Lutein and Astaxanthin Supplementation Induce Competitive Inhibition of Carotenoid Deposition in Egg Yolk
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Laying Performance and Egg Quality Measurement
2.3. Determination of Lutein and Astaxanthin Content in Egg Yolk
2.4. Carotenoid Profile Analysis in Egg Yolk
2.5. Statistical Analysis
3. Results
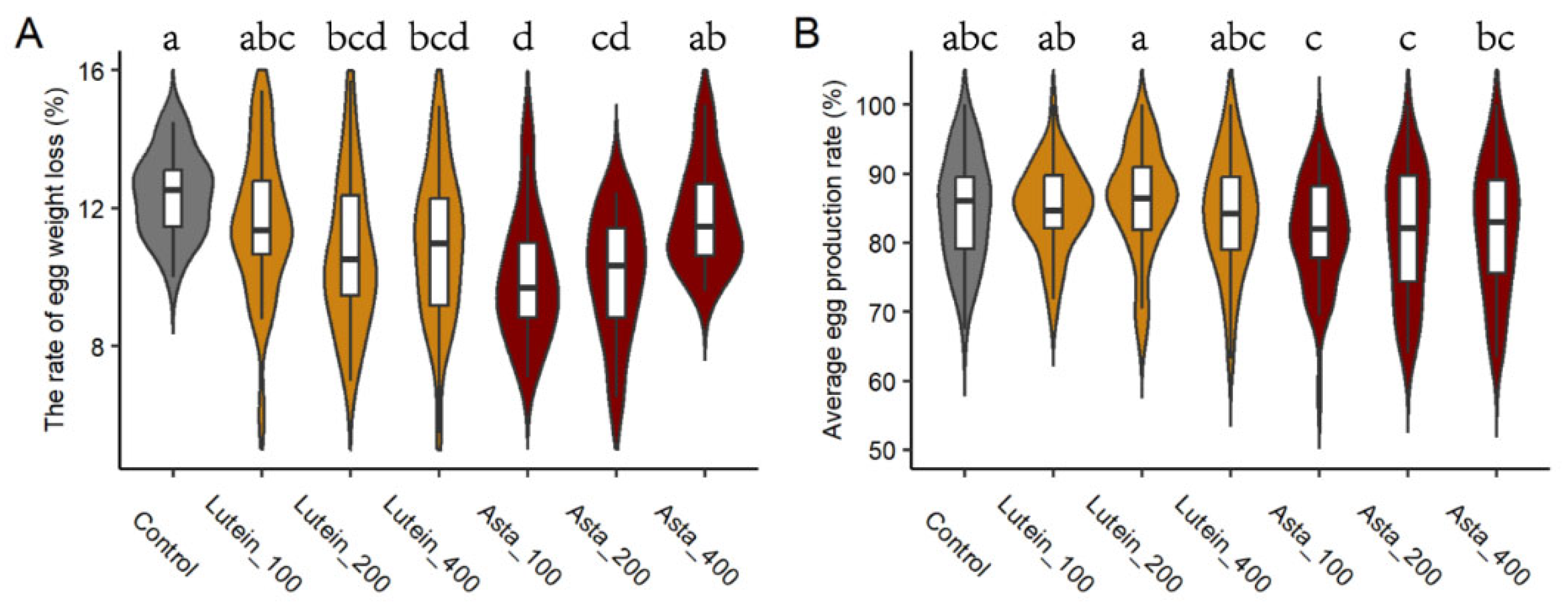
3.1. Laying Performance and Egg Quality
3.2. Astaxanthin Competitively Inhibits Lutein Deposition in Egg Yolk
3.3. Lutein and Astaxanthin Supplement Influencing Carotenoid Deposition in Egg Yolk
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| YC | Yolk Color |
| ESS | Eggshell Strength |
| EST | Eggshell Thickness |
| ESC | Eggshell Color |
| ESI | Egg Shape Index |
| EW | Egg Weight |
| AH | Albumen Height |
| HU | Haugh unit |
References
- Eggersdorfer, M.; Wyss, A. Carotenoids in human nutrition and health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Terao, J. Revisiting carotenoids as dietary antioxidants for human health and disease prevention. Food Funct. 2023, 14, 7799–7824. [Google Scholar] [CrossRef] [PubMed]
- Grčević, M.; Kralik, Z.; Kralik, G.; Galović, O. Effects of dietary marigold extract on lutein content, yolk color and fatty acid profile of omega-3 eggs. J. Sci. Food Agric. 2019, 99, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Kljak, K.; Carović-Stanko, K.; Kos, I.; Janječić, Z.; Kiš, G.; Duvnjak, M.; Safner, T.; Bedeković, D. Plant Carotenoids as Pigment Sources in Laying Hen Diets: Effect on Yolk Color, Carotenoid Content, Oxidative Stability and Sensory Properties of Eggs. Foods 2021, 10, 721. [Google Scholar] [CrossRef]
- Zhao, Y.C.; Li, X.Y.; Wang, C.C.; Yang, J.Y.; Xue, C.H.; Zhang, T.T.; Wang, Y.M. Free astaxanthin-rich diets enhanced astaxanthin accumulation in egg yolks compared to esterified astaxanthin-rich diets. Food Chem. 2023, 405, 134872. [Google Scholar] [CrossRef]
- Altmann, B.A.; Trinks, A.; Mörlein, D. Consumer preferences for the color of unprocessed animal foods. J. Food Sci. 2023, 88, 909–925. [Google Scholar] [CrossRef]
- Kojima, S.; Koizumi, S.; Kawami, Y.; Shigeta, Y.; Osawa, A. Effect of Dietary Carotenoid on Egg Yolk Color and Singlet Oxygen Quenching Activity of Laying Hens. J. Poult. Sci. 2022, 59, 137–142. [Google Scholar] [CrossRef]
