Bao, E.D. Expression of heat shock protein 90 (Hsp90) and transcription of its corresponding mRNA in broilers exposed to high temperature. Br. Poult. Sci. 2009, 50, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Masanja, F.; Xu, Y.; He, G.X.; Liang, F.L.; Liu, X.L.; Yang, K.; Mkuye, R.; Liang, J.; Deng, Y.W.; Zhao, L.Q. Exploring HSP90 as a biomarker for marine heatwaves in Pinctada maxima. Front. Mar. Sci. 2022, 9, 913920. [Google Scholar] [CrossRef]
- Mayer, M.P.; Bukau, B. Hsp70 Chaperones: Cellular functions and molecular mechanism. CMLS Cell. Mol. Life Sci. 2005, 62, 670. [Google Scholar] [CrossRef]
- Qiu, X.B.; Shao, Y.M.; Miao, S.; Wang, L. The diversity of the DnaJ/Hsp40 family, the crucial partners for Hsp70 chaperones. Cell. Mol. Life Sci. 2006, 63, 2560–2570. [Google Scholar] [CrossRef]
- Li, X.R.; Zhu, M. Genome-wide identification of the Hsp70 gene family in Penaeus chinensis and their response to environmental stress. Anim. Biotechnol. 2024, 35, 2344205. [Google Scholar] [CrossRef]
- Jacob, P.; Hirt, H.; Bendahmane, A. The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotech. J. 2017, 15, 405–414. [Google Scholar] [CrossRef]
- Liu, X.X.; Shi, H.N.; Liu, Z.; Kang, Y.J.; Wang, J.F.; Huang, J.Q. Effect of heat stress on heat shock protein 30 (Hsp30) mRNA expression in rainbow trout (Oncorhynchus mykiss). Turk. J. Fish. Aquat. Sci. 2019, 19, 681–688. [Google Scholar]
- Corey, E.; Linnansaari, T.; Cunjak, R.A.; Currie, S. Physiological effects of environmentally relevant, multi-day thermal stress on wild juvenile Atlantic salmon (Salmo salar). Conserv. Physiol. 2017, 5, cox014. [Google Scholar] [CrossRef]
- Dammark, K.B.; Ferchaud, A.-L.; Hansen, M.M.; Sørensen, J.G. Heat tolerance and gene expression responses to heat stress in threespine sticklebacks from ecologically divergent environments. J. Therm. Biol. 2018, 75, 88–96. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Wu, X.Y.; Li, P.C.; Liu, Y.; Song, M.J.; Li, F.Y.; Ou, J.; Lai, J.S. Integrated metabolomic and transcriptomic responses to heat stress in a high-altitude fish, Triplophysa siluroides. Fish Shellfish Immun. 2023, 142, 109118. [Google Scholar] [CrossRef]
- Alderson, T.R.; Kim, J.H.; Markley, J.L. Dynamical structures of Hsp70 and Hsp70-Hsp40 complexes. Structure 2016, 24, 1014–1030. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.J.; Jin, E.H.; Yin, Q.R.; Che, C.Y.; He, S.J. Boron attenuates heat stress–induced apoptosis by inhibiting endoplasmic reticulum stress in mouse granulosa cells. Biol. Trace Elem. Res. 2021, 199, 611–621. [Google Scholar] [CrossRef] [PubMed]
- Shergalis, A.G.; Hu, S.A.; Bankhead, A.; Neamati, N. Role of the ERO1-PDI interaction in oxidative protein folding and disease. Pharmacol. Therapeut. 2020, 210, 107525. [Google Scholar] [CrossRef] [PubMed]
- Vembar, S.S.; Brodsky, J.L. One Step at a Time: Endoplasmic reticulum-associated degradation. Nat. Rev. Mol. Cell Biol. 2008, 9, 944–957. [Google Scholar] [CrossRef]
- Oda, Y.; Okada, T.; Yoshida, H.; Kaufman, R.J.; Nagata, K.; Mori, K. Derlin-2 and Derlin-3 are regulated by the mammalian unfolded protein response and are required for ER-associated degradation. J. Cell Biol. 2006, 172, 383–393. [Google Scholar] [CrossRef]
- Ng, C.L.; Oresic, K.; Tortorella, D. TRAM1 is involved in disposal of ER membrane degradation substrates. Exp. Cell Res. 2010, 316, 2113–2122. [Google Scholar] [CrossRef]
- Hagiwara, M.; Ling, J.J.; Koenig, P.-A.; Ploegh, H.L. Posttranscriptional regulation of glycoprotein quality control in the endoplasmic reticulum is controlled by the E2 Ub-conjugating enzyme UBC6e. Mol. Cell. 2016, 63, 753–767. [Google Scholar] [CrossRef]