- Hussein, M.A.; Rehan, I.F.; Rehan, A.F.; Eleiwa, N.Z.; Abdel-Rahman, M.A.M.; Fahmy, S.G.; Ahmed, A.S.; Youssef, M.; Diab, H.M.; Batiha, G.E.; et al. Egg Yolk IgY: A Novel Trend of Feed Additives to Limit Drugs and to Improve Poultry Meat Quality. Front. Vet. Sci. 2020, 7, 350. [Google Scholar] [CrossRef]
- Surai, P.F.; Sparks, N.H.C. Designer eggs: From improvement of egg composition to functional food. Trends Food Sci. Technol. 2001, 12, 7–16. [Google Scholar] [CrossRef]
- Yabuzaki, J. Carotenoids Database: Structures, chemical fingerprints and distribution among organisms. Database J. Biol. Databases Curation 2017, 2017, bax004. [Google Scholar] [CrossRef]
- Doukani, K.; Selles, A.S.M.; Bouhenni, H.; Chafaa, M.; Soudani, L. Chapter4.4—Carotenoids (Xanthophylls and Carotenes). In Antioxidants Effects in Health; Nabavi, S.M., Silva, A.S., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 279–308. [Google Scholar]
- Bernstein, P.S.; Li, B.; Vachali, P.P.; Gorusupudi, A.; Shyam, R.; Henriksen, B.S.; Nolan, J.M. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog. Retin. Eye Res. 2016, 50, 34–66. [Google Scholar] [CrossRef] [PubMed]
- Medoro, A.; Davinelli, S.; Milella, L.; Willcox, B.J.; Allsopp, R.C.; Scapagnini, G.; Willcox, D.C. Dietary Astaxanthin: A Promising Antioxidant and Anti-Inflammatory Agent for Brain Aging and Adult Neurogenesis. Mar. Drugs 2023, 21, 643. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Deng, X.; Ji, X.; Liu, N.; Cai, H. Sources, dynamics in vivo, and application of astaxanthin and lutein in laying hens: A review. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2023, 13, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Heng, N.; Gao, S.; Chen, Y.; Wang, L.; Li, Z.; Guo, Y.; Sheng, X.; Wang, X.; Xing, K.; Xiao, L.; et al. Dietary supplementation with natural astaxanthin from Haematococcus pluvialis improves antioxidant enzyme activity, free radical scavenging ability, and gene expression of antioxidant enzymes in laying hens. Poult. Sci. 2021, 100, 101045. [Google Scholar] [CrossRef]
- Dansou, D.M.; Wang, H.; Nugroho, R.D.; He, W.; Zhao, Q.; Tang, C.; Zhang, H.; Zhang, J. Effects of duration and supplementation dose with astaxanthin on egg fortification. Poult. Sci. 2021, 100, 101304. [Google Scholar] [CrossRef]
- Lokaewmanee, K.; Yamauchi, K.-e.; Komori, T.; Saito, K. Enhancement of Yolk Color in Raw and Boiled Egg Yolk with Lutein from Marigold Flower Meal and Marigold Flower Extract. J. Poult. Sci. 2011, 48, 25–32. [Google Scholar] [CrossRef]
- Papadopoulos, G.A.; Chalvatzi, S.; Kopecký, J.; Arsenos, G.; Fortomaris, P.D. Effects of dietary fat source on lutein, zeaxanthin and total carotenoids content of the egg yolk in laying hens during the early laying period. Br. Poult. Sci. 2019, 60, 431–438. [Google Scholar] [CrossRef]
- Wang, H.; He, W.; Mahukpégo Dansou, D.; Zhang, H.; Dwi Nugroho, R.; Tang, C.; Guo, X.; Yu, Y.; Zhao, Q.; Qin, Y.; et al. Astaxanthin improved the storage stability of docosahexaenoic acid-enriched eggs by inhibiting oxidation of non-esterified poly-unsaturated fatty acids. Food Chem. 2022, 381, 132256. [Google Scholar] [CrossRef]
- van den Berg, H. Carotenoid interactions. Nutr. Rev. 1999, 57, 1–10. [Google Scholar] [CrossRef]
- Wang, Y.; Roger Illingworth, D.; Connor, S.L.; Barton Duell, P.; Connor, W.E. Competitive inhibition of carotenoid transport and tissue concentrations by high dose supplements of lutein, zeaxanthin and beta-carotene. Eur. J. Nutr. 2010, 49, 327–336. [Google Scholar] [CrossRef]
- DB11/T 1378-2023. Available online: https://codeofchina.com/standard/DB11T1378-2023.html (accessed on 21 June 2025).
- GB 5009.248-2016. Available online: https://www.chinesestandard.net/PDF.aspx/GB5009.248-2016 (accessed on 21 June 2025).
- Sinanoglou, V.J.; Strati, I.F.; Miniadis-Meimaroglou, S. Lipid, fatty acid and carotenoid content of edible egg yolks from avian species: A comparative study. Food Chem. 2011, 124, 971–977. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Q.; Cao, Y.-M.; Hou, M.-X.; Zhao, R.; Chen, Y.-J.; Yu, S.-T.; Wang, K.-K.; Zhang, Q.; Li, S.-J.; et al. Genome-wide association analysis identifies genetic variants associated with muscle fatty acids and amino acids in grass carp (Ctenopharyngodon idella). Agric. Commun. 2024, 2, 100043. [Google Scholar] [CrossRef]
- Dansou, D.M.; Zhang, H.; Yu, Y.; Wang, H.; Tang, C.; Zhao, Q.; Qin, Y.; Zhang, J. Carotenoid enrichment in eggs: From biochemistry perspective. Anim. Nutr. (Zhongguo Xu Mu Shou Yi Xue Hui) 2023, 14, 315–333. [Google Scholar] [CrossRef]