- Visudtiphole, V.; Watthanasurorot, A.; Klinbunga, S.; Menasveta, P.; Kirtikara, K. Molecular characterization of calreticulin: A biomarker for temperature stress responses of the giant tiger shrimp Penaeus monodon. Aquaculture 2010, 308, S100–S108. [Google Scholar] [CrossRef]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef]
- Shi, K.P.; Dong, S.L.; Zhou, Y.G.; Li, Y.; Gao, Q.F.; Sun, D.J. RNA-Seq reveals temporal differences in the transcriptome response to acute heat stress in the Atlantic salmon (Salmo salar). Comp. Biochem. Physiol. D Genom. Proteom. 2019, 30, 169–178. [Google Scholar] [CrossRef]
- Yang, X.; Wang, L.; Lu, K.L.; Li, X.S.; Song, K.; Zhang, C.X. High temperature changes the structure and function of spotted seabass (Lateolabrax maculatus) tissues and causes ER stress and mitochondrial homeostasis imbalance in liver. Aquaculture 2025, 599, 742107. [Google Scholar] [CrossRef]
- Zeeshan, H.; Lee, G.; Kim, H.-R.; Chae, H.-J. Endoplasmic reticulum stress and associated ROS. Int. J. Mol. Sci. 2016, 17, 327. [Google Scholar] [CrossRef]
- Zeng, C.; Sun, H.; Xie, P.; Wang, J.H.; Zhang, G.R.; Chen, N.; Yan, W.; Li, G.Y. The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 2014, 149, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [Google Scholar] [CrossRef]
- Topal, A.; Özdemir, S.; Arslan, H.; Çomaklı, S. How does elevated water temperature affect fish brain? (A neurophysiological and experimental study: Assessment of brain derived neurotrophic factor, cFOS, apoptotic genes, heat shock genes, ER-Stress genes and oxidative stress genes). Fish Shellfish Immun. 2021, 115, 198–204. [Google Scholar] [CrossRef]
- Liu, E.G.; Zhao, X.Q.; Li, C.J.; Wang, Y.F.; Li, L.L.; Zhu, H.; Ling, Q.F. Effects of acute heat stress on liver damage, apoptosis and inflammation of pikeperch (Sander lucioperca). J. Therm. Biol. 2022, 106, 103251. [Google Scholar] [CrossRef]
- Zong, W.X.; Li, C.; Hatzivassiliou, G.; Lindsten, T.; Yu, Q.C.; Yuan, J.Y.; Thompson, C.B. Bax and Bak can localize to the endoplasmic reticulum to initiate apoptosis. J. Cell Biol. 2003, 162, 59–69. [Google Scholar] [CrossRef]
- Okumoto, K.; Tamura, S.; Honsho, M.; Fujiki, Y. Peroxisome: Metabolic functions and biogenesis. In Peroxisome Biology: Experimental Models, Peroxisomal Disorders and Neurological Diseases; Lizard, G., Ed.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1299, pp. 3–17. ISBN 978-3-030-60203-1. [Google Scholar]
- Chang, C.H.; Mayer, M.; Rivera-Ingraham, G.; Blondeau-Bidet, E.; Wu, W.Y.; Lorin-Nebel, C.; Lee, T.H. Effects of temperature and salinity on antioxidant responses in livers of temperate (Dicentrarchus labrax) and tropical (Chanos chanos) marine euryhaline fish. J. Therm. Biol. 2021, 99, 103016. [Google Scholar] [CrossRef]
- Pei, J.; Pan, X.Y.; Wei, G.H.; Hua, Y. Research progress of glutathione peroxidase family (GPX) in redoxidation. Front. Pharmacol. 2023, 14, 1147414. [Google Scholar] [CrossRef]
- Mariana, S.; Badr, G. Impact of heat stress on the immune response of fishes. J. Surv. Fish. Sci. 2019, 5, 149–159. [Google Scholar] [CrossRef]
- Yang, C.; Dong, J.J.; Sun, C.F.; Li, W.H.; Tian, Y.Y.; Liu, Z.G.; Gao, F.Y.; Ye, X. Exposure to heat stress causes downregulation of immune response genes and weakens the disease resistance of Micropterus salmoides. Comp. Biochem. Physiol. D Genom. Proteom. 2022, 43, 101011. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, B.R. Structure of fish toll-like receptors (TLR) and nod-like receptors (NLR). Int. J. Biol. Macromol. 2020, 161, 1602–1617. [Google Scholar] [CrossRef] [PubMed]