- Chung, H.Y.; Rasmussen, H.M.; Johnson, E.J. Lutein bioavailability is higher from lutein-enriched eggs than from supplements and spinach in men. J. Nutr. 2004, 134, 1887–1893. [Google Scholar] [CrossRef]
- Berkhoff, J.; Alvarado-Gilis, C.; Keim, J.P.; Alcalde, J.A.; Vargas-Bello-Pérez, E.; Gandarillas, M. Consumer preferences and sensory characteristics of eggs from family farms. Poult. Sci. 2020, 99, 6239–6246. [Google Scholar] [CrossRef]
- Zhu, Y.; Yin, L.; Ge, J.; Wu, X.; Peng, Y.; Zhang, T.; Jiang, M. Astaxanthin supplementation enriches productive performance, physiological and immunological responses in laying hens. Anim. Biosci. 2021, 34, 443–448. [Google Scholar] [CrossRef]
- Pirgozliev, V.R.; Whiting, I.M.; Kljak, K.; Mansbridge, S.C.; Atanasov, A.G.; Rose, S.P.; Enchev, S.B. Stevia (Stevia rebaudiana) Improves Carotenoid Content in Eggs When Fed to Laying Hens. Foods 2022, 11, 1418. [Google Scholar] [CrossRef]
- Obianwuna, U.E.; Oleforuh-Okoleh, V.U.; Wang, J.; Zhang, H.J.; Qi, G.H.; Qiu, K.; Wu, S.G. Potential Implications of Natural Antioxidants of Plant Origin on Oxidative Stability of Chicken Albumen during Storage: A Review. Antioxidants 2022, 11, 630. [Google Scholar] [CrossRef]
- Wen, C.; Su, Y.; Tao, Z.; Cheng, Z.; Zhou, D.; Wang, T.; Zhou, Y. Dietary Supplementation with Microencapsulated Lutein Improves Yolk Color and Lutein Content in Fresh and Cooked Eggs of Laying Hens. J. Poult. Sci. 2021, 58, 97–102. [Google Scholar] [CrossRef]
- Walker, L.A.; Wang, T.; Xin, H.; Dolde, D. Supplementation of laying-hen feed with palm tocos and algae astaxanthin for egg yolk nutrient enrichment. J. Agric. Food Chem. 2012, 60, 1989–1999. [Google Scholar] [CrossRef]
- Ladygin, V.G. Lutein-5,6-epoxide aycle: A new xanthophyll cycle in higher plant chloroplasts. Biochem. (Mosc.) Suppl. Ser. A Membr. Cell Biol. 2008, 2, 110–118. [Google Scholar] [CrossRef]
- Fitzpatrick, N.; Chachay, V.; Bowtell, J.; Jackman, S.; Capra, S.; Shore, A.; Briskey, D. An appraisal of trials investigating the effects on macular pigment optical density of lutein and zeaxanthin dietary interventions: A narrative review. Nutr. Rev. 2022, 80, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Zivkovic, A.M.; Yiu, G.; Hackman, R.M. Potential roles of dietary zeaxanthin and lutein in macular health and function. Nutr. Rev. 2023, 81, 670–683. [Google Scholar] [CrossRef] [PubMed]
- Milani, A.; Basirnejad, M.; Shahbazi, S.; Bolhassani, A. Carotenoids: Biochemistry, pharmacology and treatment. Br. J. Pharmacol. 2017, 174, 1290–1324. [Google Scholar] [CrossRef]
- Manzoor, S.; Rashid, R.; Prasad Panda, B.; Sharma, V.; Azhar, M. Green extraction of lutein from marigold flower petals, process optimization and its potential to improve the oxidative stability of sunflower oil. Ultrason. Sonochem. 2022, 85, 105994. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, D.B.; Mercadante, A.Z.; Mariutti, L.R.B. Marigold carotenoids: Much more than lutein esters. Food Res. Int. (Ott. Ont.) 2019, 119, 653–664. [Google Scholar] [CrossRef]
- Ren, Y.; Deng, J.; Huang, J.; Wu, Z.; Yi, L.; Bi, Y.; Chen, F. Using green alga Haematococcus pluvialis for astaxanthin and lipid co-production: Advances and outlook. Bioresour. Technol. 2021, 340, 125736. [Google Scholar] [CrossRef]
- Li, Y.; Huang, J.; Sandmann, G.; Chen, F. High-Light and Sodium Chloride Stress Differentially Regulate the Biosynthesis of Astaxanthin in Chlorella zofingiensis (Chlorophyceae). J. Phycol. 2009, 45, 635–641. [Google Scholar] [CrossRef]
- Hong, M.E.; Chang, W.S.; Patel, A.K.; Oh, M.S.; Lee, J.J.; Sim, S.J. Microalgal-Based Carbon Sequestration by Converting LNG-Fired Waste CO2 into Red Gold Astaxanthin: The Potential Applicability. Energies 2019, 12, 1718. [Google Scholar] [CrossRef]
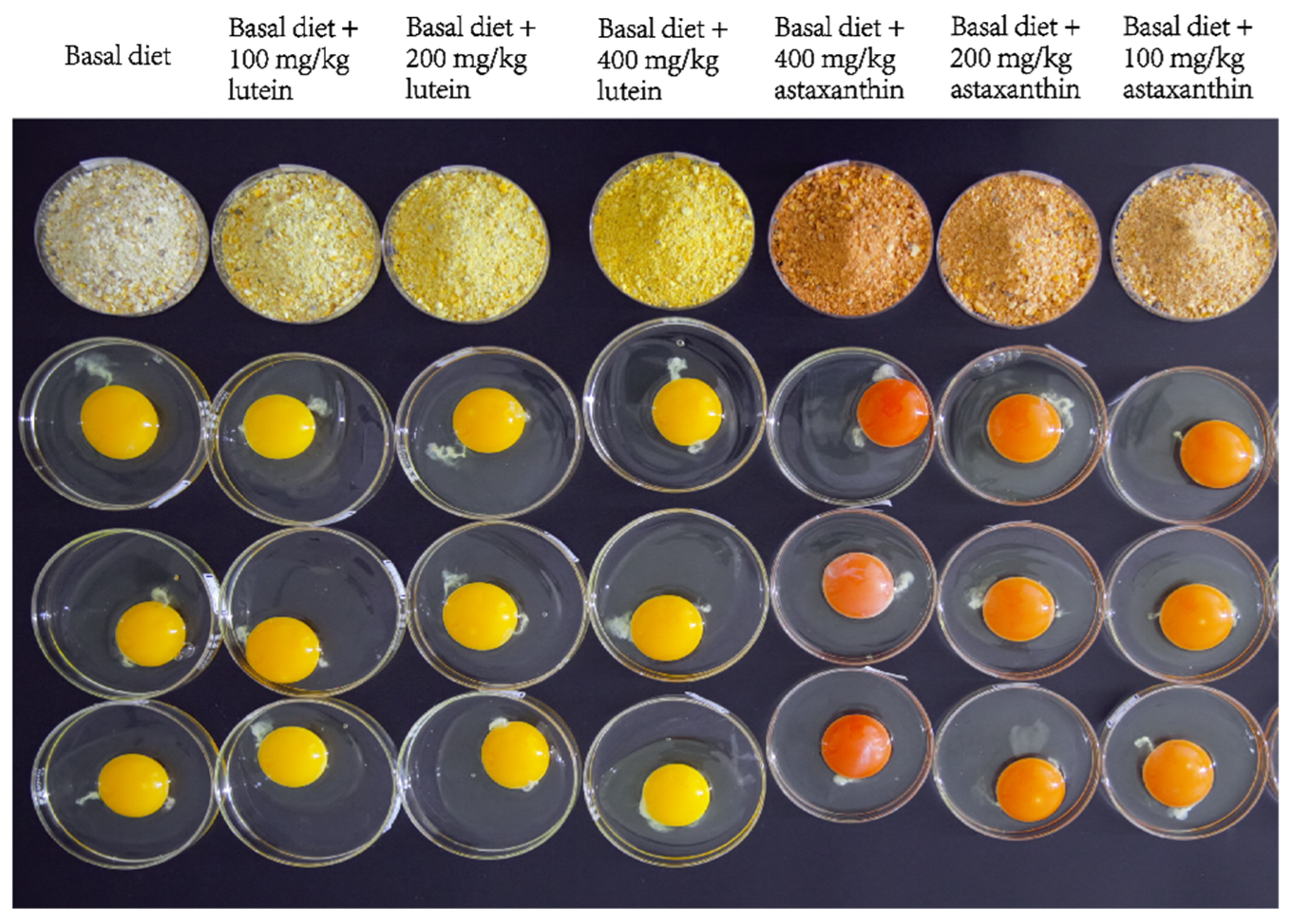

| Item | Control Group | Lutein Supplementation Group | Astaxanthin Supplementation Group | ||||
|---|---|---|---|---|---|---|---|
| Lutein_100 | Lutein_200 | Lutein_400 | Asta_100 | Asta_200 | Asta_400 | ||
| YC 1 | 9.13 ± 0.60 e | 10.5 ± 1.02 d | 11.0 ± 0.87 c | 11.4 ± 0.81 c | 16.57 ± 0.74 b | 17.68 ± 0.91 a | 18.01 ± 0.69 a |
| ESS (kg/cm2) | 3.98 ± 0.53 ab | 3.75 ± 0.65 b | 3.96 ± 0.79 ab | 3.80 ± 0.62 b | 4.24 ± 0.56 a | 4.24 ± 0.69 a | 3.83 ± 0.71 b |
| EST (cm) | 0.31 ± 0.03 | 0.30 ± 0.03 | 0.31 ± 0.02 | 0.31 ± 0.03 | 0.32 ± 0.03 | 0.31 ± 0.02 | 0.30 ± 0.02 |
| ESC-L* | 76.10 ± 4.50 abc | 75.95 ± 4.06 abc | 74.30 ± 5.21 bc | 75.22 ± 3.77 abc | 73.50 ± 11.64 c | 77.16 ± 3.72 ab | 78.10 ± 4.34 a |