- Esam, F.; Khalafalla, M.M.; Gewaily, M.S.; Abdo, S.; Hassan, A.M.; Dawood, M.A.O. Acute ammonia exposure combined with heat stress impaired the histological features of gills and liver tissues and the expression responses of immune and antioxidative related genes in Nile tilapia. Ecotox. Environ. Saf. 2022, 231, 113187. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.Y.; Wang, J.; Ren, W.H.; Zheng, S.J.; Ren, Y.C. Histological, immune, and intestine microbiota responses of the intestine of rainbow trout (Oncorhynchus mykiss) to high temperature stress. Aquaculture 2024, 582, 740465. [Google Scholar] [CrossRef]
- Zou, J.; Secombes, C.J. Teleost fish interferons and their role in immunity. Dev. Comp. Immunol. 2011, 35, 1376–1387. [Google Scholar] [CrossRef]
- Langevin, C.; Aleksejeva, E.; Passoni, G.; Palha, N.; Levraud, J.-P.; Boudinot, P. The antiviral innate immune response in fish: Evolution and conservation of the IFN system. J. Mol. Biol. 2013, 425, 4904–4920. [Google Scholar] [CrossRef]
- Xia, J.; Hu, G.B.; Dong, X.Z.; Liu, Q.M.; Zhang, S.C. Molecular characterization and expression analysis of interferon regulatory factor 5 (IRF-5) in turbot, Scophthalmus maximus. Fish Shellfish Immun. 2012, 32, 211–218. [Google Scholar] [CrossRef]
- Zhu, K.C.; Guo, H.Y.; Zhang, N.; Liu, B.S.; Guo, L.; Jiang, S.G.; Zhang, D.C. Functional characterization of IRF8 regulation of type II IFN in golden pompano (Trachinotus ovatus). Fish Shellfish Immun. 2019, 94, 1–9. [Google Scholar] [CrossRef]
- Costas, B.; Conceição, L.E.C.; Aragão, C.; Martos, J.A.; Ruiz-Jarabo, I.; Mancera, J.M.; Afonso, A. Physiological responses of senegalese sole (Solea senegalensis Kaup, 1858) after stress challenge: Effects on non-specific immune parameters, plasma free amino acids and energy metabolism. Aquaculture 2011, 316, 68–76. [Google Scholar] [CrossRef]
- Wang, W.N.; Wang, A.L.; Liu, Y.; Xiu, J.; Liu, Z.B.; Sun, R.Y. Effects of temperature on growth, adenosine phosphates, ATPase and cellular defense response of juvenile shrimp Macrobrachium nipponense. Aquaculture 2006, 256, 624–630. [Google Scholar] [CrossRef]
- Yang, S.S.; Zhao, T.T.; Ma, A.J.; Huang, Z.H.; Liu, Z.F.; Cui, W.X.; Zhang, J.S.; Zhu, C.Y.; Guo, X.L.; Yuan, C.H. Metabolic responses in Scophthalmus maximus kidney subjected to thermal stress. Fish Shellfish Immun. 2020, 103, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.T.; Ma, A.J.; Huang, Z.H.; Liu, Z.F.; Sun, Z.B.; Zhu, C.Y.; Yang, J.K.; Li, Y.D.; Wang, Q.M.; Qiao, X.W.; et al. Transcriptome analysis reveals that high temperatures alter modes of lipid metabolism in juvenile turbot (Scophthalmus maximus) liver. Comp. Biochem. Physiol. D Genom. Proteom. 2021, 40, 100887. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Wafeek, M. Fluctuations in water temperature affected waterborne cadmium toxicity: Hematology, anaerobic glucose pathway, and oxidative stress status of Nile Tilapia, Oreochromis niloticus (L.). Aquaculture 2017, 477, 106–111. [Google Scholar] [CrossRef]
- Carvalho, C.D.S.; Fernandes, M.N. Effect of copper on liver key enzymes of anaerobic glucose metabolism from freshwater tropical fish Prochilodus lineatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008, 151, 437–442. [Google Scholar] [CrossRef]
- Liang, H.L.; Xu, P.; Xu, G.C.; Zhang, L.; Huang, D.Y.; Ren, M.C.; Zhang, L. Histidine deficiency inhibits intestinal antioxidant capacity and induces intestinal endoplasmic-reticulum stress, inflammatory response, apoptosis, and necroptosis in largemouth bass (Micropterus salmoides). Antioxidants 2022, 11, 2399. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Sukhotin, A.A. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Sokolova, I.M. Energy-limited tolerance to stress as a conceptual framework to integrate the effects of multiple stressors. Integr. Comp. Biol. 2013, 53, 597–608. [Google Scholar] [CrossRef]
- Petitjean, Q.; Jean, S.; Gandar, A.; Côte, J.; Laffaille, P.; Jacquin, L. Stress responses in fish: From molecular to evolutionary processes. Sci. Total Environ. 2019, 684, 371–380. [Google Scholar] [CrossRef]
- Lu, Y.L.; Wu, Z.H.; Song, Z.C.; Xiao, P.; Liu, Y.; Zhang, P.J.; You, F. Insight into the heat resistance of fish via blood: Effects of heat stress on metabolism, oxidative stress and antioxidant response of olive flounder Paralichthys olivaceus and turbot Scophthalmus maximus. Fish Shellfish Immun. 2016, 58, 125–135. [Google Scholar] [CrossRef]

| Samples | Raw Reads | Clean Reads | Q20/% | Q30/% | Genomic Map Rate/% | GC Content% |
|---|---|---|---|---|---|---|
| T24-4d-1 | 51249386 | 48987888 | 99.56 | 97.45 | 94.76 | 48.5 |
| T24-4d-2 | 52275924 | 50194262 | 99.6 | 97.54 | 94.20 | 48.5 |
| T24-4d-3 | 50887318 | 48704476 | 99.58 | 97.59 | 93.90 | 48.5 |
| T24-13d-1 | 46950386 | 44240394 | 99.47 | 97.26 | 95.21 | 48.5 |
| T24-13d-2 | 49151540 | 46482634 | 99.51 | 97.44 | 94.14 | 48.0 |
| T24-13d-3 | 48907528 | 46191450 | 99.55 | 97.51 | 95.65 | 48.5 |
| T28-4d-1 | 53211760 | 51101020 | 97.53 | 96.03 | 94.99 | 48.5 |
| T28-4d-2 | 50579226 | 48429602 | 97.36 | 95.75 | 94.18 | 48.5 |
| T28-4d-3 | 51388726 | 49573704 | 97.24 | 96.47 | 94.64 | 48.5 |
| T28-13d-1 | 49129624 | 46689354 | 97.45 | 95.03 | 95.59 | 48.5 |
| T28-13d-2 | 51853392 | 49637240 | 97.35 | 95.73 | 95.17 | 48.5 |
| T28-13d-3 | 54456140 | 52704094 | 98.08 | 96.78 | 96.69 | 48.5 |
| T32-4d-1 | 49767488 | 47571478 | 99.56 | 97.48 | 95.49 | 48.5 |
| T32-4d-2 | 46644588 | 44232310 | 99.47 | 97.37 | 95.43 | 48.5 |
| T32-4d-3 | 44734602 | 41343752 | 99.45 | 97.34 | 95.27 | 48.5 |
| T32-13d-1 | 54273686 | 52176884 | 99.64 | 97.75 | 96.87 | 48.5 |
| T32-13d-2 | 53996890 | 51940710 | 99.78 | 98.01 | 97.11 | 48.5 |
| T32-13d-3 | 54016228 | 51907586 | 99.71 | 97.79 | 96.83 | 49.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, Y.; Li, L.; Long, H.; Zhang, D.; Wang, C.; Hao, R.; Li, H.; Ru, X.; Deng, Q.; Hu, Q.; et al. Transcriptome Analyses Reveal the Molecular Response of Juvenile Greater Amberjack (Seriola dumerili) to Marine Heatwaves. Animals 2025, 15, 1871. https://doi.org/10.3390/ani15131871
Tian Y, Li L, Long H, Zhang D, Wang C, Hao R, Li H, Ru X, Deng Q, Hu Q, et al. Transcriptome Analyses Reveal the Molecular Response of Juvenile Greater Amberjack (Seriola dumerili) to Marine Heatwaves. Animals. 2025; 15(13):1871. https://doi.org/10.3390/ani15131871
Chicago/Turabian StyleTian, Yali, Liancheng Li, Hongzhao Long, Dongying Zhang, Chen Wang, Ruijuan Hao, Hang Li, Xiaoying Ru, Qiuxia Deng, Qin Hu, and et al. 2025. "Transcriptome Analyses Reveal the Molecular Response of Juvenile Greater Amberjack (Seriola dumerili) to Marine Heatwaves" Animals 15, no. 13: 1871. https://doi.org/10.3390/ani15131871
APA StyleTian, Y., Li, L., Long, H., Zhang, D., Wang, C., Hao, R., Li, H., Ru, X., Deng, Q., Hu, Q., Huang, Y., & Zhu, C. (2025). Transcriptome Analyses Reveal the Molecular Response of Juvenile Greater Amberjack (Seriola dumerili) to Marine Heatwaves. Animals, 15(13), 1871. https://doi.org/10.3390/ani15131871