| ESC-a* | 9.00 ± 2.49 ab | 9.53 ± 2.70 ab | 10.31 ± 2.44 a | 9.96 ± 2.32 a | 9.56 ± 3.93 ab | 9.09 ± 2.41 ab | 8.28 ± 2.85 b |
| ESC-b* | 20.05 ± 3.43 ab | 21.31 ± 3.17 ab | 21.73 ± 3.24 a | 21.43 ± 3.10 ab | 20.12 ± 5.62 ab | 20.39 ± 2.71 ab | 19.25 ± 3.31 b |
| ESI | 1.31 ± 0.04 | 1.31 ± 0.05 | 1.30 ± 0.04 | 1.31 ± 0.05 | 1.30 ± 0.04 | 1.31 ± 0.04 | 1.30 ± 0.04 |
| EW (g) | 45.3 ± 3.89 ab | 44.8 ± 3.13 b | 46.4 ± 3.49 ab | 45.3 ± 2.62 ab | 45.9 ± 3.54 ab | 46.8 ± 2.95 a | 46.5 ± 3.30 ab |
| AH (mm) | 4.01 ± 0.71 | 3.74 ± 0.89 | 3.82 ± 0.64 | 3.96 ± 0.76 | 3.86 ± 0.62 | 3.96 ± 0.72 | 3.94 ± 0.53 |
| HU | 66.1 ± 6.82 | 63.2 ± 10.7 | 63.8 ± 6.06 | 65.6 ± 7.55 | 64.5 ± 6.28 | 64.7 ± 6.97 | 65.1 ± 4.72 |
| Item | Control Group | Lutein Supplementation Group | Astaxanthin Supplementation Group | ||||
|---|---|---|---|---|---|---|---|
| Lutein_100 | Lutein_200 | Lutein_400 | Asta_100 | Asta_200 | Asta_400 | ||
| AH (mm) | 2.57 ± 1.61 | 2.54 ± 1.29 | 2.32 ± 1.07 | 2.72 ± 1.30 | 2.63 ± 1.19 | 2.97 ± 1.53 | 2.61 ± 1.39 |
| HU | 51.04 ± 17.81 | 51.53 ± 14.97 | 49.45 ± 12.36 | 53.77 ± 14.72 | 51.77 ± 13.6 | 54.87 ± 15.76 | 50.76 ± 17.02 |
| Compounds | Control Group | Lutein Supplementation Group | Astaxanthin Supplementation Group | ||||
|---|---|---|---|---|---|---|---|
| Lutein_100 | Lutein_200 | Lutein_400 | Asta_100 | Asta_200 | Asta_400 | ||
| Lutein | 13.67 ± 1.13 c | 43.96 ± 0.51 b | 51.18 ± 13.38 a | 54.02 ± 4.86 a | 11.34 ± 1.45 c | 10.13 ± 1.63 c | 7.02 ± 0.17 c |
| Astaxanthin | ND | ND | ND | ND | 19.58 ± 2.16 b | 30.37 ± 4.02 a | 29.08 ± 8.44 a |
| 5,6 epoxy-lutein-caprate-palmitate | 14.31 ± 5.26 | 14.81 ± 1.19 | 16.14 ± 1.02 | 15.69 ± 0.84 | 14.56 ± 1.28 | 13.32 ± 1.83 | 12.99 ± 2.24 |
| Zeaxanthin | 11.75 ± 1.80 a | 9.55 ± 0.69 b | 8.05 ± 1.41 bc | 6.26 ± 1.43 cd | 7.45 ± 0.63 c | 6.35 ± 0.73 cd | 4.44 ± 0.26 d |
| Violaxanthin-myristate-caprate | 0.47 ± 0.14 ab | 0.38 ± 0.04 b | 0.36 ± 0.04 b | 0.47 ± 0.11 ab | 0.46 ± 0.06 ab | 0.48 ± 0.03 ab | 0.54 ± 0.06 a |
| Lutein oleate | 0.37 ± 0.05 c | 0.81 ± 0.05 b | 1.22 ± 0.09 a | 1.49 ± 0.49 a | 0.33 ± 0.09 c | 0.28 ± 0.07 c | 0.34 ± 0.05 c |
| Capsorubin | 0.32 ± 0.09 | 0.35 ± 0.06 | 0.35 ± 0.01 | 0.39 ± 0.12 | 0.33 ± 0.07 | 0.30 ± 0.03 | 0.30 ± 0.14 |
| Violaxanthin myristate | 0.31 ± 0.07 | 0.35 ± 0.09 | 0.38 ± 0.10 | 0.33 ± 0.07 | 0.35 ± 0.08 | 0.37 ± 0.01 | 0.29 ± 0.04 |
| Lutein palmitate | 0.18 ± 0.02 c | 0.25 ± 0.03 bc | 0.33 ± 0.03 ab | 0.37 ± 0.14 a | 0.17 ± 0.04 c | 0.18 ± 0.02 c | 0.23 ± 0.01 c |
| Lutein dipalmitate | 0.14 ± 0.02 | 0.12 ± 0.03 | 0.15 ± 0.04 | 0.15 ± 0.06 | 0.15 ± 0.06 | 0.13 ± 0.03 | 0.11 ± 0.01 |
| β-cryptoxanthin | 0.12 ± 0.01 ab | 0.12 ± 0.02 ab | 0.12 ± 0.02 ab | 0.11 ± 0.02 bc | 0.14 ± 0.01 a | 0.13 ± 0.01 ab | 0.09 ± 0.01 c |
| α-cryptoxanthin | 0.11 ± 0.00 bc | 0.12 ± 0.01 ab | 0.14 ± 0.02 a | 0.12 ± 0.02 bc | 0.09 ± 0.01 cd | 0.08 ± 0.01 de | 0.06 ± 0 e |
| Lutein stearate | 0.08 ± 0.00 b | 0.10 ± 0.02 ab | 0.11 ± 0.03 a | 0.12 ± 0.01 a | 0.11 ± 0.01 ab | 0.07 ± 0.01 b | 0.10 ± 0.02 ab |
| Canthaxanthin | 0.01 ± 0.01 d | 0.02 ± 0.00 d | 0.03 ± 0.00 d | 0.03 ± 0.01 d | 0.16 ± 0.02 c | 0.27 ± 0.02 b | 0.35 ± 0.03 a |
| Total | 41.79 e | 70.94 b | 78.56 a | 79.55 a | 55.22 d | 62.46 c | 55.94 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, X.; Yan, Z.; Zhang, B.; Zeng, L.; Chowdhury, U.; Pabitra, M.H.; Cao, J.; Wang, Z.; He, Y.; Liu, H.; et al. Lutein and Astaxanthin Supplementation Induce Competitive Inhibition of Carotenoid Deposition in Egg Yolk. Animals 2025, 15, 1869. https://doi.org/10.3390/ani15131869
Chen X, Yan Z, Zhang B, Zeng L, Chowdhury U, Pabitra MH, Cao J, Wang Z, He Y, Liu H, et al. Lutein and Astaxanthin Supplementation Induce Competitive Inhibition of Carotenoid Deposition in Egg Yolk. Animals. 2025; 15(13):1869. https://doi.org/10.3390/ani15131869
Chicago/Turabian StyleChen, Xia, Zhixun Yan, Bing Zhang, Lingchao Zeng, Urmita Chowdhury, Mohammad Hasanuzzaman Pabitra, Jing Cao, Zhipeng Wang, Yanghua He, Huagui Liu, and et al. 2025. "Lutein and Astaxanthin Supplementation Induce Competitive Inhibition of Carotenoid Deposition in Egg Yolk" Animals 15, no. 13: 1869. https://doi.org/10.3390/ani15131869
APA StyleChen, X., Yan, Z., Zhang, B., Zeng, L., Chowdhury, U., Pabitra, M. H., Cao, J., Wang, Z., He, Y., Liu, H., & Chu, Q. (2025). Lutein and Astaxanthin Supplementation Induce Competitive Inhibition of Carotenoid Deposition in Egg Yolk. Animals, 15(13), 1869. https://doi.org/10.3390/ani15131869





